3.5. Reactive Hazards
Reactive hazards are of considerable interests to process industries owing to their potential of causing substantial damage. The U.S. Chemical Safety and Hazard Identification Board has defined reactive chemical incidents as: A sudden event involving an uncontrolled chemical reaction with significant increases in temperature, pressure or gas evolution that has the potential to, or has caused serious harm to people, property or the environment (Murphy and Holmstrom, 2004). Identification, evaluation, and plan to control reactive hazard are recommended in all phases of a process life cycle to achieve an inherently safer design. All phases of a project typically refer to phases starting from core research and development to industrial scale plant decommissioning. Even for existing processes, understanding of reactive hazards allows one to establish appropriate mitigation techniques including implementing apropos control system. Reactive hazard exists, when changes in chemical structure have the potential to generate heat, energy, and gaseous byproducts beyond that which can be safely absorbed by the immediate surroundings (Urben, 2006). A chemical, which is stable under certain operating conditions, can initiate an undesired set of reactions when subjected to process conditions outside its operating range. The term “outside its operating range” can refer to material incompatibility, change in process-specific factors such as temperature, pressure, material quantity, and catalytic effects. Hence, reactivity hazard is not always an inherent property of an individual chemical substance, and variation in process-specific factors has the ability to generate reactive hazard. Put differently, a chemical identified safe for a specific process may pose the risk of reactive hazard in another process and hence should be reevaluated for every process. Various types of reactive hazards exist in the industry and for the sake of simplicity they can be broadly classified into two categories:
• General reactions such as with water, air, oxidation–reduction
• Self-reactions such as polymerization and decomposition
Reactive chemicals can be attributed to several incidents in the industry and characterization of reactive chemical is of utmost importance in hazard evaluation activity of a process unit. In most reactivity-related incidents, the lack of knowledge on reactive chemistry of the chemical of interest has been found to be the primary root cause (Aldeeb et al., 2003). A report (U.S. Chemical Safety and Hazard Investigation Board, 2003) by CSB suggested that the lack of information on reactivity of chemicals was responsible for over 50% of the studied reactive incidents. The report emphasized on methods and approaches, to better understand and predict reactivity hazard. As mentioned before, a change in process conditions can translate most stable chemical to a reactive material. System contamination resulting in material incompatibility can be a source of triggering a reactive incident. As an example, catalyzed by rust (iron and iron oxide) from an equipment, ethylene hydrogenation has resulted in a runaway reaction (Halle and Vadekar, 1991). Classification of reactive chemicals is subjective and different groups have classified reactive chemicals in their own ways as illustrated in Table 3.7.
However, it is important to find parameters that correlate strongly to the reactive nature of a chemical as a function of process conditions and its surroundings. As is noted later, both qualitative and quantitative methods exist in assessing reactive hazards. Table 3.8, re-created from Johnson et al. (2010), provides examples and definitions of various types of intrinsic reactive hazards.
To assess chemical reactivity hazard, in addition to performing experiments, detailed level modeling with process specific conditions has the potential to provide valuable and usable information. Besides, computational modeling approaches have also the ability to furnish insight inaccessible to its experimental counterpart.
Table 3.7
Classification of Reactive Chemicals
| Mannan et al. (Wei et al., 2004b) | CSB Data Analysis for Reactive Chemicals Classification (U.S. Chemical Safety and Hazard Investigation Board, 2003) |
|
1. Pyrophoric 2. Peroxide forming chemicals 3. Water reactive chemicals 4. Oxidizers 5. Self-reactive chemicals including decomposition, polymerization, and rearrangement 6. Incompatible materials |
1. Incompatibility 2. Runaway reaction 3. Impact or thermally sensitive materials |
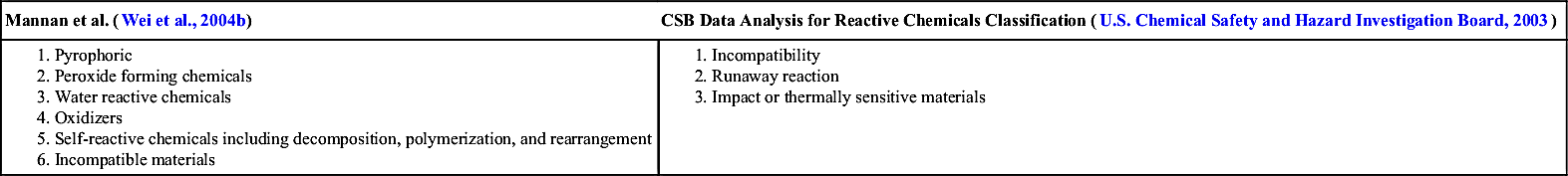
Table 3.8
Examples of Intrinsic Reactive Hazards (Johnson et al., 2010)
| Cause of Reactivity Hazard | Definition | Examples |
| Unstable | Has the tendency to break down (decompose) over time or when exposed to conditions such as heat, sunlight, shock, friction, or a catalyst with the resulting decomposition products often being toxic or flammable. Decomposition can be rapid enough to give an explosive energy release and can generate enough heat and gases for fires/explosions. | Trinitrotoluene (TNT), dibenzoyl peroxide, ethylene oxide, acetylene, picric acid, hydrogen peroxide (concentrated) |
| Polymerizing | Has the tendency to self-react to form larger molecules, while possibly generating enough heat/gases to burst a container. | Acrylic acid, styrene, 1,3-butadiene |
| Pyrophoric | Will ignite spontaneously when exposed to air. | Phosphorus, silane |
| Peroxide former | Has the tendency to slowly react with oxygen, such as from being exposed to air, to form unstable organic peroxides. | 1,3-Butadiene, isopropyl ether |
| Water reactive | Will react with water or moisture. Some react slowly; others violently. Heat and flammable/toxic gases may be produced. | Sodium, sulfuric acid, acetic anhydride |
| Oxidizer | Will give up oxygen easily or readily oxidize other materials. | Chlorine, nitric acid |
Generally speaking, there is no standard procedure for evaluating chemical reaction hazards (Barton and Rogers, 1997). Two main branch of approaches has been identified by CSB (U.S. Chemical Safety and Hazard Investigation Board, 2003), with companies relying on employing varying degree of both qualitative and quantitative approaches to evaluate reactive hazards for their processes. In qualitative approaches, for example, companies conducting reactions in batch reactors may perform HAZOP studies to identify reactive hazards. Executing “What-If” PHA study belongs to the same category. Although there is little guidance on systematically identifying and evaluating a worst-case scenario for a quantitative reactive hazard assessment, methodologies do exist to estimate helpful parameters, as recommended by CCPS and IChemE, to evaluate reactive hazards.
Figure 3.12 illustrates one of the recommended approaches for chemical hazard evaluation by quantifying chemical reactivity through calculation of seven different parameters.
Among these factors, using 13 sets of calorimeter data, covering polymerization and decomposition reactions, heat of reaction has been found to be the most important factor in assessing the reactivity of a chemical from calorimetric data (Crowl and Elwell, 2004), showing a 77% agreement in the screening test results within the domain of the study. The study set was chosen to represent a very wide range of chemistries and illustrate the complexities arising from several exotherms and changing concentrations. Even though the study could not emerge with a single criterion in assessing the reactivity of chemical mixtures, it suggested following conclusions in regards to reactivity of a chemical mixture:
1. High total heat release or high rates of heat release will represent a greater hazard, as it will produce more heat than can be removed and can potentially lead to a runaway reaction.
2. Generation of noncondensable gases at high rates can lead to high pressure and represent a hazard.
However, systems defined as hazardous due to noncondensable gas generation, are not as common compared to hazardous scenarios arising from heat generation effects (Crowl and Elwell, 2004). This emphasizes the importance of accurate estimation of heat of reaction and heat release rate in assessing reactivity hazards associated with a chemical under defined conditions. Other than having a runaway reaction, in some scenarios, reactivity hazards can result in producing toxic gas, such as TiCl4 that produces hydrogen chloride gas on in contact with moisture in air (Kapias and Griffiths, 2005). Dealing with such scenarios, that is, generation of toxic gases has been dealt with in earlier here.

Figure 3.12 Chemical hazard evaluation adopted from (Aldeeb et al., 2003).
3.5.1. Reactivity Hazard Assessment Criterion
Parameters and combination of parameters, which are used to determine the reactivity of chemical, are summarized (Crowl and Elwell, 2004) and are shown in Table 3.9. It consists of 12 different parameter values, incorporating both theoretically and experimentally obtained results as separate indicators as shown here:
Table 3.9
Criterion Used for Reactivity Hazard Assessment (Crowl and Elwell, 2004)
| Criterion | Comments | |
| 1 | NFPA instability number | As per NFPA 49 (1975), |
| 325M (2002), or 704 (2001). | ||
| The highest NFPA for any of the pure reactants was selected. | ||
| Based mostly on expert judgment. Limited mostly to a small number of pure components. | ||
| 2 | Experimental determination of total heat released | Based on temperature versus time data and total reaction mass. |
| Requires heat capacity of reaction mass. | ||
| Determined using DSC or closed-cell calorimeter. | ||
| Includes Phi factor for calorimeter. | ||
| 3 | Experimental heat of reaction | Based on dominant or limiting reactant. Computed from total heat released. Determined using DSC or closed-cell calorimeter. |
| 4 | Instantaneous power density | As described by NFPA 704 (2001) |
| Requires rate determination at 250 °C | ||
| Determined using closed-cell adiabatic calorimeter. | ||
| Designed to track NFPA numbers. | ||
| 5 | Reaction onset temperature | Highly instrument dependent—best to compare values from the same calorimeter. |
| A lower value means a greater hazard, but operating below this value is no guarantee of safety. | ||
| Determined from temperature–time data | ||
| Determined from closed-cell adiabatic calorimeter. | ||
| 6 | Total temperature change | Determined from temperature–time data |
| Determined using closed-cell adiabatic calorimeter. | ||
| 7 | Total pressure change | Determined from pressure–time data |
| Determined using closed-cell adiabatic calorimeter. | ||
| 8 | Maximum rate of change of temperature with respect to time | Determined from temperature–time data |
| Determined using closed-cell adiabatic calorimeter. | ||
| 9 | Maximum rate of pressure change with time | Determined from pressure–time data determined using closed-cell adiabatic calorimeter. |
| 10 | Energy release as calculated by CHETAH | Based on maximum for any stable products. |
| 11 | Calculated heat of reaction | Calculated from heats of formation of reaction and equilibrium products. |
| 12 | Computed adiabatic heat of reaction | Calculated from heat of reaction and heat capacities of products, at equilibrium. Assumes constant enthalpy during reaction. |

Note that, the criterions mentioned above are not completely independent of each other. From the above set of criterion, input data requirements for determining reactivity hazard can be lumped into a set of four major different types:
1. NFPA instability number
a. Expert opinion
b. Instantaneous power density
2. Heat of reaction/heat released in a reaction
a. Experimental/calculated
b. Adiabatic
3. Analysis of time–temperature data
a. Reaction onset temperature
b. Change in total temperature
c. Rate of change of temperature
4. Analysis of time–pressure data
a. Total pressure change
b. Rate of change of pressure
Based on screening methods as discussed in the 2003 version of the CCPS concept book (Johnson et al., 2010) on reactivity hazards, Mannan et al. (Wei et al., 2004b) analyzed 167 reactive incidents spanning over 20 years, from the period of June 1980 to January 2001, as reported by the CSB (U.S. Chemical Safety and Hazard Investigation Board, 2003). The study was performed by addressing the questions as posed by the CCPS book through using MSDSs and NOAA reactivity worksheet (NOAA). Put differently, the approach is to estimate various parameters, based on information available from experiments or through calculations, in order to provide a response to the set of questions asked by CCPS concept book on assessing the reactivity of a chemical. Consequently, an important part in evaluating reactive hazard is, estimating answers to the questions presented, such as finding out heat of reaction, time–temperature characteristics and the like, as precisely as possible. So the applicability of different experimental and modeling techniques depends on the factors or parameters that are estimated. As an example, the purpose of employing a calorimeter in reactivity hazard assessment is to use a small quantity of a reactive substance to assess its hazards, through estimating heat of reaction and heat capacity. Nonetheless, a caveat in using experimental techniques is that the data collected is truly an artifact of the apparatus and the procedure (Crowl and Elwell, 2004). Another limitation, in this case, is that the calorimeter conditions, should match with the intended process conditions, which is often not possible in reality, especially in the process design phase.
Unlike estimation of flash point or flammability limits, assessment of reactive hazard is more complicated due to the nature of the hazard, that is, material–process combination specific hazard. Owing to the fundamental nature of data requirements in assessing reactivity hazard, for the estimation methods to be accurate, it needs to incorporate molecular structural information and its corresponding changes during a reaction in a case-by-case basis for different process-specific conditions. This can only be achieved through direct and carefully devised experiments or molecular modeling level techniques. In here, we have focused on various multiscale modeling methodologies that can be utilized to help assessment of reactive hazard. Since fundamentally reactivity hazard is very much a function of the molecules involved and surrounding conditions (temperature, pressure), there is no well-established model at the macroscale to predict reactivity hazard. Based on the types of reactivity hazards as depicted before, two main approaches on assessing reactivity hazards are through experiments and molecular structure based methods.
Before we delve into the specifics of applicability of multiscale modeling approaches to estimate reactive hazards, let us revisit input data requirements which will help us to assess reactive hazards. Keeping requirement for qualitative input information aside (such as input in the form of expert opinion for estimating NFPA instability number), requirement of measurable information includes, as pointed out earlier in Figure 3.12:
• Heat of reaction
• Gibbs free energy of reaction
• Initiation temperature of significant exothermic reaction
• Maximum adiabatic temperature increase
• Arrhenius parameters (activation energy and frequency parameter) for power law kinetics
• Order of reaction
• Adiabatic time to maximum rate
Note that, some parameters, such as instantaneous power density, as required for calculating NFPA instability number can be computed as
![]()
The following section provides illustrations on how molecular structural information can be utilized to address reactivity hazard–related concerns through estimating some of these measurable parameters in the absence of experimental results.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
