Diffusion fires are a subset of fires in which the fuel and oxidizer are physically separated with a region of rapid combustion between them. In this sense, many solid material fires are diffusional in nature with pyrolysis vapor diffusing away from a material surface where it then reacts with oxygen (
Drysdale, 2011). More traditional examples of diffusion fires include jet fires, which are a release of flammable material from an orifice into ambient conditions, where it ignites and forms a fire jet.
Jet fires
Jet fires occur when a pressurized flammable material is released from an opening to a lower pressure exterior. Jet fires can be further categorized as either laminar or turbulent. The transition between these two types of fire is categorized by a dimensionless quantity called the Reynolds number, shown in
Eqn (2.8).
Re=ρvDμ=inertial effectsviscous effects
 (2.8)
(2.8)
where
Re is the Reynolds number,
ρ is the density of the fluid exiting the orifice,
v is the velocity of fluid flow out of the orifice,
D is the characteristic length of the orifice, and
μ is the viscosity of the fluid exiting the orifice.
The dimensionless Reynolds number makes it simple and unambiguous to classify jet flames, regardless of scale. Laminar jet fires have Reynolds numbers less than 2000 at the orifice opening, while turbulent jet fires occur at substantially higher Reynolds numbers (
Hottel and Hawthorne, 1949). These flammable jets have been shown experimentally to have a flame height dependency on the square root of the volumetric flow rate. However, because these fires usually occur at low Froude numbers, buoyant factors play a large role in flame behavior (
Jost, 1939). Therefore, different materials have the potential to have noticeably different laminar jet flames.
At the micro-scale, most jet flames are classified as laminar because of their low Reynolds numbers. However, as the scale of jet flames decrease to the micro-scale, new complications arise. Mixing becomes increasingly poor and dominated by diffusion, which can lead to flame instabilities and complicated transient behavior (
Miesse et al., 2005). Additionally, the smaller size of a jet flame means a greater surface area to volume ratio and an increased amount of heat loss, making micro jet flame behavior deviate from conventional laminar jet flames even more.
The second set of jet fire, turbulent jet fires, occurs at Reynolds numbers much greater than 2000 (
Hottel and Hawthorne, 1949). As the velocity of the fuel increases, the flame begins to break up at the end (opposite of the fuel release). As the velocity increases further, this point of flame breakup gets closer and closer to the nozzle but never reaches it (
Hottel and Hawthorne, 1949).
Interestingly, the efficiency of fuel burning is also increased in turbulent jet fires. High turbulence correlates to less soot formation and less heat loss in the fire overall, creating a flame that burns more completely and at higher temperatures (
Delichatsios and Orloff, 1988). The flame height dependencies differ between turbulent and laminar jet fires. Instead of being related to volumetric flow rate, the height of turbulent jet fires is linearly dependent on the release orifice (
Kanury, 1975).
Another important hazard parameter to calculate is the mass flow rate of flammable material during a jet fire. Since this value will affect burning rates and incident severities, determining the amount of flammable material release during a jet flame is important. Analytical and semianalytical models have been derived in the past for the release of pressurized fluids through orifices by analyzing the transport phenomena taking place. One such model is derived from a momentum balance around the orifice and relates the mass loss rate to general process conditions and material properties (
American Institute of Chemical Engineers. Center for Chemical Process Safety. 2003). This model, shown in
Eqn (2.9), assumes adiabatic expansion, which seems reasonable because the flammable gas is released and expands very quickly.
M=CdρaAh2Ppρp(kk−1)[1−(PaPp)k−1k]−−−−−−−−−−−−−−−−−−−−⎷
 (2.9)
(2.9)
where
M is the mass flow rate,
Cd is the coefficient of discharge (0.85 typically for gas release),
Ah is the area of the discharge opening,
ρa is the density of the ambient air,
ρp is the density of the process flammable fluid under pressure (the fluid that will be released),
Pa is the ambient pressure,
Pp is the
pressure of the flammable process fluid under pressure, and
k is the isentropic expansion factor defined in
Eqn (2.10) as
Pvk=c
 (2.10)
(2.10)
where P is the pressure of the flammable fluid, v is the specific volume of the flammable fluid, k is the isentropic expansion factor, and c is a constant.
However, there exists a specific situation where a maximum mass release is achieved and increasing the process pressure of the fluid or reducing the ambient pressure does not increase the mass release rate further. When the maximum release rate is achieved, it is called choked flow. Finding the maximum mass flow rate with respect to downstream pressure yields the criteria for choked flow shown in
Eqn (2.11).
(PaPp)choked=(2k+1)k/(k−1)
 (2.11)
(2.11)
When choked flow condition is achieved,
Eqn (2.9) is simplified and expressed as
Eqn (2.12) and is no longer dependent on ambient conditions.
Mmax=CdAhPpρpk(2k+1)(k+1)/(k−1)−−−−−−−−−−−−−−−−−√
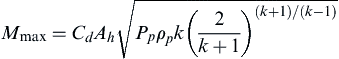 (2.12)
(2.12)
where Mmax is the choked release rate.
After the release rate of the jet fire is established, the heat release rate is calculated simply by multiplying the mass flow rate by the heat of combustion for the flammable fluid. This heat release rate assumes complete combustion making it a conservative estimate. If the ambient air is relatively stationary and fairly laminar, the flame length can be calculated by
Eqn (2.13) (
Beyler, 2002).
L=0.2Q˙25
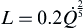 (2.13)
(2.13)
where
Q˙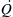
is the heat release rate, and
L is the flame length.
As ambient turbulence increases, the flame length become affected more and this model fails to provide useful results. It should also be noted that if the path of the jet flame is obstructed, it can greatly change the size and shape of the flame.
Pool fires
Another class of diffusional flame is the pool fire. As the name implies, a pool fire occurs when a pool of material forms and produces enough vapor to create an ignitable fuel source above the pool. Pool fires are generally a liquid, although gas and solid pool fires (such as a dense pool of gas or a solid polymer burning) also exist. Like some other types of diffusional flames, pool fires have three distinct regions: the fuel-rich core, the intermediate zone, and the downstream plume (
Bouhafid et al., 1898). These three regions are similar to the regions discussed in other natural diffusion fires.
The fuel-rich core lies directly above the burning material surface and contains vaporized flammable materials. The boundaries of this region are formed by the air-entrained eddies above in the intermediate zone. This region contains a relatively small amount of combustion because the vapor is so rich (
Smith and Cox, 1992). As the flammable vapor moves away from the material surface, it enters the intermediate zone where combustion is rapid. The movement of the flame up entrains cool, oxygen-rich air that fuels the fire as it moves upwards. Soot and products from incomplete combustion can be formed here as well. It is in this intermittent zone of a pool fire where the majority of heat is generated (
Smith and Cox, 1992).
As the heated combustion products and vortices continue to rise, they exit the visible region of the flame and continue through to the downstream plume, which contains heated combustion products mixing with ambient air. As expected, this downstream plume broadens and cools as it rises from the fire. As the temperature drops, the kinetics of the combustion reactions slow exponentially and the formation of combustion products stops (
Smith and Cox, 1992).
If the burning rate of the pool fire is assumed to be relatively constant (steady state), a simple approximation can be used to determine the steady-state diameter of the pool fire resulting from a given leak rate given in
Eqn (2.14) (
Spouge, 1999).
Dss=(4V˙Leakρπm˙′′)0.5
 (2.14)
(2.14)
where
Dss is the steady-state diameter of the pool fire,
V˙Leak
is the volumetric flow rate,
ρ is the density of the fuel, and
m˙′′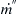
is the constant mass burning rate (
Spouge, 1999).
Heskestad proposed a simple model for the height for a pool fire dependent on the heat released by the fire and the diameter of the pool fire (
Heskestad, 1981,
1983). However, it should be noted that this model represented in
Eqn (2.15) is a simplification of pool fire phenomena and does not directly take into account many possible factors such as the vapor flow rate away from the burning material to the combustion region, effects of gravity, and time-dependent transient effects of the pool fire to name a few.
H=0.23Q˙2/5−1.02D
 (2.15)
(2.15)
where
D is the pool fire diameter, and the heat release rate,
Q˙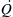
, can be written for a simplified pool fire as
Eqn (2.16).
Q˙=m˙ΔHc=m˙′′AΔHc
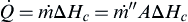 (2.16)
(2.16)
where
m˙
is the mass loss rate for the pool fire,
m˙′′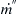
is the mass loss flux for the pool fire, and
A is the area (
American Institute of Chemical Engineers, Center for Chemical Process Safety, 2003). The fundamental heat, mass, and momentum transport make actual pool fire behavior very complicated, so resorting to dimensionless analysis is useful for pool fire analysis. Furthermore, the use of dimensionless variables allows fires at any scale to be comparable using the same measureable quantities. An important dimensionless parameter in the study of pool fire behavior is the dimensionless Froude number shown in
Eqn (2.17).
Fr=v2gD=inertial effectsbouyant effects
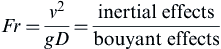 (2.17)
(2.17)
where Fr is the Froude number, v is the fluid velocity, g is gravity, and D is the characteristic length of the pool fire (the diameter).
The Froude number is useful in establishing whether buoyant effects from the heated combustion products or the velocity of the rising gases dominate the fluid behavior of the flame.
The models derived by Orloff and Ris show that the pool fire can move laterally back and forth over the boundaries of the burning fuel, but the amount of lateral movement at the base of the fire is dependent on the Froude number. At low values of Fr, the boundary of the fire is able to move farther laterally than at higher Fr values (
Orloff and Ris, 1982,
1983). With high Fr numbers, it seems the relatively high gas velocity up prevents the flame from deviating laterally compared with lower relative gas velocities associated with more rapid burning. Numerous studies have been conducted to determine the source of the pulsing behavior of diffusional flames with some progress; however, the true source of the instability of pool fires is still debated (
Hertzberg, Cashdollar et al., 1978;
Buckmaster and Peters, 1986;
Bejan, 1991). Another dimensionless variable that appears to be useful in pulsating pool fires is the Strouhal number, shown in
Eqn (2.18).
St=fDv
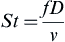 (2.18)
(2.18)
where
St is the Strouhal number,
f is the frequency of eddie shedding,
D is the characteristic length, and
v is the fluid velocity.
The Strouhal number contains information about the frequency of oscillations within the fluid flow. Substantial research has been done to correlate the Strouhal number and Froude number with relatively good results. Hamins showed that the relation between the Froude and Strouhal numbers as shown in
Eqn (2.19) was valid for various flames (
Hamins et al., 1992).
St∝Fr−0.38
 (2.19)
(2.19)
In addition to how a pool fire behaves, understanding the pool fires dimensions is important to fully understand the hazards they present. To first define the size of a fire, it is necessary to define the fire boundary. It is common to represent a fire as the region that emits visible light, but for turbulent flames with pulsating regions, this representation can become difficult. One way to combat this is to use a length measurement in which 50% of the flame would occur above that level and 50% of the flame would occur below that level. Using a dimensionless version of this flame length and a dimensionless heat release quantity, defined in
Eqns (2.20) and
(2.21), Heskestad developed a correlation for flame height as shown in
Eqn (2.22) that fits experimental data closely (
Heskestad, 1983;
Zukoski et al., 1984).
N=(rCpT0Hc)3Q˙2D
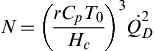 (2.20)
(2.20)
where
N is a dimensionless parameter,
r is the mass based stoichiometric air-to-fuel ratio,
Cp is the heat capacity of ambient air,
T0 is the ambient air temperature,
Hc is the fuel heat of combustion, and
Q˙D
is dimensionless heat released as defined in
Eqn (2.21) (
Heskestad, 1983).
Q˙D=Q˙ρ0CpT0(gD5)0.5
 (2.21)
(2.21)
where
Q˙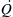
is the heat release rate of the fire,
ρ0 is the density of the ambient air,
g is the acceleration due to gravity, and
D is the characteristic length of the fire (
Heskestad, 1983).
ZfD=−1.02+15.6N1/5
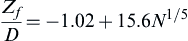 (2.22)
(2.22)
where Zf is the flame height, and D is pool diameter.
Although Heskestad's correlation seems to fit the majority of data very well, acetylene (C
2H
2) data do not match
Eqn (2.22) nearly as well as other mixtures (
Hamins et al., 1992). Because acetylene burning forms a significant amount of soot, length scales could be redefined within the fire to more closely fit
Eqn (2.22) (
Delichatsios et al., 1992). Therefore, caution should be used when applying Heskestad's correlation to sooty flammable mixtures.
The key hazard from pool fires is the heat produced. Heat in a pool fire can have three ultimate destinations. First, heat can be transferred from the buoyant flame and plume to the adjacent ambient air. Second, heat can be transferred from the flame to the fuel source. Third, heat can be transferred across open spaces via radiation (
Hamins et al., 1995).
The heat transferred from the buoyant plume to the surrounding ambient air is calculated through a simple energy balance and shown in
Eqn (2.23).
Qambient=vpApρpCpΔT
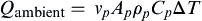 (2.23)
(2.23)
where
Qambient is the heat transferred to the ambient air,
vp is the velocity of the bouyant plume,
Ap is the average area of the plume,
ρp is the average density of the plume,
Cp is the specific heat of the plume, and Δ
T is the change in temperature of the plume from the hottest point of the plume to its coldest.
This equation is derived from a simple energy balance in which it is assumed that all the heat required to change in temperature of the buoyant plume is transferred to the ambient air with no energy losses or entropic considerations, so it is an approximate calculation (
Hamins et al., 1995).
Aside from heating the ambient air, a fire can also radiate heat over open space to heat other objects. Radiant heating is especially hazardous because it acts in all directions, unlike conductive and convective heating that are limited in this sense. A series of studies with heptane measured the radiant heat released by using a series of radiometers to obtain vertical and radial heat flux profiles. These profiles were then used to estimate radiant heat from a fire. The results show that the fraction of heat released as radiant energy in a heptane pool fire is significant and varies depending on the diameter of the pool fire. Under 2
m, the fraction of radiant heat is relatively constant, but as the diameter grows above 2
m, the fraction of radiant heat drops. The cause for this drop at 2
m is attributed to the soot formation within the fire. Cleaner burning fuels do not exhibit a drop in radiant heat as they grow because soot particles in the flame do not block radiant heat. However, as the size of a heptane pool fire increases, a lack of available oxygen causes incomplete combustion and the formation of soot that blocks radiant heat (
Hamins et al., 1995). This is similar to how standing in the shade on a hot day blocks heat from the sun. These data show that fires that form soot and carbonaceous particles are more likely to be colder than nonsooty counterparts.
A more direct way of determining the radiant heat released from a fire to an object is to use
Eqn (2.24) (
Shokri and Beyler, 1989).
Equation (2.24) requires only two parameters to calculate the heat flux and provides a relatively straightforward method for determining radiant energies.
q''=EF12
 (2.24)
(2.24)
where
q''
is the radiant heat flux,
F12 is the dimensionless view factor for the radiant energy, and
E is the total emissive energy flux from the fire given in
Eqn (2.25) as
E=58(10−0.00823D)
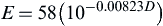 (2.25)
(2.25)
where D is the diameter of the fire.
The view factor is a complicated parameter that essentially determines the fraction of radiant energy from a fire that reaches a target. Fortunately, previous algebraic analysis of these systems has yielded plots to obtain the view factor easily. Primarily, the view factor is dependent on the ratio of flame height to radius, distance from the center of the fire to the object, and the height of the target object (to be heated by a fire). A series of plots for various combinations of these parameters is found in
Guidelines for Fire Protection in Chemical, Petrochemical, and Hydrocarbon Processing Facilities (
American Institute of Chemical Engineers, Center for Chemical Process Safety, 2003). Once the maximum emissive heat released from the fire is calculated assuming complete combustion of all flammable material, this can be multiplied by the view factor for the specific scenario to find the heat flux on an object.
Aside from heat transfer from the plume to the ambient air and radiant heating from the fire to surrounding objects, the third destination for heat from a fire is to the fuel. When a fire burns, heat is
transferred back toward the flame in a process called feedback. Feedback causes increased fuel vaporization, which in turn increases the burning rate and, thus, increases fuel vaporization further. A general expression for the heat transferred to the fuel can be written as
Eqn (2.26), although it is of limited use because the heat lost
(Q˙lost)
and heat associated with setting up a thermal boundary layer
(Q˙liquid)
are complex (
Hamins et al., 1995).
Q˙fuel=m˙Hg+Q˙lost+Q˙liquid
 (2.26)
(2.26)
where
Q˙fuel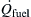
is the heat transferred to the fuel,
m˙
is the mass loss rate,
Hg is the heat of gasification,
Q˙lost
is the heat lost to the surroundings, and
Q˙liquid
is the heat associated with heating the liquid in the pool fire and establishing a boundary layer.
Without further detailed knowledge of the heat transfer in the fuel, it is difficult to calculate the rate of vaporization and thus the rate of burning in a pool fire. Empirical relations from literature suggest that mass flux is directly related to the
B number of the material as shown in
Eqn (2.27).
B=ΔHcΔHg
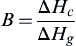 (2.27)
(2.27)
where B is the B number, ΔHc is the heat of combustion, and ΔHg is the heat required to gasify a material.
The wind effects of pool fires have been empirically observed and used to develop a model for the angle of flame and the dimensionless wind velocity. When the wind impinges on a fire, the vertical axis of the flame will tilt and rotate an angle θ around the base of the fire in the direction of the wind velocity. This correlation is outlined in
Eqns (2.28) through
(2.30) (
Society of Fire Protection Engineers, 2002):
cosθ=1foru∗≤1
 (2.28)
(2.28)
cosθ=1u∗√foru∗>1
 (2.29)
(2.29)
where θ is the angle of the flame due to the wind, and
u∗ is the dimensionless wind velocity given in
Eqn (2.30) as:
u∗=uw(gm˙′′D/ρv)1/3
 (2.30)
(2.30)
where
D if effective fire diameter,
uw is the wind speed measured at a height of 1.6
m,
g is the acceleration due to gravity,
m˙′′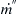
is the mass burning rate,
D is the pool fire diameter, and
ρv is the vapor density of the flammable vapor at the boiling point (
Society of Fire Protection Engineers, 2002).
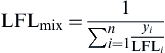 (2.1)
(2.1) (2.2)
(2.2)![]() (2.3)
(2.3)![]() (2.4)
(2.4)![]() (2.5)
(2.5)![]() (2.6)
(2.6)![]() (2.7)
(2.7)![]() (2.8)
(2.8) (2.9)
(2.9)![]() (2.10)
(2.10) (2.11)
(2.11)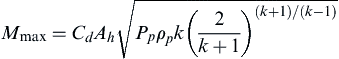 (2.12)
(2.12)![]() (2.13)
(2.13) (2.14)
(2.14)![]() (2.15)
(2.15)![]() (2.16)
(2.16)![]() (2.17)
(2.17)![]() (2.18)
(2.18)![]() (2.19)
(2.19)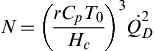 (2.20)
(2.20) (2.21)
(2.21)![]() (2.22)
(2.22)![]() (2.23)
(2.23)![]() (2.24)
(2.24)![]() (2.25)
(2.25)![]() (2.26)
(2.26)![]() (2.27)
(2.27)![]() (2.28)
(2.28)![]() (2.29)
(2.29)![]() (2.30)
(2.30)![]() (2.31)
(2.31)![]() (2.32)
(2.32)![]() (2.33)
(2.33)![]() (2.34)
(2.34)
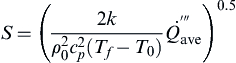 (2.35)
(2.35) (2.36)
(2.36)