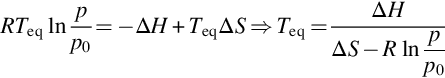Sorption Heat Storage
H.A. Zondag Department of Mechanical Engineering, Eindhoven University of Technology, Eindhoven, The Netherlands
6.1 Characteristics of Different Types of Heat Storage
6.1.1 Introduction
Heat storage provides a method to overcome the mismatch in thermal demand and thermal supply, both in time and in power. Heat can be stored as sensible heat (increasing the temperature), as latent heat (melting), or as thermochemical heat (endothermic decomposition reaction). If these processes are reversible, it is then possible to discharge the storage by respectively cooling down, solidification, or the reverse exothermic reaction. For example, if a solid block of CaCl2⋅ 6H2O(s) is raised in temperature, the result is sensible heat storage. If this block of CaCl2⋅ 6H2O(s) is molten according to the reaction CaCl2⋅ 6H2O(s) ↔ CaCl2⋅ 6H2O(l), the result is latent heat storage. If this block is dehydrated according to the reaction CaCl2⋅ 6H2O(s) ↔ CaCl2⋅ 2H2O(s) + 4H2O(g), the result is thermochemical heat storage.
An overview of the different methods is shown in Figure 6.1. This chapter will focus on thermochemical heat storage, which is also known as sorption heat storage. First, the general principles of sorption will be explained. Next, the different sorption materials will be explained, also indicating the difference between adsorption and absorption materials. Finally, an overview of sorption heat storage systems will be presented.

6.1.2 Introduction of Sorption Heat Storage
In sorption heat storage, heat is stored in a reversible (de)composition reaction, by means of the following reaction:
In this equation, A is the charged sorbent (solid or liquid), B is the sorbate (vapor), and AB is the discharged sorbent, while the subscripts s, l, and g stand for the solid, liquid, and gaseous state, respectively. By heating the discharged sorbent to a temperature above its equilibrium temperature, the composite material AB is separated into its components (charging the sorbent). If the material is cooled to below its equilibrium temperature, the reverse reaction will take place, and the components A and B will react again under the release of heat (discharging the sorbent). However, the reverse reaction can be postponed by separating the components after charging. The heat is thus stored in chemical form, minimizing heat loss. The charged sorbent can be stored at ambient temperature, and the heat is only generated when the sorbate is taken up again.
For the sorbent material, solid adsorption materials (e.g., zeolites or silicagel), solid absorption materials (e.g., salt hydrates, salt hydroxides, or salt ammoniates), and liquid absorption materials (e.g., concentrated solutions of LiBr, LiCl, or NaOH) can be used. For liquid sorption, on absorption, the liquid is going from a concentrated hygroscopic solution to a weak solution, and the reverse on desorption.
For the sorbate material, water is most often applied. In closed systems, the sorbate is stored after desorption. This storage is normally in condensed form, which strongly reduces the required volume. The condensation heat is released at ambient temperature. On sorption, the sorbate is normally evaporated again with low temperature heat (e.g., ambient temperature heat from a borehole) before it is taken up by the sorbent. In principle, the sorbate can also be applied to the sorbent in liquid form, but this strongly reduces the energy released on the sorption reaction, because in that case a large fraction of the reaction energy is taken up by breaking the bonds between the sorbate molecules. For hydrates, this is typically 70-75% of the total energy released. Therefore, it is important that the sorbate is supplied as vapor; if it were supplied as liquid, the energy released on sorption would be much lower, and typical energy densities for these materials would be strongly reduced.
Therefore, in sorption heat storage, a large part of the energy density results from the evaporation energy of the vapor. In this sense, sorption heat storage can be compared to latent heat storage, but now using the evaporation energy instead of the melting energy. Because evaporation energy is typically much larger than melting energy, sorption heat storage can reach much higher energy densities than are possible for latent heat storage, depending on how compact the vapor can be stored in adsorbed or absorbed form.
6.1.3 Comparison of Different Types of Heat Storage
An overview of typical storage densities of the different materials is given in Figure 6.2. Note that sorption energies and evaporation energies can be of the order 103 MJ/m3, melting enthalpies are of the order 102 MJ/m3, and specific heats of the order 100 MJ/m3⋅ K. In other words, for the case of MgCl2⋅ 6H2O, the sorption energy is equivalent to about 13 times the melting enthalpy, and to an amount of sensible heat equivalent to heating the material over a temperature range in the order of ~ 1000 °C.
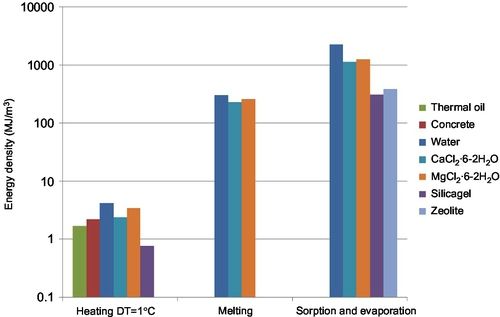
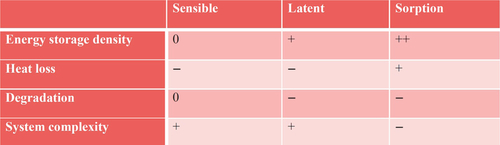
Advantages and disadvantages of sorption heat storage compared to other types of heat storage are shown in Figure 6.3. Sorption materials have the highest energy storage density, and in addition, once the material has been charged, it can be used for long-term loss-free storage of heat. However, sorption systems are relatively complex due to the fact that the storage material consists of two components that have to be stored separately after charging. Also, sorption materials may degrade over time due to changes in chemical or mechanical composition over many sorption/desorption cycles.
6.2 Principles of Sorption Heat Storage
In sorption heat storage, heat is stored in a reversible (de)composition reaction between vapor and a solid or liquid. An important aspect for sorption materials is the equilibrium temperature, which is a function of vapor pressure. Above this temperature, the sorbent desorbs the sorbate, while below this temperature the sorbent absorbs (or adsorbs) the sorbate. The equilibrium temperature can be calculated by the Clausius-Clapeyron equation, involving the enthalpy ΔH and entropy ΔS of the reaction per mole of sorbate, which for chemical equillibrium can be written in the following form:
In the equation, Teq is the equilibrium temperature, p is the vapor pressure, p0 is the reference pressure of 1 bar, and R is the universal gas constant 8.31 J/mol K.
For a sorbate taken up by a sorbent, the entropy change ΔS is a measure for the change in disorder in the system, and is mostly determined by the transition of the sorbate from the disordered gas phase to the more ordered solid phase (for solid sorbents) or liquid phase (for liquid sorbents). Therefore, on selecting a working pair, ΔS is largely determined by the choice of the sorbate, but almost independent of the choice of sorbent. On the other hand, ΔH strongly depends on the choice of the sorbent, related to how strongly the sorbate is bound to the sorbent. As an illustrative example, for a hydration reaction, ΔS is typically 150 J/mol/K, while ΔH is typically 55-80 kJ/mol. For a water vapor pressure of 1 bar, this gives equilibrium temperatures in the range 100-250 °C. For a water vapor pressure of 10 mbar (corresponding to a source temperature of ~ 8 °C), this gives equilibrium temperatures in the range of 20-150 °C, as can be calculated from the Clausius-Clapeyron equation.
In Figure 6.4, the vapor pressure curves of different common sorbates (water, ammonia, and methanol) are compared. Because water is a strongly polar liquid with hydrogen bonding between the water molecules, water molecules are relatively strongly bound to each other. The binding between CH3OH molecules—and even more between NH3 molecules—is weaker. Consequently, water has a lower vapor pressure than the other two liquids, and a relatively high heat of evaporation of 44.0 kJ/mol as compared to 37.4 kJ/mol for methanol and 19.9 kJ/mol for ammonia (at 25 °C reference temperature).

In a similar way as for sorbates, vapor pressure curves can be drawn for sorbents such as salt hydrates, hygroscopic liquids, and adsorption materials, as a function of their fractional loading. In this case, the vapor pressure curve gives the equilibrium between sorbate vapor and the sorbate absorbed/adsorbed in the sorbent. The equilibrium pressure depends on the strength of the bonding between the sorbate and the sorbent; a stronger binding results in a lower vapor pressure.
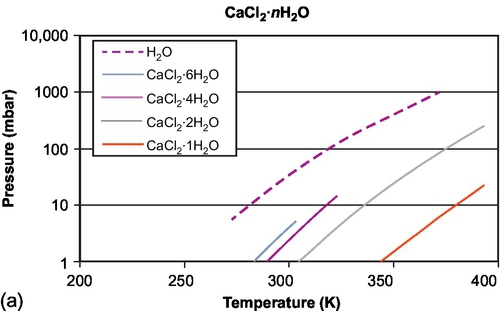
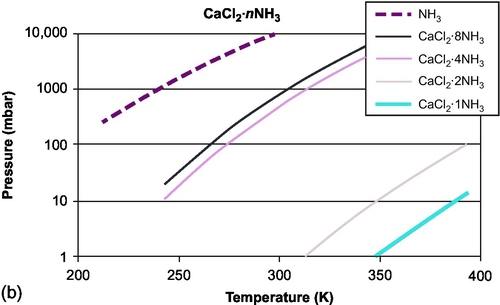
The equilibrium composition in the reversible reaction A(s) + B(g) ↔ AB(s) + heat is a function of temperature and vapor pressure. This can be plotted in the isostere graph (concentration as a function of temperature and pressure). As an example, the equilibrium curves for CaCl2 are shown in Figure 6.5 for absorption of water and for absorption of ammonia. The figures show different equilibrium lines, each line resembling one equilibrium loading of the material. The different lines are due to the different binding energies with which the water is bound to the salt molecule. The first water molecules are bound stronger than subsequent water molecules. As can be seen from Figure 6.5b, if pure ammonia is used in the evaporator, even at ambient temperatures already high vapor pressures of over 8 bar result, leading to high power densities. This makes ammonia very suitable for chilling applications, and as such it is widely applied in industrial chillers. On the other hand, the use of ammonia involves safety issues, restricting the use of this material to industrial environments. For use at higher temperatures, ammonia is usually not applied in its pure form, but is absorbed in other materials, which leads to lower vapor pressure.
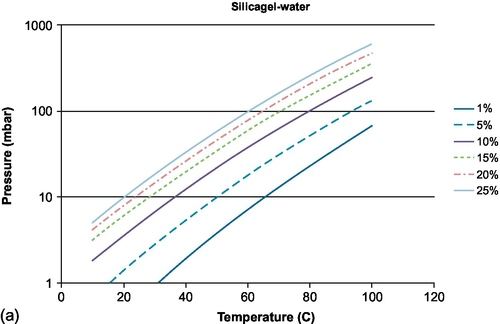
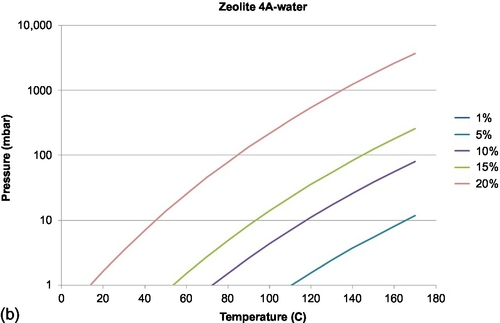
For absorption in solids, the sorption process shows discrete steps in the amount of sorbate that can be taken up, related to how many molecules of sorbate can be taken up by one molecule of sorbent. This is in contrast to absorption in liquids and adsorption in solids, which have a more continuous character, with a large amount of equilibrium lines showing the fractional loading of the material. In Figure 6.6, examples are shown for water sorption on silicagel and water sorption on zeolite 4A.
6.3 Sorption Heat Storage Materials
6.3.1 Introduction
Different types of sorption reactions exist, as shown in Figure 6.7. Absorption in liquids relates to the absorption of water vapor in hygroscopic salt solutions, but also to the absorption of gasses like ammonia vapor in water. For solid sorption, a distinction can be made between absorption in solids, in which the sorbate is integrated into the crystal lattice of the sorbent, and adsorption in solids, in which the sorbate is attached to the internal surface area of the sorbent.

6.3.2 Physisorption Materials—Zeolites, Silicagel
In adsorption, the molecule adheres to the surface of the sorbent by van der Waals interaction. Typical adsorption materials therefore have a large internal surface area, due to their extensive pore structure. Because the sorption takes place at the surface, the structure of the sorbent is unaffected and the sorbent does not expand on adsorption, which has a positive effect on the mechanical stability over multiple cycles. Many different adsorption materials exist, such as different types of zeolites, silicagel, activated carbon, activated alumina, aluminum phosphates, and metal-organic frameworks (MOFs). Some of these are in wide use (zeolites, silicagel), while others are still largely in the research and development phase (MOFs).
The performance of adsorption materials is mainly determined by their pore size distribution and their polarity. Adsorption materials like activated carbon, activated alumina, or silicagel have a wide range of pore sizes, from small micropores (< 2 nm) to large macropores (> 50 nm). On the other hand, molecular sieve adsorption materials such as zeolites have a well-defined pore width, ranging from 0.4 nm for zeolite 4A to 1.0 nm for zeolite 13X. Most sorbents are polar, such as activated alumina, silicagel, and zeolites, increasing the sorption enthalpy for polar sorbates such as water. On the other hand, activated carbon is nonpolar, resulting in relatively low sorption enthalpy.
Among the different adsorption materials, zeolite is presently the main adsorption material under research for sorption heat storage, because of its relatively high discharge temperature (much higher than silicagel, for example, as can be seen in Figure 6.6). Zeolites consist of mainly of SiO2 and Al2O3, in which part of the Al is replaced by elements such as Na+, K+, Ca2 +, and so on, increasing the polarity of the zeolites. Typically, they can absorb an amount of water up to about 22–27% of their dry weight, limiting the energy storage density that can be obtained, compared to salt hydrates, for example. On the other hand, zeolites are more stable than most salt hydrates, and also have fast kinetics. Efforts have been carried out to improve the characteristics of zeolite by impregnation with MgCl2 and CaCl2 (Jänchen et al., 2004) and MgSO4 (Hongois et al., 2010).
6.3.3 Sorption in Liquids
The main practical differences between absorption in solids and in liquids is that on one hand liquids have a lower temperature step, but on the other hand they have much better transport properties. Therefore, in liquid systems the sorbent is usually not reacting in the storage vessel, but is pumped through a separate reactor in which the sorbent is charged or discharged, allowing a decoupling between storage and power generation. Typical hygroscopic liquids are solutions of LiBr, LiCl, NaOH, and H2SO4. On charging the liquid (desorbing), too high concentration may cause the liquid to crystallize, which may result in blocking of pipes and pumps. On the other hand, low concentrations reduce the energy density, and the temperature effect will become too small to be useful.
Work on an absorption chiller with integrated heat storage using an LiCl solution has been carried out by SERC in Sweden (Bales, 2006), using a special design that allowed partial crystallization of the solution to enhance the effective energy density. N'Tsoukpoue et al. (2010) carried out a systems study for an LiBr solution-based seasonal heat storage system. He concludes that crystallization is probably necessary for the competiveness of the process, to enhance the effective energy storage density, especially because LiBr is expensive and large volumes are required to reach 100% solar fraction. For seasonal heat storage, EMPA in Switzerland focuses on a hygroscopic solution of NaOH, which is much cheaper than LiBr (Weber and Dorer, 2008; Fumey et al., 2014). According to Fumey et al. (2014), the NaOH solution can be charged to a concentration of 50% (limited by crystallization) and discharged down to a concentration of 38% (assuming a 24 °C return temperature and a 2 °C evaporator temperature). For this case, the energy density of the solution is indicated to be three times the energy density of a water vessel over a temperature range of 65 °C. Another approach is followed at the University of Minnesota (Quinnell et al., 2010), using a CaCl2 solution for both thermochemical and sensible heat storage. In order to increase the effective energy density on the system level, a single tank is used to store both the diluted and the strong solution, separated by a thermocline. Researchers indicate that the material energy storage density of the closed system is about twice that of sensible storage with water.
6.3.4 Weak Chemisorption—Hydrates
Higher values for the effective energy density per mass can be obtained by absorption in solids. The absorption of water vapor typically has a ΔH per mole of water in the range of 55-80 kJ/mol, similar to polar adsorption materials. However, the fractional water loading can be much higher for absorption in solids, in the range of 50–100% of the dry sorbent mass, increasing the potential energy storage density. On the other hand, the water sorption in the crystal lattice causes expansion and contraction of the material over the sorption-desorption cycles, reducing the mechanical stability.
For seasonal heat storage, several research institutes focus on salt hydrates, because of their relatively low cost, high potential storage density, and high temperature step. A comparison of different materials for thermochemical heat storage was presented by Van Essen (2009), indicating that CaCl2 and MgCl2 hydrates have a better performance and particularly faster hydration kinetics than MgSO4 and Al2(SO4)3 hydrates. Research at the Energy Research Centre of the Netherlands (ECN) focused on MgCl2 (Zondag et al., 2013). Unfortunately, instability of MgCl2 hydrates reduced the performance over multiple cycles (Ferchaud et al., 2014). Mauran et al. (2008) presented research on SrBr2. Work on an open sorption system containing a packed bed with KAl(SO4)2⋅ 12H2O was carried out by CEA in France (Marias et al., 2011). Studies on crystal level into the detailed mechanisms involved in the hydration-dehydration transition were carried out by Ferchaud et al. (2014) and Lan et al. (2014).
6.3.5 Strong Chemisorption—Hydroxides
In hydroxides, a much stronger water bonding occurs than in hydrates. A typical hydroxide reaction is given by AO + H2O → A(OH)2, in which AO is an oxide. ΔS values are still typically about 150 J/mol/K, similar as for hydrates, as expected. However, the ΔH values show a much larger range. Typically, for the formation of hydroxides from oxides, values of about 50 kJ/mol are found for metal oxides, while values of 80-150 kJ/mol are found for the earth alkali metals of group 2 of the periodical system, with an increase in ΔH for heavier molecules. These high values of the reaction enthalpy make these materials suitable for high temperature heat storage for industrial applications.
Hydroxides have been investigated mainly for high temperature heat pump applications (e.g., Kariya and Kato (2014), examining a heat pump based on the reaction H2O + CaO → Ca(OH)2). However, increasingly, these materials are also investigated for industrial heat storage. Research into improving the kinetics of these reactions, and thereby reducing the effective charging temperature, is carried out by Aristov and Shkatulov, showing a significant improvement of the kinetics for Mg(OH)2 on doping with LiNO3 and for Ca(OH)2 on doping with KNO3 (Shkatulov and Aristov, 2014). At DLR in Germany, tests are carried out on high temperature heat storage based on CaO + H2O ↔ Ca(OH)2, with discharge temperatures as high as 550 °C and an energy density of 323 kWh/m3 or 1.1 GJ/m3 (Laing and Wörner, 2013).
6.4 Sorption Heat Storage System Designs
6.4.1 Introduction
Thermochemical storage systems are all still in the R&D phase. In commercial applications, sorption materials are mostly used for industrial process applications such as gas cleaning and separation processes, and on a smaller scale for absorption heat pumps and chillers.
6.4.2 Principles of Sorption Systems
Sorption heat storage systems can be classified in two ways, leading to four different types of storage for the case of seasonal sorption heat storage, as shown in Figures 6.8 and 6.9. A first classification distinguishes between packed bed systems and separate reactor systems:

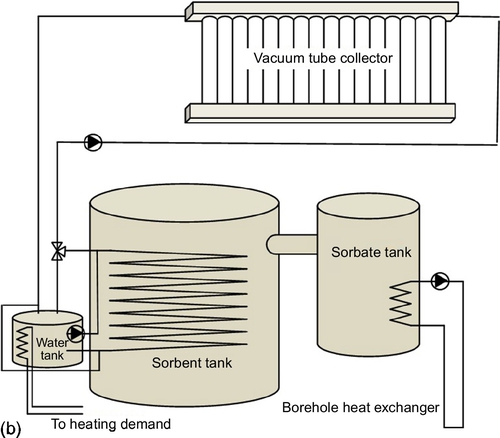


• In packed bed systems, the packed bed of sorbent functions both as storage and as reactor vessel, without transport of the sorbent.
• In separate reactor systems, on charging, the sorbent is taken from the storage tank with discharged material, is flown through a separate reactor in which the charging reaction is taking place, and is finally stored in the storage tank with charged material. On discharging, this process flow is reversed. In this way, storage and power generation are decoupled.
Generally, systems using solid sorption are of the packed bed type (e.g., Zondag et al., 2013), while systems using liquid sorption are of the separate reactor type (e.g., Weber and Dorer, 2008). However, in the literature, a few solid sorption reactors of the separate reactor type are also found, using a granulate flow of sorbent (Kerskes et al., 2011).
A second classification is between open systems taking the sorbate from the ambient (e.g., water vapor from the air) and closed systems in which the sorbate remains within the system, and only the evaporation heat is taken from the ambient:
• Open systems are necessarily at ambient pressure and can only use as sorbate components already present in the air, such as water vapor. In an open system, the water vapor is transported as moist air by a fan.
• In closed systems, not only water can be used as sorbate, but also other liquids such as ammonia or methanol. All inert gasses are removed as much as possible by prior evacuation of the system, and the system pressure under operation will be determined by the equilibrium pressure of the sorbate. The water vapor is transported by the difference in vapor pressure between the sorbent and the evaporator.
The storage systems in Figures 6.8 and 6.9 show a different number of tanks for the packed bed open system (one sorbent tank), the packed bed closed system (one sorbent tank and one sorbate tank), the separate reactor open system (two sorbent tanks, one for charged and one for discharged sorbent), and the separate reactor closed system (two sorbent tanks and one sorbate tank). Because multiple tanks increase the total volume of the system, and thereby reduce the effective energy storage density on system level, it is important to keep the design as compact as possible, preferably combining tanks. Furthermore, the closed systems in the figures are connected to a borehole, supplying low temperature evaporation heat to generate the required water vapor. While in a closed system a low temperature heat source for evaporation is inevitable, in an open system, this is not strictly necessary as long as the ambient air provides sufficient water vapor. In open systems, a critical component is the air-to-air heat exchanger that is providing the heat recovery in the charge and discharge air loops. While these loops are drawn in the figure as separate loops (for clarity), in practice the system design is normally such that the same loop is used for both charge and discharge. Finally, in all systems, the sorption heat storage is coupled to a small water tank to deliver peak power; in this way, the sorption system itself can be designed at lower power, which improves its performance.
In the literature, research has been carried out on both closed systems and open systems for heat storage. Closed systems tend to have higher output temperatures and have lower electrical power requirements for fans, while open systems tend to be cheaper (no vacuum tanks or vacuum pumps required) and more robust (no leakage issues), and tend to have better heat transfer characteristics compared to closed packed bed systems (due to the large contact area in the packed bed). It is expected that both types of systems will in time have their own markets and applications where they perform best.
6.4.2.1 Closed System
In a closed system, the maximum temperature rise that can be obtained on absorption can be obtained from the isostere plot. In Figure 6.10, a simplified isostere graph is shown, with curves for the equilibrium vapor pressure of the sorbate (in this case water) and the vapor pressure of the sorbent (in this case a salt hydrate). Because the salt hydrate is more hygroscopic than the liquid water, the vapor pressure for the salt hydrate is lower than for water at the same temperature. On absorption, the temperature rise that can be obtained between the evaporator temperature and the sorbent temperature is the temperature difference between these isosteres at the given vapor pressure, corrected for pressure drop in the system. In a closed system, on discharging, the evaporator temperature determines the evaporator vapor pressure and thereby the discharge temperature of the sorbent. On charging, the condenser temperature determines the system vapor pressure and thereby the desorption temperature.

Closed sorption heat storage systems are often found for short-term heat storage applications, but have also been applied for seasonal heat storage. Typically, the system consists of a packed bed with a large heat exchanger area integrated in the bed. The limited heat transfer in the packed bed is often a bottleneck for the performance of these systems. Therefore, the conductivity may be enhanced by impregnating the sorbent into a conductive matrix such as expanded graphite.
Solid adsorption silicagel chillers are commercially available. In addition, several prototypes of solid absorption heat pumps with integrated heat storage have been realized, for example, with water absorption by SrBr2 (Mauran et al., 2008), Na2S (De Boer et al., 2004), and CaO (Kariya and Kato, 2014), as well as with CO2 absorption in the system PbCO3-CaO (Kato et al., 1998). In several cases, expanded graphite was used to improve the effective heat transfer through the bed. Mauran et al. (2008) presented research on SrBr2 impregnated in graphite for an air conditioning system application with integrated heat/cold storage; a 1 m3 prototype was built with 40 kWh cold storage (60 kWh heat storage). In the Modestore project, carried out by AEE Intec in Austria, a closed evacuated sorption storage was built and monitored in a field test. The sorption storage contained a large copper sheet-and-tube spiral as heat exchanger, which was filled with silicagel beads. The silicagel was dried using flat-plate solar thermal collectors. Unfortunately, it had to be concluded that most of the moisture captured by the silicagel did not result in sufficient temperature lift, resulting in a low effective energy density.
Closed liquid sorption differs from closed solid sorption due to the fact that the liquid can easily be transported through the system. This allows separation of the storage of the sorbent (in a vessel with liquid) and the power generation (in a separate sorption reactor). Typically, the principle of a liquid sorption reactor is similar to a chemical absorption column, generating a large contact area between the gas and the fluid by application of dedicated internal structures. Much research has been conducted into liquid sorption systems. Some recent developments have been achieved by Bales (2006) and Weber and Dorer (2008). To enhance the evaporation area, Bales built a prototype in which the liquid sorbent is sprayed over a heat exchanger, thereby generating effective contact between the sorbent and the vapor. Weber and Dorer (2008) indicates a reactor consisting of several trays to improve the contact area.
6.4.2.2 Open System
In an open system, the temperature rise in the bed is limited by the relatively large thermal mass of the inert carrier gas flow (air). Depending on the mass ratio of inert gas over vapor, this may significantly reduce the temperature rise that can be obtained; for a seasonal heat storage system operating at 10 mbar vapor pressure (corresponding to saturated air of 8 °C), the effective temperature rise over the sorption bed is limited to about 25-30 °C, which is much lower than the equilibrium temperature. In order to reach a high output temperature, it is necessary to preheat the airflow into the bed. This can be realized with an air-to-air heat recovery system (Zondag et al., 2013), preheating the incoming airflow with the exhaust airflow that exits the system after heating the load, as shown in Figures 6.8a and 6.9a.
In the Monosorp project, carried out by ITW Stuttgart, an open sorption storage system was developed, based on extruded blocks of zeolite 4A with integrated channels, to reduce pressure drop (Kerskes et al., 2007). During summer, the zeolite was dried with high temperature heat from the solar collector system. During winter, the moist ventilation exhaust air was led through the sorption storage, in which the moisture was captured by the zeolite, heating the exhaust air. This air was subsequently led over an air-to-air heat exchanger, heating the incoming ventilation air. Mette et al. (2012) found that if the hot air for charging the zeolite was pre-dried by means of a sorption wheel, the charging temperature of the zeolite could be reduced from 180 °C down to 130 °C, thereby improving the overall system efficiency (increasing solar collector efficiency and reducing thermal losses).
Salt hydrates have a higher potential energy storage density than adsorption materials like silicagel and zeolite, and in addition are often substantially cheaper. Therefore, salt hydrates are considered an interesting option for seasonal heat storage. In this field, ECN and Eindhoven University of Technology (TU/e) in the Netherlands are involved in both material research and system development. Initially, the focus was on sorption storage using MgCl2⋅ 6H2O. A lab scale prototype built at ECN is shown in Figure 6.11. However, in the later European Energy Hub project, due to problems with instability of the MgCl2 hydrate (Ferchaud et al., 2014), ECN built an open sorption prototype based on 200 l zeolite 13X (De Boer et al., 2014). Although the zeolite showed a stable performance, the high required charging temperature led to significant heat losses during charging. Also, the pressure drop in the system required significant auxiliary fan power.


6.5 Overall System Aspects
As previously indicated, the main advantage of sorption heat storage is potentially high energy density, combined with the fact that losses are negligible once the energy is properly stored. This makes sorption heat storage very interesting for compact long-term storage of large amounts of heat. This is reflected in the fact that much of the research on sorption heat storage has focused on seasonal storage of solar heat. Unfortunately, seasonal storage of solar heat is also an area in which the economics of the storage is a difficult issue, because a large amount of storage material is required, which is charged and discharged only once per year. Therefore, this application sets very strong constraints on the cost of the storage material, as well as the system installation as a whole, to keep the cost per amount of energy delivered sufficiently low. In addition, careful design is required to minimize the electrical energy use of the system (borehole pump, fan in open system, solar collector pump, vacuum pump in closed system) to make sure that the effective coefficient of performance (COP) of the system remains sufficiently high, as it should at least be significantly higher than in a conventional heat pump system (e.g., above 10).
A different application in which compactness is important is mobile transport of industrial waste heat. Compared to seasonal heat storage, the sorption material has to undergo many more cycles, affecting both the economics and the requirements for stability of the materials. The use of sorption is particularly interesting for industrial drying, as the sorption material on discharge is absorbing moisture to generate heat. This principle is applied in a project by ZAE in Germany, in which a mobile zeolite sorption heat storage was developed for transport of industrial waste heat from a waste incineration plant (MVA Hamm) to an industrial drying installation for plastics (Krönauer, 2013).
Furthermore, several applications focus on absorption heat pumps or absorption chillers with integrated heat storage. This is an interesting application when mismatch exists between the heat supply and the required heating/chilling.
Finally, because of its loss-free storage of heat, sorption heat storage is also highly suitable for small autonomous applications for one-time use, requiring instantaneous heating or cooling. This has led to the development of applications such as self-heating coffee mugs or self-cooling beer vessels. Although this is a small niche market, it opens new opportunities and a range of applications that can be expanded much further.
6.6 Conclusions
Sorption heat storage has the potential of very compact storage of heat, as well as very low losses after charging of the storage. Several materials are under investigation, such as hygroscopic NaOH solutions, zeolites, and salt hydrates. Also, different system types are under development, in which the distinction between open systems and closed systems is most prominent. The most challenging aspect presently is to find economically sound applications for sorption heat storage and to develop optimized systems for these applications, both in terms of material selection and also in terms of performance and auxiliary energy use.