Exercises
Supporting Files and Up-to-Date Exercise Material Is Available on the Book's Companion Website
11.1. Problems
![]()
A (2-octanol) + B (nitrosonium ion) → P (2-octanone) + 2B (nitrosonium ion) …(11.1)
P (2-octanone) + B (nitrosonium ion) → E (carboxylic acid) …(11.2)
Table 11.1
LFL Values for Chemicals in Example 1
| Chemical | LFL Values (Vol.% in Air) | |
| Lewis and Elbe (2013) | National Fire Protection Association (1986) | |
| Ethyl acetate | 2.5 | 2.0 |
| Ethyl alcohol | 4.3 | 3.3 |
| Toluene | 1.4 | 1.2 |

Table 11.2
| Parameters | Reaction (R1) | Reaction (R2) | Reaction (R3) |
| K (m3 mol−1 s−1) | 96,000 | 9600 | 960 |
| E (J mol−1) | 75,362 | 82,899 | 91,189 |
| −91,360 | −111,000 | −244,600 |
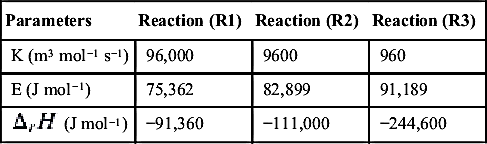
Table 11.3
Initial Conditions of the Reactor
| CPropylene oxide,0 (mol/s) | 13 |
| CWater,0 (mol/s) | 6.8 |
| T0 (C) (feed temperature) | 26 |
| T0,1 (K) | 470 |
| T0,2 (K) | 425.7 |
| T0,3 (K) | 400 |
| Vi=1,2,3 (m3) | 1 |
Table 11.4
Initial Conditions around Heat Exchanger
| UA (kW/K) | 10 |
| Cooling medium | Water |
| Fwater (mol/s) | 105 |
Table 11.5
Quantitative Representation of Qualitative HAZOP Keywords
| Parts/Components | Qualitative Failure Mode | Quantitative Representation |
| Valve | Open failure/closed failure | |
| Heat exchanger | Fouling, plugging | |
| Separator | Open failure/closed failure |
![]()
![]()
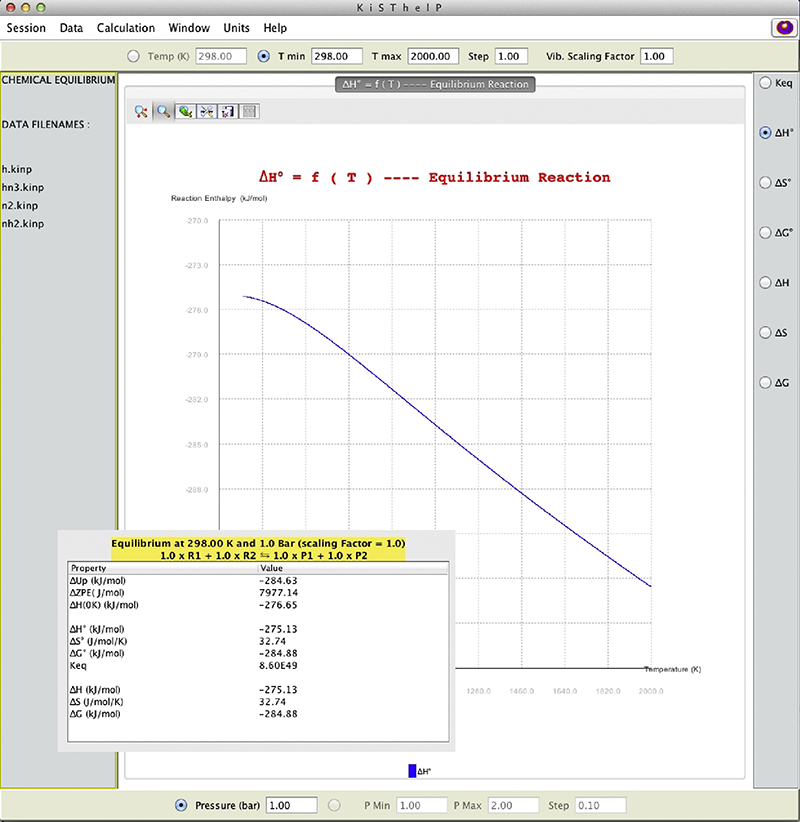
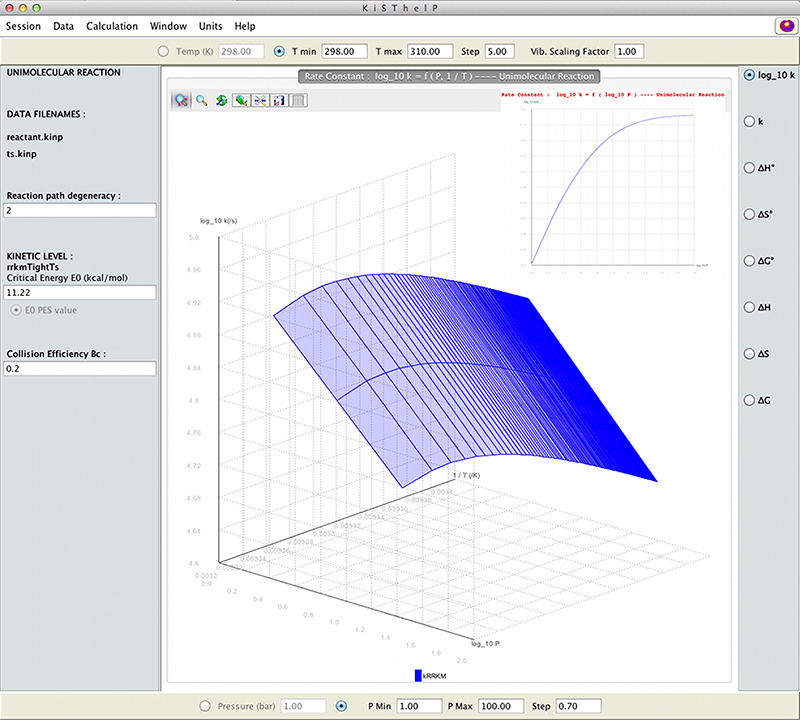
11.2. Application of KiSThelP Software for Explosive Decomposition Reaction
H + HN3 → NH2 + N2
11.2.1. Context
11.2.2. Aim
11.2.3. General Methodology
H + HN3 → H2N3 → NH2 + N2
11.3. Application to a Reaction of Atmospheric Interest
CH3OCH2OCH2O → H + CH3OCH2OCHO