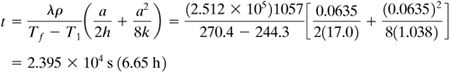5.5. CHILLING AND FREEZING OF FOOD AND BIOLOGICAL MATERIALS
5.5A. Introduction
Unlike many inorganic and organic materials which are relatively stable, food and other biological materials decay and deteriorate more or less rapidly with time at room temperature. This spoilage is due to a number of factors. Tissues of foods such as fruits and vegetables continue to undergo metabolic respiration after harvesting, and ripen and eventually spoil. Enzymes of the dead tissues of meats and fish remain active and induce oxidation and other deteriorating effects. Microorganisms attack all types of foods by decomposing the foods so that spoilage occurs; chemical reactions also occur, such as the oxidation of fats.
At low temperatures the growth rate of microorganisms will be slowed if the temperature is below that which is optimum for growth. Enzyme activity and chemical reaction rates are also reduced at low temperatures. The rates of most chemical and biological reactions in storage of chilled or frozen foods and biological materials are reduced by factors of ![]() to
to ![]() for each 10 K (10°C) drop in temperature.
for each 10 K (10°C) drop in temperature.
Water plays an important part in these rates of deterioration, and it is present to a substantial percentage in most biological materials. To reach a temperature low enough for most of these rates to approximately cease, most of the water must be frozen. Materials such as food do not freeze at 0°C (32°F), as pure water does, but at a range of temperatures below 0°C. However, because of some of the physical effects of ice crystals and other effects, such as concentrating of solutions, chilling of biological materials is often used for preservation instead of freezing.
Chilling of materials involves removing the sensible heat and heat of metabolism and reducing the temperature, usually to a range of 4.4°C (40°F) to just above freezing. Essentially no latent heat of freezing is involved. The materials can be stored for a week or so up to a few months, depending on the product stored and the gaseous atmosphere. Each material has its optimum chill storage temperature.
In the freezing of food and biological materials, the temperature is reduced so that most of the water is frozen to ice. Depending on the final storage temperature, down to as low as −30°C, the materials can be stored for up to a year or so. Often in the production of frozen foods, they are first treated by blanching or scalding to destroy enzymes.
5.5B. Chilling of Food and Biological Materials
In the chilling of food and biological materials, the temperature of the materials is reduced to the desired chill storage temperature, which can be about −1.1°C (30°F) to 4.4°C (40°F). For example, after slaughter, beef has a temperature of 37.8°C (100°F) to 40°C (104°F), and it is often cooled to about 4.4°C (40°F). Milk from cows must be chilled quickly to temperatures just above freezing. Some fish fillets at the time of packing are at a temperature of 7.2°C (45°F) to 10°C (50°F) and are chilled to close to 0°C.
These rates of chilling or cooling are governed by the laws of unsteady-state heat conduction discussed in Sections 5.1 to 5.4. The heat is removed by convection at the surface of the material and by unsteady-state conduction in the material. The fluid outside the foodstuff or biological materials is used to remove this heat; in many cases it is air. The air has previously been cooled by refrigeration to −1.1°C to +4.4°C, depending on the material and other conditions. The convective heat-transfer coefficients, which usually include radiation effects, can also be predicted by the methods in Chapter 4; for air the coefficient varies from about 8.5 to 40 W/m2 · K (1.5 to 7 btu/h · ft2 · °F), depending primarily on air velocity.
In some cases the fluid used for chilling is a liquid flowing over the surface, and the values of h can vary from about 280 to 1700 W/m2 · K (50–300 btu/h · ft2 · °F). In other cases, a contact or plate cooler is used, where chilled plates are in direct contact with the material. Then the temperature of the surface of the material is usually assumed to be equal or close to that of the contact plates. Contact freezers are used for freezing biological materials.
Where the food is packaged in boxes or the material is tightly covered by a film of plastic, this additional resistance must be considered. One method for doing this is to add the resistance of the package covering to that of the convective film:
Equation 5.5-1
![]()
where RP is the resistance of covering, RC the resistance of the outside convective film, and RT the total resistance. Then, for each resistance,
Equation 5.5-2
![]()
Equation 5.5-3
![]()
Equation 5.5-4
![]()
where hc is the convective gas or liquid coefficient, A is the area, Δ× is the thickness of the covering, k is the thermal conductivity of the covering, and h is the overall coefficient. The overall coefficient h is the one to use in the unsteady-state charts. This assumes a negligible heat capacity of the covering, which is usually the case. Also, it assumes that the covering closely touches the food material so there is no resistance between the covering and the food.
The major sources of error in using the unsteady-state charts are inadequate data on the density, heat capacity, and thermal conductivity of the foods and the prediction of the convective coefficient. Food materials are irregular anisotropic substances, whose physical properties are often difficult to evaluate. Also, if evaporation of water occurs on chilling, latent heat losses can affect the accuracy of the results.
EXAMPLE 5.5-1. Chilling Dressed BeefHodgson (H2) gives physical properties of beef carcasses during chilling of ρ = 1073 kg/m3, cp = 3.48 kJ/kg · K, and k = 0.498 W/m · K. A large slab of beef 0.203 m thick and initially at a uniform temperature of 37.8°C is to be cooled so that the center temperature is 10°C. Chilled air at 1.7°C (assumed constant) with an h = 39.7 W/m2 · K is used. Calculate the time needed. Solution: The thermal diffusivity α is
Then, for the half-thickness x1 of the slab,
For the center of the slab,
Also,
Using Fig. 5.3-6 for the center of a large flat plate,
Solving, t = 6.95 × 104 s (19.3 h). |
5.5C. Freezing of Food and Biological Materials
1. Introduction
In the freezing of food and other biological materials, the removal of sensible heat in chilling occurs first and then the removal of the latent heat of freezing. The latent heat of freezing water of 335 kJ/kg (144 btu/lbm) is a substantial portion of the total heat removed on freezing. Other slight effects, such as the heats of solution of salts and so on, may be present but are quite small. Actually, when materials such as meats are frozen to −29°C, only about 90% of the water is frozen to ice, with the rest thought to be bound water (B1).
Riedel (R1) gives enthalpy–temperature–composition charts for the freezing of many different foods. These charts show that freezing does not occur at a given temperature but extends over a range of several degrees. As a consequence, there is no one freezing point with a single latent heat of freezing.
Since the latent heat of freezing is present in the unsteady-state process of freezing, the standard unsteady-state conduction equations and charts given in this chapter cannot be used for prediction of freezing times. A full analytical solution of the rate of freezing of food and biological materials is very difficult because of the variation of physical properties with temperature, the amount of freezing varying with temperature, and other factors. An approximate solution by Plank is often used.
2. Approximate solution of Plank for freezing
Plank (P2) has derived an approximate solution for the time of freezing which is often sufficient for engineering purposes. The assumptions in the derivation are as follows. Initially, all the food is at the freezing temperature but is unfrozen. The thermal conductivity of the frozen part is constant. All the material freezes at the freezing point, with a constant latent heat. The heat transfer by conduction in the frozen layer occurs slowly enough that it is under pseudo-steady-state conditions.
In Fig. 5.5-1 a slab of thickness a m is cooled from both sides by convection. At a given time t s, a thickness of x m of frozen layer has formed on both sides. The temperature of the environment is constant at T1 K and the freezing temperature is constant at Tf. An unfrozen layer in the center at Tf is present.
Figure 5.5-1. Temperature profile during freezing.

The heat leaving at time t is q W. Since we are at pseudo-steady state, at time t, the heat leaving by convection on the outside is
Equation 5.5-5
![]()
where A is the surface area. Also, the heat being conducted through the frozen layer of x thickness at steady state is
Equation 5.5-6
![]()
where k is the thermal conductivity of the frozen material. In a given time dt s, a layer dx thick of material freezes. Then multiplying A times dx times ρ gives the kg mass frozen. Multiplying this by the latent heat λ in J/kg and dividing by dt,
Equation 5.5-7
![]()
where ρ is the density of the unfrozen material.
Next, to eliminate TS from Eqs. (5.5-5) and (5.5-6), Eq. (5.5-5) is solved for TS and substituted into Eq. (5.5-6), giving
Equation 5.5-8
![]()
Equating Eq. (5.5-8) to (5.5-7),
Equation 5.5-9
![]()
Rearranging and integrating between t = 0 and x = 0, to t = t and x = a/2,
Equation 5.5-10

Integrating and solving for t,
Equation 5.5-11
![]()
To generalize the equation for other shapes,
Equation 5.5-12
![]()
where a is the thickness of an infinite slab (as in Fig. 5.5-1), diameter of a sphere, diameter of a long cylinder, or smallest dimension of a rectangular block or brick. Also,

For a rectangular brick having dimensions a by β1a by β2a, where a is the shortest side, Ede (B1) has prepared a chart to determine the values of P and R to be used to calculate t in Eq. (5.5-12). Equation (5.5-11) can also be used for calculation of thawing times by replacing the k of the frozen material by the k of the thawed material.
EXAMPLE 5.5-2. Freezing of MeatSlabs of meat 0.0635 m thick are to be frozen in an air-blast freezer at 244.3 K (−28.9°C). The meat is initially at the freezing temperature of 270.4 K (−2.8°C). The meat contains 75% moisture. The heat-transfer coefficient is h = 17.0 W/m2 · K. The physical properties are ρ = 1057 kg/m3 for the unfrozen meat and k = 1.038 W/m · K for the frozen meat. Calculate the freezing time. Solution: Since the latent heat of fusion of water to ice is 335 kJ/kg (144 btu/lbm), for meat with 75% water,
The other given variables are a = 0.0635 m, Tf = 270.4 K, T1 = 244.3 K, ρ = 1057 kg/m3, h = 17.0 W/m2 · K, k = 1.038 W/m · K. Substituting into Eq. (5.5-11),
|
3. Other methods for calculating freezing times
Neumann (C1, C2) has derived a complicated equation for freezing in a slab. He assumes the following conditions. The surface temperature is the same as the environment, that is, no surface resistance. The temperature of freezing is constant. This method suffers from the limitation that a convection coefficient cannot be used at the surface, since it assumes no surface resistance. However, the method does include the effect of cooling from an original temperature, which may be above the freezing point.
Plank's equation does not make provision for an original temperature, which may be above the freezing point. An approximate method for calculating the additional time necessary to cool from temperature T0 down to the freezing point Tf is as follows. Using the unsteady-state charts calculate the time for the average temperature in the material to reach Tf, assuming that no freezing occurs and using the physical properties of the unfrozen material. If there is no surface resistance, Fig. 5.3-13 can be used directly for this. If a resistance is present, the temperature at several points in the material will have to be obtained from the unsteady-state charts and the average temperature calculated from these point temperatures. This may be partially trial and error, since the time is unknown and must be assumed. If the average temperature calculated is not at the freezing point, a new time must be assumed. This is an approximate method since some material will actually freeze.