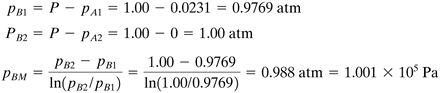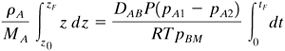6.2. MOLECULAR DIFFUSION IN GASES
6.2A. Equimolar Counterdiffusion in Gases
In Fig. 6.2-1 a diagram is given of two gases A and B at constant total pressure P in two large chambers connected by a tube where molecular diffusion at steady state is occurring. Stirring in each chamber keeps the concentrations in each chamber uniform. The partial pressure pA1 > pA2 and pB2 > pB1. Molecules of A diffuse to the right and B to the left. Since the total pressure P is constant throughout, the net moles of A diffusing to the right must equal the net moles of B to the left. If this is not so, the total pressure would not remain constant. This means that
Equation 6.2-1
![]()
Figure 6.2-1. Equimolar counterdiffusion of gases A and B.

The subscript z is often dropped when the direction is obvious. Writing Fick's law for B for constant c,
Equation 6.2-2
![]()
Now since P = pA + pB = constant, then
Equation 6.2-3
![]()
Differentiating both sides,
Equation 6.2-4
![]()
Equating Eq. (6.1-3) to (6.2-2),
Equation 6.2-5
![]()
Substituting Eq. (6.2-4) into (6.2-5) and canceling like terms,
Equation 6.2-6
![]()
This shows that for a binary gas mixture of A and B, the diffusivity coefficient DAB for A diffusing into B is the same as DBA for B diffusing into A.
EXAMPLE 6.2-1. Equimolar CounterdiffusionAmmonia gas (A) is diffusing through a uniform tube 0.10 m long containing N2 gas (B) at 1.0132 × 105 Pa pressure and 298 K. The diagram is similar to Fig. 6.2-1. At point 1, pA1 = 1.013 × 104 Pa and at point 2, pA2 = 0.507 × 104 Pa. The diffusivity DAB = 0.230 × 10−4 m2/s.
Solution: Equation (6.1-13) can be used, where P = 1.0132 × 105 Pa, z2 − z1 = 0.10 m, and T = 298 K. Substituting into Eq. (6.1-13) for part (a),
Rewriting Eq. (6.1-13) for component B for part (b) and noting that pB1 = P − pA1 = 1.0132 × 105 − 1.013 × 104 = 9.119 × 104 Pa and pB2 = P − pA2 = 1.0132 × 105 − 0.507 × 104 = 9.625 × 104 Pa,
The negative value for |
6.2B. General Case for Diffusion of Gases A and B Plus Convection
Up to now we have considered Fick's law for diffusion in a stationary fluid; that is, there has been no net movement or convective flow of the entire phase of the binary mixture A and B. The diffusion flux ![]() occurred because of the concentration gradient. The rate at which moles of A passed a fixed point to the right, which will be taken as a positive flux, is
occurred because of the concentration gradient. The rate at which moles of A passed a fixed point to the right, which will be taken as a positive flux, is ![]() kg mol A/s · m2. This flux can be converted to a velocity of diffusion of A to the right by
kg mol A/s · m2. This flux can be converted to a velocity of diffusion of A to the right by
Equation 6.2-7
![]()
where νAd is the diffusion velocity of A in m/s.
Now let us consider what happens when the whole fluid is moving in bulk or convective flow to the right. The molar average velocity of the whole fluid relative to a stationary point is νM m/s. Component A is still diffusing to the right, but now its diffusion velocity νAd is measured relative to the moving fluid. To a stationary observer A is moving faster than the bulk of the phase, since its diffusion velocity νAd is added to that of the bulk phase νM. Expressed mathematically, the velocity of A relative to the stationary point is the sum of the diffusion velocity and the average or convective velocity:
Equation 6.2-8
![]()
where νA is the velocity of A relative to a stationary point. Expressed pictorially,

Multiplying Eq. (6.2-8) by cA,
Equation 6.2-9
![]()
Each of the three terms represents a flux. The first term, cAνA, can be represented by the flux NA kg mol A/s · m2. This is the total flux of A relative to the stationary point. The second term is ![]() , the diffusion flux relative to the moving fluid. The third term is the convective flux of A relative to the stationary point. Hence, Eq. (6.2-9) becomes
, the diffusion flux relative to the moving fluid. The third term is the convective flux of A relative to the stationary point. Hence, Eq. (6.2-9) becomes
Equation 6.2-10
![]()
Let N be the total convective flux of the whole stream relative to the stationary point. Then,
Equation 6.2-11
![]()
Or, solving for vM,
Equation 6.2-12
![]()
Substituting Eq. (6.2-12) into (6.2-10),
Equation 6.2-13
![]()
Since ![]() is Fick's law, Eq. (6.1-7),
is Fick's law, Eq. (6.1-7),
Equation 6.2-14
![]()
Equation (6.2-14) is the final general equation for diffusion plus convection to use when the flux NA is used, which is relative to a stationary point. A similar equation can be written for NB.
Equation 6.2-15
![]()
To solve Eq. (6.2-14) or (6.2-15), the relation between the flux NA and NB must be known. Equations (6.2-14) and (6.2-15) hold for diffusion in a gas, liquid, or solid.
For equimolar counterdiffusion, NA = −NB and the convective term in Eq. (6.2-14) becomes zero. Then, NA = ![]() = −NB =×
= −NB =×![]() .
.
6.2C. Special Case for A Diffusing Through Stagnant, Nondiffusing B
The case of diffusion of A through stagnant or nondiffusing B at steady state often occurs. In this case one boundary at the end of the diffusion path is impermeable to component B, so it cannot pass through. One example, shown in Fig. 6.2-2a, is in the evaporation of a pure liquid such as benzene (A) at the bottom of a narrow tube, where a large amount of inert or nondiffusing air (B) is passed over the top. The benzene vapor (A) diffuses through the air (B) in the tube. The boundary at the liquid surface at point 1 is impermeable to air, since air is insoluble in benzene liquid. Hence, air (B) cannot diffuse into or away from the surface. At point 2 the partial pressure pA2 = 0, since a large volume of air is passing by.
Figure 6.2-2. Diffusion of A through stagnant, nondiffusing B: (a) benzene evaporating into air, (b) ammonia in air being absorbed into water.

Another example, shown in Fig. 6.2-2b, occurs in the absorption of NH3 (A) vapor which is in air (B) by water. The water surface is impermeable to the air, since air is only very slightly soluble in water. Thus, since B cannot diffuse, NB = 0.
To derive the case for A diffusing in stagnant, nondiffusing B, NB = 0 is substituted into the general Eq. (6.2-14):
Equation 6.2-16
![]()
The convective flux of A is (cA/c)(NA + 0). Keeping the total pressure P constant, substituting c = P/RT, pA = xAP, and cA/c = pA/P into Eq. (6.2-16),
Equation 6.2-17
![]()
Rearranging and integrating,
Equation 6.2-18
![]()
Equation 6.2-19
![]()
Equation 6.2-20
![]()
Equation (6.2-20) is the final equation to be used to calculate the flux of A. However, it is often written in another form. A log mean value of the inert B is defined as follows. Since P = pA1 + pB1 = pA2 + pB2, pB1 = P − pA1, and pB2 = P − pA2,
Equation 6.2-21
![]()
Substituting Eq. (6.2-21) into (6.2-20),
Equation 6.2-22
![]()
EXAMPLE 6.2-2. Diffusion of Water Through Stagnant, Nondiffusing AirWater in the bottom of a narrow metal tube is held at a constant temperature of 293 K. The total pressure of air (assumed dry) is 1.01325 × 105 Pa (1.0 atm) and the temperature is 293 K (20°C). Water evaporates and diffuses through the air in the tube, and the diffusion path z2 − z1 is 0.1524 m (0.5 ft) long. The diagram is similar to Fig. 6.2-2a. Calculate the rate of evaporation at steady state in lb mol/h · ft2 and kg mol/s · m2. The diffusivity of water vapor at 293 K and 1 atm pressure is 0.250 × 10−4 m2/s. Assume that the system is isothermal. Use SI and English units. Solution: The diffusivity is converted to ft2/h by using the conversion factor from Appendix A.1:
From Appendix A.2 the vapor pressure of water at 20°C is 17.54 mm, or pA1 = 17.54/760 = 0.0231 atm = 0.0231(1.01325 × 105) = 2.341 × 103 Pa, pA2 = 0 (pure air). Since the temperature is 20°C (68°F), T = 460 + 68 = 528°R = 293 K. From Appendix A.1, R = 0.730 ft3 · atm/lb · mol·°R. To calculate the value of pBM from Eq. (6.2-21),
Since pB1 is close to pB2, the linear mean (pB1 + pB2)/2 could be used and would be very close to pBM. Substituting into Eq. (6.2-22) with z2 − z1 = 0.5 ft (0.1524 m),
|
EXAMPLE 6.2-3. Diffusion in a Tube with Change in Path LengthDiffusion of water vapor in a narrow tube is occurring as in Example 6.2-2 under the same conditions. However, as shown in Fig. 6.2-2a, at a given time t, the level is z m from the top. As diffusion proceeds, the level drops slowly. Derive the equation for the time tF for the level to drop from a starting point of z0 m at t = 0 to zF at t = tF s as shown. Solution: We assume a pseudo-steady-state condition since the level drops very slowly. As time progresses, the path length z increases. At any time t, Eq. (6.2-22) holds; but the path length is z and Eq. (6.2-22) becomes as follows, where NA and z are now variables: Equation 6.2-23
Assuming a cross-sectional area of 1 m2, the level drops dz m in dt s, and ρA(dz · 1)/MA is the kg mol of A that have left and diffused. Then, Equation 6.2-24
Equating Eq. (6.2-24) to (6.2-23), rearranging, and integrating between the limits of z = z0 when t = 0 and z = zF when t = tF, Equation 6.2-25
Solving for tF, Equation 6.2-26
|
The method shown in Example 6.2-3 has been used to experimentally determine the diffusivity DAB. In this experiment the starting path length z0 is measured at t = 0 together with the final zF at tF. Then Eq. (6.2-26) is used to calculate DAB.
6.2D. Diffusion Through a Varying Cross-Sectional Area
So far, in the cases at steady state we have considered NA and ![]() as constants in the integrations. In these cases the cross-sectional area A m2 through which the diffusion occurs has been constant with varying distance z. In some situations the area A may vary. Then it is convenient to define NA as
as constants in the integrations. In these cases the cross-sectional area A m2 through which the diffusion occurs has been constant with varying distance z. In some situations the area A may vary. Then it is convenient to define NA as
Equation 6.2-27
![]()
where ![]() is kg moles of A diffusing per second or kg mol/s. At steady state,
is kg moles of A diffusing per second or kg mol/s. At steady state, ![]() will be constant but not A for a varying area.
will be constant but not A for a varying area.
1. Diffusion from a sphere
To illustrate the use of Eq. (6.2-27), the important case of diffusion to or from a sphere in a gas will be considered. This situation appears often, in such cases as the evaporation of a drop of liquid, the evaporation of a ball of naphthalene, and the diffusion of nutrients to a sphere-like microorganism in a liquid. In Fig. 6.2-3a is shown a sphere of fixed radius r1 m in an infinite gas medium. Component (A) at partial pressure pA1 at the surface is diffusing into the surrounding stagnant medium (B), where pA2 = 0 at some large distance away. Steady-state diffusion will be assumed.
Figure 6.2-3. Diffusion through a varying cross-sectional area: (a) from a sphere to a surrounding medium, (b) through a circular conduit that is tapered uniformally.

The flux NA can be represented by Eq. (6.2-27), where A is the cross-sectional area 4πr2 at point r distance from the center of the sphere. Also, ![]() is a constant at steady state:
is a constant at steady state:
Equation 6.2-28
![]()
Since this is a case of A diffusing through stagnant, nondiffusing B, Eq. (6.2-18) will be used in its differential form and NA will be equated to Eq. (6.2-28), giving
Equation 6.2-29
![]()
Note that dr was substituted for dz. Rearranging and integrating between r1 and some point r2 a large distance away,
Equation 6.2-30
![]()
Equation 6.2-31
![]()
Since ![]() , 1/r2 ≅ 0. Substituting pBM from Eq. (6.2-21) into Eq. (6.2-31),
, 1/r2 ≅ 0. Substituting pBM from Eq. (6.2-21) into Eq. (6.2-31),
Equation 6.2-32
![]()
This equation can be simplified further. If pA1 is small compared to P (a dilute gas phase),PBM ≅ P . Also, setting 2r1 = D1, diameter, and cA1 = pA1/RT, we obtain
Equation 6.2-33
![]()
This equation can also be used for liquids, where DAB is the diffusivity of A in the liquid.
EXAMPLE 6.2-4. Evaporation of a Naphthalene SphereA sphere of naphthalene having a radius of 2.0 mm is suspended in a large volume of still air at 318 K and 1.01325 × 105 Pa (1 atm). The surface temperature of the naphthalene can be assumed to be at 318 K and its vapor pressure at 318 K is 0.555 mm Hg. The DAB of naphthalene in air at 318 K is 6.92 × 10−6 m2/s. Calculate the rate of evaporation of naphthalene from the surface. Solution: The flow diagram is similar to Fig. 6.2-3a. DAB = 6.92 × 10−6 m2/s, pA1 = (0.555/760)(1.01325 × 105) = 74.0 Pa, pA2 = 0, r1 = 2/1000 m, R = 8314 m3· Pa/kg mol · K, pB1 = P − pA1 = 1.01325 × 105 − 74.0 = 1.01251 × 105 Pa, pB2 = P − pA2 = 1.01325 × 105 − 0. Since the values of pB1 and pB2 are close to each other,
Substituting into Eq. (6.2-32),
|
If the sphere in Fig. 6.2-3a is evaporating, the radius r of the sphere decreases slowly with time. The equation for the time it takes for the sphere to evaporate completely can be derived by assuming pseudo-steady state and by equating the diffusion flux equation (6.2-32), where r is now a variable, to the moles of solid A evaporated per dt time and per unit area as calculated from a material balance. (See Problem 6.2-9 for this case.) The material-balance method is similar to Example 6.2-3. The final equation is
Equation 6.2-34
![]()
where r1 is the original sphere radius, ρA the density of the sphere, and MA the molecular weight.
2. Diffusion through a conduit of nonuniform cross-sectional area
In Fig. 6.2-3b component A is diffusion at steady state through a circular conduit which is tapered uniformally as shown. At point 1 the radius is r1 and at point 2 it is r2. At position z in the conduit, for A diffusing through stagnant, nondiffusing B,
Equation 6.2-35
![]()
Using the geometry shown, the variable radius r can be related to position z in the path as follows:
Equation 6.2-36
![]()
This value of r is then substituted into Eq. (6.2-35) to eliminate r and the equation integrated:
Equation 6.2-37

A case similar to this is given in Problem 6.2-10.
6.2E. Diffusion Coefficients for Gases
1. Experimental determination of diffusion coefficients
A number of different experimental methods have been used to determine the molecular diffusivity for binary gas mixtures. Several of the important methods are as follows. One method is to evaporate a pure liquid in a narrow tube with a gas passed over the top, as shown in Fig. 6.2-2a. The fall in liquid level is measured with time and the diffusivity calculated from Eq. (6.2-26).
In another method, two pure gases having equal pressures are placed in separate sections of a long tube separated by a partition. The partition is slowly removed and diffusion proceeds. After a given time the partition is reinserted and the gas in each section analyzed. The diffusivities of the vapor of solids such as naphthalene, iodine, and benzoic acid in a gas have been obtained by measuring the rate of evaporation of a sphere. Equation (6.2-32) can be used. See Problem 6.2-9 for an example of this.
A method often used is the two-bulb method (N1). The apparatus consists of two glass bulbs with volumes V1 and V2 m3 connected by a capillary of cross-sectional area A m2 and length L whose volume is small compared to V1 and V2, as shown in Fig. 6.2-4. Pure gas A is added to V1 and pure B to V2 at the same pressures. The valve is opened, diffusion proceeds for a given time, and then the valve is closed and the mixed contents of each chamber are sampled separately.
Figure 6.2-4. Diffusivity measurement of gases by the two-bulb method.

The equations can be derived by neglecting the capillary volume and assuming each bulb is always of a uniform concentration. Assuming quasi-steady-state diffusion in the capillary,
Equation 6.2-38
![]()
where c2 is the concentration of A in V2 at time t and c1 in V1. The rate of diffusion of A going to V2 is equal to the rate of accumulation in V2:
Equation 6.2-39
![]()
The average value cav at equilibrium can be calculated by a material balance from the starting compositions ![]() and
and ![]() at t = 0:
at t = 0:
Equation 6.2-40
![]()
A similar balance at time t gives
Equation 6.2-41
![]()
Substituting c1 from Eq. (6.2-41) into (6.2-39), rearranging, and integrating between t = 0 and t = t, the final equation is
Equation 6.2-42
![]()
If c2 is obtained by sampling at t, DAB can be calculated.
2. Experimental diffusivity data
Some typical data are given in Table 6.2-1. Other data are tabulated in Perry and Green (P1) and Reid et al. (R1). The values range from about 0.05 × 10−4 m2/s, where a large molecule is present, to about 1.0 × 10−4 m2/s, where H2 is present at room temperatures. The relation between diffusivity in m2/s and ft2/h is 1 m2/s = 3.875 × 104 ft2/h.
| System | Temperature | Diffusivity [(m2/s)104 or cm2/s] | Ref. | |
|---|---|---|---|---|
| °C | K | |||
| Air–NH3 | 0 | 273 | 0.198 | (W1) |
| Air–H2O | 0 | 273 | 0.220 | (N2) |
| 25 | 298 | 0.260 | (L1) | |
| 42 | 315 | 0.288 | (M1) | |
| Air–CO2 | 3 | 276 | 0.142 | (H1) |
| 44 | 317 | 0.177 | ||
| Air–H2 | 0 | 273 | 0.611 | (N2) |
| Air–C2H5OH | 25 | 298 | 0.135 | (M1) |
| 42 | 315 | 0.145 | ||
| Air–CH3COOH | 0 | 273 | 0.106 | (N2) |
| Air–n-hexane | 21 | 294 | 0.080 | (C1) |
| Air–benzene | 25 | 298 | 0.0962 | (L1) |
| Air–toluene | 25.9 | 298.9 | 0.086 | (G1) |
| Air–n-butanol | 0 | 273 | 0.0703 | (N2) |
| 25.9 | 298.9 | 0.087 | ||
| H2–CH4 | 25 | 298 | 0.726 | (C2) |
| H2–N2 | 25 | 298 | 0.784 | (B1) |
| 85 | 358 | 1.052 | ||
| H2–benzene | 38.1 | 311.1 | 0.404 | (H2) |
| H2–Ar | 22.4 | 295.4 | 0.83 | (W2) |
| H2–NH3 | 25 | 298 | 0.783 | (B1) |
| H2–SO2 | 50 | 323 | 0.61 | (S1) |
| H2–C2H5OH | 67 | 340 | 0.586 | (T1) |
| He–Ar | 25 | 298 | 0.729 | (S2) |
| He–n-butanol | 150 | 423 | 0.587 | (S2) |
| He–air | 44 | 317 | 0.765 | (H1) |
| He–CH4 | 25 | 298 | 0.675 | (C2) |
| He–N2 | 25 | 298 | 0.687 | (S2) |
| He–O2 | 25 | 298 | 0.729 | (S2) |
| Ar–CH4 | 25 | 298 | 0.202 | (C2) |
| CO2–N2 | 25 | 298 | 0.167 | (W3) |
| CO2–O2 | 20 | 293 | 0.153 | (W4) |
| N2–n-butane | 25 | 298 | 0.0960 | (B2) |
| H2O–CO2 | 34.3 | 307.3 | 0.202 | (S3) |
| CO–N2 | 100 | 373 | 0.318 | (A1) |
| CH3Cl–SO2 | 30 | 303 | 0.0693 | (C3) |
| (C2H5)2O–NH3 | 26.5 | 299.5 | 0.1078 | (S4) |
3. Prediction of diffusivity for gases
The diffusivity of a binary gas mixture in the dilute gas region, that is, at low pressures near atmospheric, can be predicted using the kinetic theory of gases. The gas is assumed to consist of rigid spherical particles that are completely elastic on collision with another molecule, which implies that momentum is conserved.
In a simplified treatment it is assumed that there are no attractive or repulsive forces between the molecules. The derivation uses the mean free path λ, which is the average distance that a molecule has traveled between collisions. The final equation is
Equation 6.2-43
![]()
where ū is the average velocity of the molecules. The final equation obtained after substituting expressions for ū and λ into Eq. (6.2-43) is approximately correct, since it correctly predicts DAB proportional to 1/pressure and approximately predicts the temperature effect.
A more accurate and rigorous treatment must consider the intermolecular forces of attraction and repulsion between molecules as well as the different sizes of molecules A and B. Chapman and Enskog (H3) solved the Boltzmann equation, which uses a distribution function instead of the mean free path λ. To solve the equation, a relation between the attractive and repulsive forces between a given pair of molecules must be used. For a pair of nonpolar molecules, a reasonable approximation to the forces is the Lennard–Jones function.
The final relation for predicting the diffusivity of a binary gas pair of A and B molecules is
Equation 6.2-44
![]()
where DAB is the diffusivity in m2/s, T temperature in K, MA molecular weight of A in kg mass/kg mol, MB molecular weight of B, and P absolute pressure in atm. The term σAB is an “average collision diameter” and ΩD,AB is a collision integral based on the Lennard–Jones potential. Values of σA and σB as well as ΩD,AB can be obtained from a number of sources (B3, G2, H3, R1).
The collision integral ΩD,AB is a ratio giving the deviation of a gas with interactions as compared to a gas of rigid, elastic spheres. This value would be 1.0 for a gas with no interactions. Equation (6.2-44) predicts diffusivities with an average deviation of about 8% up to about 1000 K (R1). For a polar–nonpolar gas mixture, Eq. (6.2-44) can be used if the correct force constant is used for the polar gas (M1, M2). For polar–polar gas pairs, the potential-energy function commonly used is the Stockmayer potential (M2).
The effect of concentration of A in B in Eq. (6.2-44) is not included. However, for real gases with interactions, the maximum effect of concentration on diffusivity is about 4% (G2). In most cases the effect is considerably less, and hence it is usually neglected.
Equation (6.2-44) is relatively complicated to use, and often some of the constants such as σAB are not available or are difficult to estimate. Hence, the semiempirical method of Fuller et al. (F1), which is much more convenient to use is often preferred. The equation was obtained by correlating many recent data and uses atomic volumes from Table 6.2-2, which are summed for each gas molecule. The equation is
Equation 6.2-45
![]()
| Atomic and structural diffusion volume increments, v | |||
| C | 16.5 | (C1) | 19.5 |
| H | 1.98 | (S) | 17.0 |
| O | 5.48 | Aromatic ring | −20.2 |
| (N) | 5.69 | Heterocyclic ring | −20.2 |
| Diffusion volumes for simple molecules, Ε v | |||
| H2 | 7.07 | CO | 18.9 |
| D2 | 6.70 | CO2 | 26.9 |
| He | 2.88 | N2O | 35.9 |
| N2 | 17.9 | NH3 | 14.9 |
| O2 | 16.6 | H2O | 12.7 |
| Air | 20.1 | (CCl2F2) | 114.8 |
| Ar | 16.1 | (SF6) | 69.7 |
| Kr | 22.8 | (Cl2) | 37.7 |
| (Xe) | 37.9 | (Br2) | 67.2 |
| Ne | 5.59 | (SO2) | 41.1 |
| Source: Reprinted with permission from E. N. Fuller, P. D. Schettler, and J. C. Giddings, Ind. Eng. Chem., 58, 19(1966). Copyright by the American Chemical Society. | |||
[*] Parentheses indicate that the value listed is based on only a few data points.
where Ε vA = sum of structural volume increments, Table 6.2-2, and DAB = m2/s. This method can be used for mixtures of nonpolar gases or for a polar–nonpolar mixture. Its accuracy is not quite as good as that of Eq. (6.2-44).
The equation shows that DAB is proportional to 1/P and to T1.75. If an experimental value of DAB is available at a given T and P and it is desired to have a value of DAB at another T and P, one should correct the experimental value to the new T and P by means of the relationship DAB ∝ T1.75/P.
4. Schmidt number of gases
The Schmidt number of a gas mixture of dilute A in B is dimensionless and is defined as
Equation 6.2-46
![]()
where µ is viscosity of the gas mixture, which is viscosity of B for a dilute mixture in Pa · s or kg/m · s, DAB is diffusivity in m2/s, and ρ is the density of the mixture in kg/m3. For a gas the Schmidt number can be assumed independent of temperature over moderate ranges and independent of pressure up to about 10 atm or 10 × 105 Pa.
The Schmidt number is the dimensionless ratio of the molecular momentum diffusivity μ/ρ to the molecular mass diffusivity DAB. Values of the Schmidt number for gases range from about 0.5 to 2. For liquids Schmidt numbers range from about 100 to over 10 000 for viscous liquids.
EXAMPLE 6.2-5. Estimation of Diffusivity of a Gas MixtureNormal butanol (A) is diffusing through air (B) at 1 atm abs. Using the Fuller et al. method, estimate the diffusivity DAB for the following temperatures and compare with the experimental data:
Solution: For part (a), P = 1.00 atm, T = 273 + 0 = 273 K, MA (butanol) = 74.1, MB (air) = 29. From Table 6.2-2,
Substituting into Eq. (6.2-45),
This value deviates by +10% from the experimental value of 7.03 × 10−6 m2/s from Table 6.2-1. For part (b), T = 273 + 25.9 = 298.9. Substituting into Eq. (6.2-45), DAB = 9.05 × 10−6 m2/s. This value deviates by +4% from the experimental value of 8.70 × 10−6 m2/s. For part (c), the total pressure P = 2.0 atm. Using the value predicted in part (a) and correcting for pressure,
|