5
2D Nanomaterials for Cancer Therapy
Naresh Kuthala
National Tsing Hua University, Department of Chemistry, No. 101, Section 2, Guangfu Road, East District, Hsinchu, 300044, Taiwan ROC
5.1 Introduction
Since decades, conventional treatment modalities such as surgery, chemotherapy, and radiotherapy (RT) have been implemented to cancer eradication [1–7]. All these modalities with combined or standalone approaches resulted in the poor prognosis of this deadly disease and side effects due to (i) highly energetic electromagnetic radiations results in the major side effects and healthy tissue damage and (ii) conventional diagnosis methods need vast amounts of imaging agents injected into patients [8, 9]. Hence, these higher doses of conventional imaging agents result in nephrotoxicity, which is the leading drawback of the clinical imaging agents Gd‐DTPA (diethylenetriamine pentaacetate), Gd‐DOTA (tetraazacyclododecanetetraacetic acid), etc. [10–13]. Numerous clinical and preclinical therapies with chemotherapy resulted in the limited applications due to the growth of chemo or drug resistance in the cancer cells. It is highly impossible to eradicate the tumors completely with such kind of standalone cancer therapy, which will further raise the complications such as therapeutic side effects [14, 15]. There are extensive efforts dedicated toward the development of nanomaterials with the optical properties ranging between the biological windows I (650–950 nm), biological windows IIa (1000–1350 nm), and biological windows IIb (1500–1870 nm) [16–18]. Especially, 2D nanomaterials exhibiting extraordinary photo‐matter interaction enable them as excellent plasmonic 2D nanomaterials for their applications in the field of photothermal therapy (PTT), photodynamic therapy (PDT), or synergistic therapies.
2D nanomaterials have attracted interest as emerging nanomaterials since the invention of graphene with exfoliation in 2004 [19–22]. To date, numerous family of 2D nanomaterials has been synthesized, such as transition metal dichalcogenides (TMDs), graphene‐based nanomaterials, black phosphorus (BP), palladium (Pd) nanosheets, and transition metal nitrides, carbides, and carbonitrides (MXenes), and other 2D nanomaterials [23, 24]. Undoubtedly, due to the unique features of 2D nanomaterials such as excellent chemical, mechanical, and optical properties, shape, tunable size, biocompatibility, and biodegradability have attracted the scientific world in many fields of applications. They have been successfully applied for the biomedical applications and cancer treatment [25]. Additionally, with the large surface areas and nanosheet structures compared with other nanomaterials enable them to grab fluorescent probes, nucleic acids, drugs, proteins, etc. by noncovalent or covalent interactions, and controlled release under the external stimuli [21]. Although various nanoparticles, such as AuNPs, TiO2 NPs, Fe3O4 NPs, and quantum dots, effectively integrated onto the nanosheet surface of these 2D nanomaterials enable them for the additional functionalities like imaging, magnetic, and radiological properties, etc. [21]. Furthermore, the nanosheet structures of 2D nanomaterials endowed them to absorb near‐infrared (NIR) light for the phototherapy applications such as PTT and PDT. Most of the previous reports fabricated 2D nanomaterials for the application of monotherapy, whereas it is well documented that monotherapies are not as effective as dual or synergistic therapies, due to tumor recurrence as well as metastasis of tumor cells [26]. Here, in this chapter, we have discussed current trend and future prospects of 2D nanomaterials for the applications in biomedical field with the combination or synergistic therapy of PTT with other treatment modalities.
5.2 2D Nanomaterials for Cancer Therapy
In the present chapter, the state of the art of 2D nanomaterials in cancer therapy is presented by depicting the five main scenarios of advanced nanotechnology‐based approaches in cancer, namely PTT, PDT, radiotherapy, sonodynamic therapy (SDT), and immunotherapy (ImT).
5.2.1 2D Nanomaterials for Combination PTT with PDT
- Photothermal therapy (PTT): Most of the nanomaterials act as efficient PTT agents, based on their absorption capability in the UV‐vis‐NIR region. PTT is a type of localized hyperthermia treatment modality that depends on the existence of a nanomaterial or photosensitizer (PS) that can act as an optical absorbing agent, which can further absorb energy and transform it into heat upon irradiation with an electromagnetic radiation, such as microwaves or radiofrequency or NIR energy source [27]. The main features of PTT include the ability of higher penetration depths and minimal invasiveness to the healthy tissue surrounding the tumor, which is far more beneficial than the conventional chemotherapy or radiotherapy [27]. The photothermal properties of metal nanoparticles are based on their photophysical and photochemical properties. In case of metal nanoparticles, the working principle of PTT involves plasmonic excited states of metal nanoparticles that reach to the ground states through electron–phonon interactions with in 10−12 seconds and emits the excited state energy in the form of thermal heat [16]. In general, the metal nanoparticles, when compared with light absorbing organic PSs, exhibit 3–7 orders higher molar extinction coefficients that enable them as efficient photothermal agents for the conversion of photon to thermal heat.
In the PTT scenario, overheating of the nanomaterial or PS leads to hyperthermia that may produce toxicity to the cancer cells or tumors, such as evaporation of cytosol, protein aggregation and denaturation, cell lysis, necrosis, and apoptosis. An ideal nanomaterial or PS has (i) higher targeting ability toward the tumor, (ii) higher absorption capability for wide range of optical wavelengths, (iii) lower toxicity, (iv) easy functionalization with targeting moieties, and (v) good compatibility in biological fluids [16]. PTT becomes popular phototherapy in the field of nanomedicine stand‐alone or in combination with therapy models such as radiotherapy, PDT, SDT, ImT, and imaging modalities (Figure 5.1).
- Photodynamic therapy (PDT): In addition to the chemotherapy, radiotherapy and phototherapy are two exciting treatment modalities that can cure cancer very effectively. Among them, phototherapeutic approach has many advantages such as noninvasiveness, excellent spatial–temporal selectivity, low side effects, and cost effective as compared to other treatment modalities. PDT requires the use of organic PSs to absorb and transfer the photon energy to molecular oxygen (3O2) in normal tissues, to generate cytotoxic singlet oxygen (1O2) and kill cancer cells. [28, 29]. In general, PDT can be further classified into type‐I and type‐II. The light‐triggered PS excited to singlet excited state and produces long‐lived triplet excited state with intersystem crossing phenomenon. In the presence of chromophore/biological substrates, the resultant excited triplet excited state releases radical anions with electron transfer mechanisms; finally, these radical anions interact with molecular oxygen to produce oxygenated reactive oxygen species (ROS) such as O2−˙ (superoxide) and OH radicals (type‐I PDT mechanism). In the similar manner, the triplet excited state PS can transmit its excited state energy to the molecular oxygen to produce the singlet oxygen, i.e. 1O2 (type‐II PDT mechanism) [16]. In addition to the type‐I and II PDT, recently, oxygen‐independent generation pathway for PDT was reported, which may be termed type‐III PDT (oxygen‐independent type‐I PDT). The mechanism for such unique process of generating ROS, i.e. OH radical, involves water as the source of oxygen species instead of molecular oxygen. Hence, compared to conventional chemotherapeutic methods, which leads to the acute systemic toxicity, in the radiotherapy, radiation beam induces the damage to the healthy tissue surrounding the tumor location. All types of PDT involve nontoxic components such as non‐ionizing light source, water/molecular oxygen, and PS/metal nanoparticles. Hence, PDT becomes the popular cancer therapeutic modality for tumor‐specific and patient‐friendly [30]. In clinical practice, PS has been employed. But conventional organic PS‐based PDT has many limitations, such as poor water solubility, severe photo‐bleaching issues, restriction to UV or visible light activation, which has low tissue penetration depths, and therefore not applicable to deep tissue‐buried tumors. To treat deep tissue‐buried tumors, NIR light with far larger tissue penetration depths has to be used. To address such issues, the research has to be dedicated to the development of new class of nanomedicine/metal nanoparticles with broad NIR light absorption with the features of both PTT and PDT for the production of synergistic effects.
Figure 5.1 The key reasons for the cancer.
Most of the tumor micro environments (TMEs) are highly hypoxic, resulting in the suppression of ROS generation rate, which restricts the therapeutic efficacy of PDT [31]. To overcome such limitations of PDT, PDT combined with PTT leads to the effective combination therapy to establish synergistic photo‐triggered therapy, and it may produce the great‐additive therapeutic outcome. In this combination therapy, mild hyperthermia generated with PTT results in the enhancement of the intracellular concentration of PSs by improving membrane permeability, hence increasing the uptake of PS‐loaded nanoplatforms by cancer cells [32, 33]. The advantages of mild hyperthermia may lead to stimulate blood flow and enhance concentration of saturated oxygen in the blood vessels, hence further ease the generation of ROS in PDT type‐II pathway which is oxygen‐dependent [34, 35]. Therefore, nanotechnology‐based 2D nanomaterials have attracted more attention as ideal multifunctional nanomedicine for the applications in the field of combined PTT‐PDT therapy to destruct the cancer tumors. Most of the 2D nanomaterials adopt two common strategies for the synergistic PTT‐PDT therapy. The first one includes the innate PDT and PTT properties within the single 2D nanoparticles such as Ti3C2 nanodots, 2D BP nanosheets, etc. In the second method, 2D nanomaterials act as a drug shipper to load organic PSs; hence, the 2D nanomaterial and PS can adopt both the combination of photothermal and photodynamic properties, which is also the almost frequently used methodology. Recently, graphene and graphene oxide (GO) were reported as drug carriers to load organic PSs, thus accomplishing the synergistic therapy of PTT and PDT. Recently, various 2D nanomaterials fabricated for their synergistic applications of PDT‐PTT due to their ease of biocompatibility and surface modification properties [36]. The 2D nanomaterial TMD, for example, MoS2‐polyethylene glycol (PEG) nanosheets loaded with chlorin e6 (Ce6), i.e. MoS2‐PEG@Ce6 NSs applied for the synergistic PDT‐PTT against tumors with in vivo experiments [37]. The MoS2 nanosheets were synthesized via exfoliation method and modified with lipoic acid (LA)‐PEG. The in vitro experiments showed the superiority of the therapy with MoS2‐PEG@Ce6 NSs as compared with the Ce6 alone. Overall, MoS2‐PEG@Ce6 NSs act as positive contrast agents for photoacoustic (PA) imaging and effectively results in the synergistic PDT‐PTT therapy as compared with the monotherapies alone [37]. Similarly, same strategy is applied to design Ce6‐loaded black phosphorus nanosheets (BP NSs), which have been applied for combination therapy against tumors with PDT and PTT (Figure 5.2) [38]. BP NSs have relatively higher surface area, and further BP@PEG NSs are capable of loading Ce6 into higher extents. BP@PEG‐Ce6 NSs reported with excellent advantages such as biocompatibility, physiological stability, higher tumor accumulation and targeting ability, and higher fractions of photothermal conversion efficiency (PTCE) of 43.6%. Overall, BP@PEG‐Ce6 NSs can efficiently act as PDT‐PTT synergistic therapy of cancer cells, with the release of ROS ability in the presence of Ce6. Moreover, pH‐dependent 2D nanomaterials designed with toluidine blue O (TBO) integrated into MoS2 nanosheets, which is successfully applied for combined PDT‐PTT for cancer eradication [39]. MoS2NSs@TBO showed poor fluorescence, and light‐triggered ROS generation nature of TBO is controlled by MoS2 NSs under physiological pH or normal pH values. While under tumor mimicking acidic conditions such as pH 5.5, the MoS2NSs@TBO showed greater antitumor response and light‐triggered ROS generation, clearly showing the synergistic PDT‐PTT cancer therapy. It is well known that most of the solid tumors severely suffer from the hypoxia and almost all the organic PSs have limitations with hydrophobic nature which renders the effective therapeutic outcomes of PDT [34, 35]. To overcome these impediments, Pd@Pt‐PEG‐Ce6 nanoplatform fabricated for more advanced results with PDT [40]. In addition, Pd@Pt‐PEG nanoplatform showed catalase activity and can convert hydrogen peroxide to produce molecular oxygen. Furthermore, Pd@Pt‐PEG‐Ce6 nanoplatform had excellent PTCE and biocompatibility and higher tumor accumulation. Higher PTCE of Pd@Pt‐PEG‐Ce6 leads to the release of Ce6 to the greater extent, and higher degree of decomposition of hydrogen peroxide leads to the effective PDT effect. Overall, Pd@Pt‐PEG‐Ce6 nanoplatform can effectively resolve the hypoxia, synergistic PDT‐PTT, and higher PTCE.
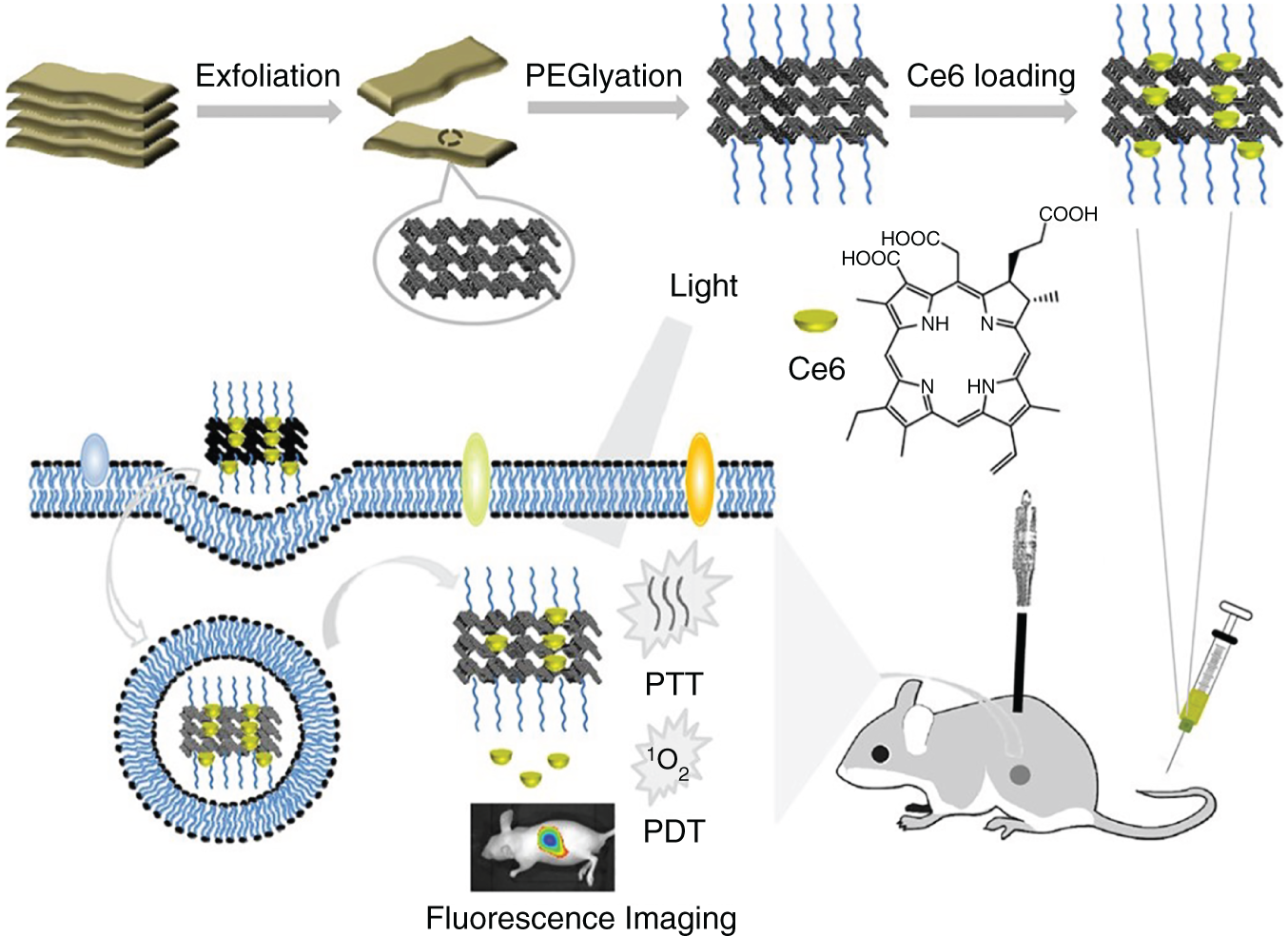
Figure 5.2 The combination therapy of PTT‐PDT. Synthesized BP@PEG/Ce6 NS acts as PTT‐PDT agents in the presence of external stimuli light.
Source: Yang et al. [38]. Reprinted with permission from American Chemical Society.
5.2.2 2D‐Nanomaterials for Combination PTT Therapy with Radiotherapy (RT)
Radiation or radiotherapy is a therapeutic tool, which is employed to abolish cancer cells. Among all the cancer treatment modalities, 50% of the cancer patients all over the world undergoes the radiotherapy standalone or in combination with the other therapy modalities such as surgery, chemotherapy, and so on [41, 42]. The radiotherapy involves the radiations such as x‐rays, γ‐rays, and electron or proton or neutron beams for the treatment options. X‐rays or γ‐rays can be termed ionizing radiation because it develops charged particles or ions and accumulates energy in the normal cells or cancer cells of the tissues where it passes through. This credited energy may kill cancerous cells as well as the normal cells and finally cause deoxyribonucleic acid (DNA) damages that lead to the genetic alterations causing cell death [43].
Highly energetic radiation costs for the damage of genetic material such as DNA of cells and hence arrests their cell cycle capability which helps the cells to split and multiply further to the formation of new cells [43]. Hence, the major task is to control the demerit of the radiation therapy that damages the normal cells or healthy cells to intensify the radiation dose at the cancer tumor sites and to nullify the radiation exposure to the healthy tissue around the tumor location or in the traveling path of the ionization radiation beam within the body. The major advantage of normal or healthy cells gifted with self‐repair at a moderate or quicker rate and get back their usual functions as compared with the cancerous cells. Hence, radiation therapy needs to use low energetic x‐rays or γ‐rays to reduce the effect of the damage to the normal tissue; at the same time, the low dose of these ionization radiations can effectively kill cancer cells. In general, cancer cells are not efficient in recovery of the damage caused by the radiation treatment as compared with normal cells [42]. Even though neutron source leaves the healthy surrounding tissue around the tumor in the treatment modality such as boron neutron capture therapy (BNCT), but very few boron drugs or 10B‐enriched nanoparticles are reported [44]. To overcome such limitation of radiation therapy applied to the cancer with the treatment modalities, such as PDT, ImT for the synergistic effects to control, and eradicate cancer.
In the radiation therapy for clinical practice, the radiation beam can be delivered to the location of cancer in two different ways. The most popular approach in the clinical practice is to pass the high energetic radiation beam from outside the body to the tumor location. The second method includes the brachytherapy or internal radiation where the radioactive resource was located at the tumor site; this method practiced particularly in prostate, gynecological malignancies.
The recent nanotechnology is developed to address the issues of high‐dose radiation therapy by the introduction of higher atomic numbered elements such as Au, Gd, Bi, and W, which have the higher X‐ray attenuation probability for the enhanced deposition of energy to facilitate the release of ROS [45]. Additionally, 2D nanomaterials with the higher PTCE and X‐ray sensitizer heavy elements can produce better therapeutic results for the synergistic therapy of PTT radiotherapy.
For example, WS2 nanoflakes integrated with Gd for the combined RT and PTT against cancer tumor models [46]. In this combined nanoplatform, WS2 nanoflakes showed the NIR absorbance which converts the incident light into PTT, whereas heavier elements W and Gd efficiently convert X‐ray radiation to improved ROS generation, which could result in the synergistic therapy model with PTT‐RT with eradication of in vitro as well as in vivo tumor cells to improve the monotherapy with RT alone. Further, multifunctional 64Cu‐tagged‐FeSe2/Bi2Se3‐PEG nanoplatform reported for combined PTT and RT against tumors [47]. Due to excellent X‐ray attenuation with the heavier element Bi, NIR absorption leads to the successful eradication of tumor models with synergistic therapy.
For example, Ti3C2@Au was synthesized as multifunctional 2D nanomaterial via seed growth methodology and successfully applied for the combination therapy PTT‐RT of tumors (Figure 5.3) [48]. The integration of Au NPs into Ti3C2 nanosheets enables Ti3C2@Au to exhibit higher X‐ray attenuation properties, making the nanoplatform feasible for RT of tumors. Overall, Ti3C2@Au nanocomposites reported for the synergistic therapy of PTT‐RT as compared with the Ti3C2 therapy alone. The same strategy followed for the 2D nanomaterials such as WS2 quantum dots/nanosheets [46], MoS2/Bi2S3‐PEG nanocomposites [49], MoS2 quantum dot@polyaniline nanohybrids [50], ultrathin TeO2/(NH4)xWO3 nanoribbons [51], ReS2 nanosheets [52], BP/Bi2O3 heterostructures [53], and selenocysteine‐modified Bi2Se3 nanoparticles [54] has been extensively used in the synergistic therapy of PTT‐RT, showing excellent antitumor effects.

Figure 5.3 The combination therapy of PTT‐RT. Synthesized Ti3C2@Au acts as PTT‐RT agents in the presence of external stimuli light as well as X‐rays.
Source: Tang et al. [48]. Reprinted with permission from American Chemical Society.
Furthermore, 2D nanocomposites loaded with radio‐labeled nuclides such as 131I or 188Re have also been fabricated to apply for the PTT and internal radiotherapy (iRT) combined therapy without using the external high energetic X‐ray dose. For example, 131I‐RGO‐PEG 2D nanocomposites are designed for the eradication of metastatic cancer tumor models in combination with PTT and iRT. Overall, due to the presence of 131I radioactive nuclide release, the internal radiation and reduced graphene oxide (RGO) corresponding to the strong NIR absorbance enables 131I‐RGO‐PEG 2D nanocomposites in remarkable synergistic iRT‐PTT therapeutic efficiency [55]. Similarly, 188Re‐WS2‐PEG fabricated as 2D nanocomposites are applied for the combination PTT‐enhanced iRT of cancer tumors. Surprisingly, the internal radiation released from the radio nuclide 188Re could be effectively captured by the heavier W element without applying the external high‐dose X‐ray radiation. Overall, excellent PTCE with WS2 and self‐sensitization nature enables 188Re‐WS2‐PEG 2D nanocomposites for excellent combination therapy effect with RT‐PTT [56].
5.2.3 2D Nanomaterials for Combination PTT Therapy with Sonodynamic Therapy (SDT)
ROS, i.e. reactive oxygen species, are oxygen containing highly active species, such as singlet oxygen, hydroxyl radicals, peroxides, and superoxide. In a biological system, higher levels of ROS can result in the cellular damage, with the production of oxidative stress in the endogenous environments in the cells at different levels. The main possible ROS production causes are heat, ionizing radiation, light, and sound. The later source sound in other words called ultrasound (US) become an alternative to produce ROS for cancer therapeutic treatments by effectively damaging targeted tumor cells, such unique technique termed SDT. US is a form of energy that disrupts and generates bubbles in the propagation media and causes oscillations and ultimately disrupts with the acoustic field. The major driving phenomenon for SDT is the formation of cavitation [57–60]. SDT was discovered in the late 1980s, which is in similarity with PDT, and it is simply replacing the light source with US for the generation of ROS [61]. At the initial stage of SDT, the similar kind of PDT drugs (used as sonosensitizers) adopted for the US‐triggered ROS generation. The clinical PDT drugs such as porphyrin and its derivative drugs have been developed to generate ROS when exposed with US source [58, 62]. Photofrin is an approved clinical drug used in PDT [63–65]. It has been proven that Xanthene dyes showed the capability to produce ROS with the exposure to US [66]. Overall, these drugs showed the immense effect on human health in the form of side effects due to their higher and rapid accumulations by the spleen and liver, which leads to their restrictions for the applications. Furthermore, it was reported that amphiphilic agents showed the increased uptake at the targeted tumor sites, hence these drugs introduced as the new class of sonosensitizers. In literature, the methods of alkylation and carboxylation lead to the synthesis of rose bengal derivatives [67] as the resulting drug is known as a rose bengal derivative and effectively used as the sonosensitizers. After these classic sonosensitizers lead to the production of numerous derivatives of the same class with wide range of US parameters, it can be tuned for different applications of therapy. The effective frequency of US to initiate the production of ROS from most of the sonosensitizers ranges within 0.4–3 MHz, which is especially convenient for cancer therapeutic treatments due to the acceptable ratio in between the accuracy and propagation depth. The reported US power densities typically range between 0.5 and 10 W/cm2, and high intense US has also been used. In the case of PS that are applied to use in the SDT may have serious issues such as photo bleaching to the atmospheric light, poor water solubility due to hydrophobic nature, and rapid accumulations at liver. Hence to avoid such issues, SDT can be introduced with new kind of sonosensitizers such as nanoparticles. Nanotechnology‐based synthesis of nanoparticles or nanomedicine introduced a unique way for the generation of sonosensitizers and shown advancement in the field of cancer treatment. Compared to organic PSs, inorganic‐based nanomaterials have the advantages such as (i) tunable particle size and shape, (ii) superior physiological and chemical stability, and (iii) higher accumulations at tumor site with efficient targeting ability [68–72].
PDT‐PTT treatment in the biological windows I, II is suffering from the penetration depth of the light into the tumor tissue. To overcome such limitations of PDT, SDT become an alternative therapy that offers deep penetrations of tumor tissue, which uses the nontoxic energy form of sound waves, which are already well known in the clinical practice for the US imaging [73]. Additionally, SDT delivers the ultrasonic energy within the focused area of tumor regions and damages the blood vessels at tumor which leads effective eradication of tumor leaving unharmed to the surrounding normal tissues [74, 75]. 2D nanocomposites or nanomaterials are widely used for enhanced SDT results due to their exceptional PTCE, greater surface to volume ratio, and higher conductivity. For instance, 2D graphene successfully integrated with TiO2 for the improved sonocatalytic activity and also sonosensitizer ability, which is feasible for the efficient SDT [76] (Figure 5.4). Graphene is an electrically conductive material, which enables the holes and electrons well separated and to avoid them from recombining under US radiation. Overall, compared with the single sonosensitizer TiO2, 2D‐graphene@TiO2 showed excellent and improved SDT outcomes with higher PTCE of graphene with the sonosensitizer TiO2. Furthermore, Au@TiO2 has synthesized with different shell thicknesses to act as a multifunctional cancer therapy agent [77]. The formation of shell TiO2 over the Au nanoplates successfully induces (i) Localized surface plasmon resonance (LSPR) shifted from NIR I to NIR II BW (biological window), (ii) achieved excellent absorption at the wavelength 1064 nm, and (iii) enhanced PTCE such as 42.05%. Au@TiO2 acts as a metal semiconductor interface, which enables the holes and electrons well separated and to avoid them from recombining under US radiation. Overall, compared with the single sonosensitizer TiO2, 2D‐Au@TiO2 showed excellent SDT outcomes with enhanced ROS generation as well as with higher PTCE. In conclusion, as compared with PTT and PDT, SDT can give a deep penetration of tumor tissues. However, very few research reports available for 2D nanomaterials in the direction of combination therapy PTT‐SDT model, which may lead to the dedication of research on 2D nanomaterials for the future breakthroughs in the direction of synergistic PDT‐SDT therapeutic tumor models.

Figure 5.4 The combination therapy of PTT‐SDT. Synthesis procedure of MnOx/TiO2‐GR‐PVP nanocomposites and its application as both PTT‐SDT agents in the presence of light as well as US.
Source: Dai et al. [76]. Reprinted with permission from American Chemical Society.
5.2.4 2D Nanomaterials for Combination PTT Therapy with Immune Therapy (ImT)
Since few decades, cancer ImT has expanded remarkably as a potent therapeutic option to the present types of cancer therapies. In this treatment, immune therapeutic drugs modulate and hence enhance the capability of immune responses, further instigating immunological evocation from natural mechanisms without damaging the normal or healthy cells. Cancer ImT modality effectively inhibits the metastasis of malignant tumors and instigates immunological memory to stipulate durable protection over a feasible reoccurrence or regrown of the tumor.
The Nobel Prize in cancer ImT (medicine and physiology) has been awarded jointly to the scientists James P. Allison of The University of Texas MD Anderson Cancer Center and Dr. Tasuku Honjo of Kyoto University in Japan. The innovation of their research is to activate the immune system to attack the cancer malignant tumors, which leads to the breakthrough in the innovations of new cancer treatment modalities. Overall, their discoveries had enormous impact for the improvement of several drugs which can be considered for the routine treatment of the cancer ImT for the effective therapeutic outcomes. The mechanisms involved in ImT to treat cancer are as follows.
Tumor‐associated antigens (TAAs) are produced during the process of cancer cells necrosis [78–80]. Mostly, cancer tumor cells expressed with the tumor‐specific antigens (TSAs), including TAAs, are responsible for the immunogenic signals. These antigens have been processed by dendritic cells (DCs), stimulated CD4 T, and CD8 T lymphocytes, which are termed antigen‐presenting cells (APCs) that activate potent immune responses to the antitumor and lead to combat cancer [78, 81]. TME and targeted cancer cells reorganize themselves to block the tumoricidal responses. Targeted cancer cells can lower the regulation of tumor‐assisted antigens to avoid the detection by the immune system, such as altering the expression levels of antigen processing and presentation associated components or expression levels of MHC I. The other way of regulating or stimulating the immune response is with the cytotoxic T‐lymphocyte‐associated antigen (CTLA‐4) or programmed death‐1 (PD‐L1/PD‐1) antigens, which acts as negative control over T‐cell immune functions. For the effective immune response, these immune check points can be blocked to achieve the better therapeutic results with immune check point blockades such as anti‐CTLA‐4 and anti PD‐L1/PD‐1.
Nanotechnology becomes an alternative method to improve the capability to deliver the therapeutic agents inside the tumor tissues and demonstrated excellent results in ImT to treat cancer over the past several decades. Nanotechnology stipulates opportunities to conquer restrictions on regular treatments and to propagate an efficient immune therapeutic response for malignant tumors. In addition, nanotechnology‐based nanoparticles can shield their consignment during circulation through blood and other fluidics inside the body for an extended period and transport it successfully to the target sites, nanoparticles can also travel from numerous biological barriers and reach extracellular space within the tumor site. Nanocarriers can efficiently transmit antigens to the immune cells with no noticeable degradation by endogenous enzymes, hence induce an antitumor immune response and regulates an immunosuppressive tumor microenvironment for effective cancer immunotherapeutic response [82–84]. Cancer ImT has its limitations along with the advantages, such as the stimulation of severe therapeutic side effects as a result of usage of multiple doses to attain sufficient and effective concentration in the tumor site, expensive, and extended processing are considered limitations of immunotherapeutic techniques. Hence, it is considered to innovate methods that have concentrated on technologies to conquer present hurdles and improve the efficacy and safety of ImT [85–87].
It is noteworthy that PTT‐hyperthermia promotes the necrosis and apoptosis of cancer cells. Further, tumor antigens were produced from apoptotic and necrotic cancer cells, which leads to initiation of APCs, such as DCs and circulate T cells, to suppress or completely eradicate metastatic and residual tumors [88]. The abnormal expression of programmed cell death ligand 1 (PD‐L1) within the cancer cells is able to interact with the programmed cell death protein 1 (PD‐1), leading to T cell consumption. Hence, PTT in combination with PD‐1 or CTLA‐4 antibodies as the checkpoint blockade could rejuvenate the exhausted T cells, thus exceptionally enhancing the immune response results in the effective antitumor effect. Recently, the various 2D nanomaterials for PTT therapy combination with immune checkpoint blockade have been studied. For example, BP quantum dot, i.e. BPQDs, fabricated to produce necrosis and apoptosis of tumor triggered with NIR light irradiation to activate the immune antigens to effectively eradicate the residual and metastatic tumor cells (Figure 5.5) [89]. The BPQDs modified with erythrocyte membranes (RMs) enhance the circulation period and tumor‐targeting efficiency. The PTT‐hyperthermia can effectively produce the tumor antigens via apoptosis and necrosis and train DCs to get the better antitumor immune response. Moreover, BPQD‐RMNV (red blood cell membrane nanovesicles) with PD‐1, combined with PTT, effectively prevent the exhaustion of T‐cells and significantly control the residual and metastatic tumor cells. Further, introduction of indoleamine‐2,3‐dioxygenase inhibition (IDOi) into ImT attracted better antitumor responses. RGO nanosheets loaded with IDOi, with multifunctional physicochemical properties, destroy cancer tumors under shining with 808 nm light and triggers in situ immune response for the synergistic PTT and ImT against tumors [90]. Furthermore, in vivo tumor model demonstrated enhanced immune response with the detection of improved tumor‐infiltrating lymphocytes, such asCD8+ T cells, CD45+ leukocytes, CD4+ T cells, and natural killer cells; the release of INF‐γ; and the control of the immune suppressive activity of regulatory T cells. Moreover, the synergistic therapy with PD‐L1 blockade, PTT, and IDOi based on rGO could effectively damage the growth of primary tumors and also the distant tumors with no PTT therapy. Recently, GO is loaded with the oligonucleotide for the stimulation of immune response with the combination of PTT therapy of cancer tumors [91]. Cytosine phosphate guanine (CpG) has been widely used to boost the immune system because most of the mammalians immune system easily recognize CpG through the toll‐like receptor‐9 (TLR9). The polymer‐modified GO introduced as a highly loaded CpG (GO‐PEG‐PEI‐CpG) nanotransporter efficiently into targeted tumor regions without undergoing intracellular degradation. Interestingly, PTT‐hyperthermia leads to significant improvement in the immune stimulation activity of CpG. Overall, GO‐PEG‐PEI‐CpG induces synergistic immunological and photothermal effects under photo irradiation and results in the better efficiency as compared with monotherapy alone. In the same way, MoS2 NSs showed multifunctional properties such as synergistic photothermal and ImT for cancer treatment [92]. In conclusion, 2D nanomaterials mediated PTT in combination with immune checkpoint blockade therapy or immune stimulatory oligonucleotides, leading to the significant enhancement in the immune effects at the tumor regions, which could result in the synergistic antitumor effect and lead to the significant improvement of the ImT efficiency.
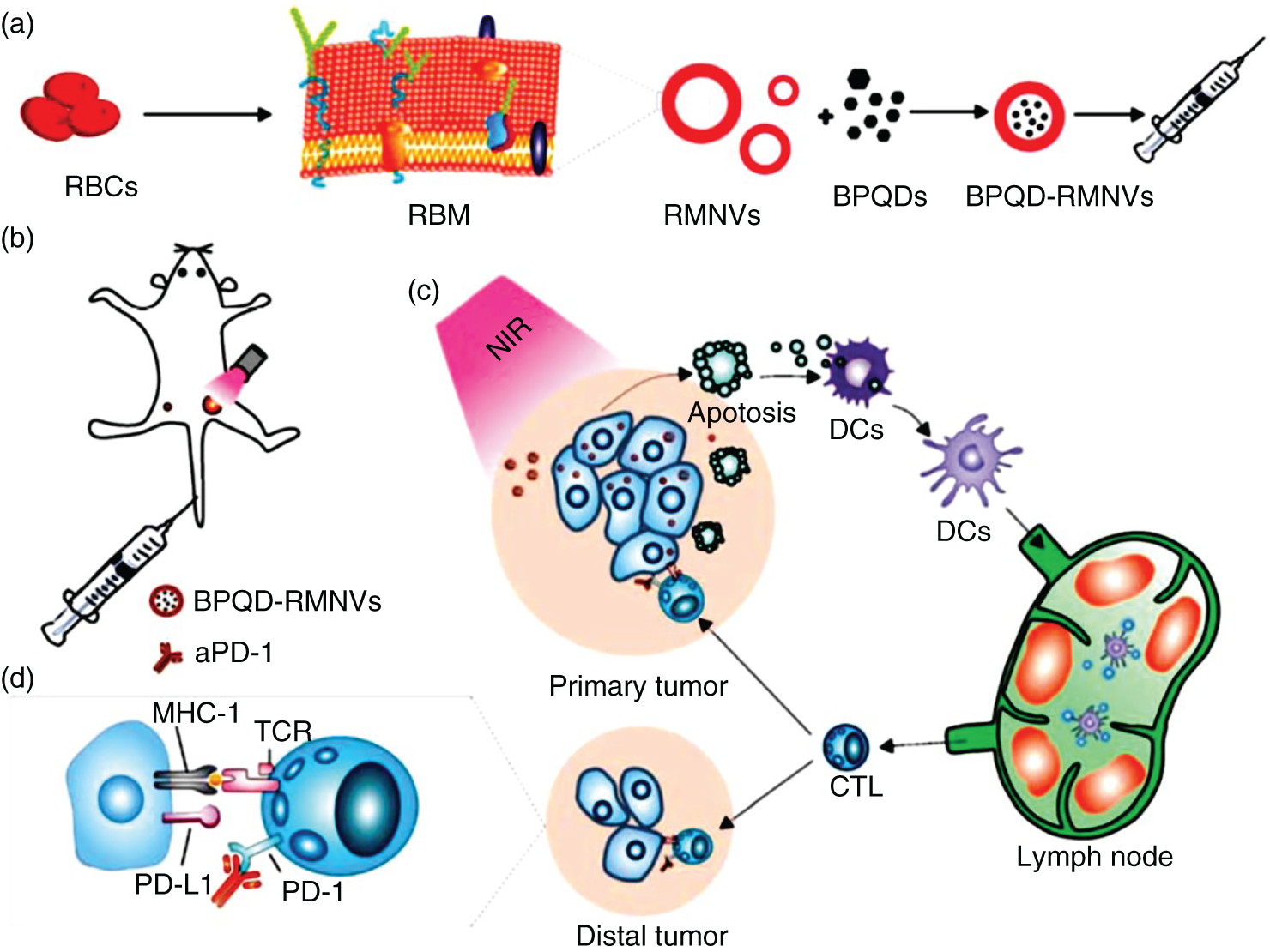
Figure 5.5 Schematic representation of combination therapy of PTT‐ImT mediated by BPQD‐RMNVs and aPD‐1. (a) Extrusion synthesis of BPQD‐RMNVs (b) aPD‐1 and PTT treatment of mice tumor model with BPQD‐RMNVs. (c) Tumor antigen release and in situ generation of tumor cell apoptosis. (d) Role of aPD‐1 for protecting tumor infiltrating CD8+ T cells.
Source: Liang et al. [89]. Reprinted with permission from Elsevier.
5.3 Summary and Future Perspectives
Nanotechnology becomes more popular from some decades especially in the synthesis of nanomedicine such as 2D nanomaterials attracting the scientific world due to their unique properties such as excellent chemical, mechanical, and optical properties, shape, tunable size, biocompatibility, and biodegradability. Conventional phototherapies using PSs failed due to the hydrophobic nature of PS and unable to treat the deep‐seated tumor models. More precisely, efficient 2D nanomaterials or nanocomposites can impart excellent prognosis and disease control with the NIR radiation absorption, which opens a new era of treatment of the deep‐seated tumor from NIR‐I to NIR‐II BW as compared with the other nanomaterials. The combination therapy of PTT for cancer treatment using 2D nanomaterials has a broad impact and important applications in the biomedical field. But most of the reported 2D nanomaterials are in the preclinical stage. To successfully move these 2D nanomaterials into clinical stage trials, it should overcome and studied in a detailed manner to translate into the clinical trials. The concerns are (i) the biosafety and long‐term cytotoxicity of the 2D nanomaterials to be addressed, (ii) clearance or excretion and biodegradation of 2D‐nanomaterials are the challenging tasks, and (iii) 2D‐nanomaterials synthesis scale up and quality of the product for clinical applications. However, nanoparticle formulations that can exactly target the tumors sites are not readily available for the treatment of cancer patients in clinical cancer therapy. Overall, 2D nanomaterials with all these issues resolved could be applied from preclinical studies to the future clinical studies.
References
- 1 Chabner, B.A. and Roberts, T.G. (2005). Nat. Rev. Cancer 5: 65–72.
- 2 Ediriwickrema, A. and Saltzman, W.M. (2015). ACS Biomater. Sci. Eng. 1: 64–78.
- 3 DeVita, V.T. and Chu, E. (2008). Cancer Res. 68: 8643–8653.
- 4 Montanari, M., Fabbri, F., Rondini, E. et al. (2012). Tumori J. 98: 696–701.
- 5 Stylianopoulos, T. and Jain, R.K. (2015). Nanomed. Nanotechnol. Biol. Med. 11: 1893–1907.
- 6 Wennstig, A.‐K. (2020). Comprehensive summary thesis. Doctoral thesis. Umeå Universitet (Umeå).
- 7 Lehmann, H.C., Staff, N.P., and Hoke, A. (2020). Exp. Neurol. 326: 113140.
- 8 Brigger, I., Dubernet, C., and Couvreur, P. (2012). Adv. Drug Delivery Rev. 64: 24–36.
- 9 Cavaletti, G., Cornblath, D.R., Merkies, I.S.J. et al. (2019). J. Peripheral Nerv. Syst. 24: 111–119.
- 10 Faucon, A.‐L., Bobrie, G., and Clément, O. (2019). Eur. J. Radiol. 116: 231–241.
- 11 Schultz, T.E. and Lynch, A.C. (2019). J. Oncol. Pharm. Pract. 25: 993–997.
- 12 Huang, J., Lyu, Y., Li, J. et al. (2019). Angew. Chem. Int. Ed. 58: 17796–17804.
- 13 Zhang, L., Liu, Z., Liu, Y. et al. (2020). Biomaterials 230: 119655.
- 14 He, W., Wang, S., Yan, J. et al. (2019). Adv. Funct. Mater. 29: 1807736.
- 15 Thakor, A.S. and Gambhir, S.S. (2013). CA: Cancer J. Clin. 63: 395–418.
- 16 Vankayala, R. and Hwang, K.C. (2018). Adv. Mater. 30: e1706320.
- 17 Hong, G., Antaris, A.L., and Dai, H. (2017). Nat. Biomed. Eng. 1: 0010.
- 18 Kuthala, N., Vankayala, R., Chiang, C.‐S., and Hwang, K.C. (2020). Adv. Funct. Mater. 30: 2002940.
- 19 Chen, Y., Fan, Z., Zhang, Z. et al. (2018). Chem. Rev. 118: 6409–6455.
- 20 Tan, C., Cao, X., Wu, X.‐J. et al. (2017). Chem. Rev. 117: 6225–6331.
- 21 Ji, D.‐K., Ménard‐Moyon, C., and Bianco, A. (2019). Adv. Drug Delivery Rev. 138: 211–232.
- 22 Zhang, H., Chhowalla, M., and Liu, Z. (2018). Chem. Soc. Rev. 47: 3015–3017.
- 23 Zhang, H., Cheng, H.‐M., and Ye, P. (2018). Chem. Soc. Rev. 47: 6009–6012.
- 24 Chen, Y., Wu, Y., Sun, B. et al. (2017). Small 13: 1603446.
- 25 Chen, Y., Tan, C., Zhang, H., and Wang, L. (2015). Chem. Soc. Rev. 44: 2681–2701.
- 26 Gu, Z., Zhu, S., Yan, L. et al. (2019). Adv. Mater. 31: e1800662.
- 27 Liu, Y., Bhattarai, P., Dai, Z., and Chen, X. (2019). Chem. Soc. Rev. 48: 2053–2108.
- 28 Dolmans, D.E., Fukumura, D., and Jain, R.K. (2003). Nat. Rev. Cancer 3: 380–387.
- 29 Castano, A.P., Mroz, P., and Hamblin, M.R. (2006). Nat. Rev. Cancer 6: 535–545.
- 30 Chen, G., Roy, I., Yang, C., and Prasad, P.N. (2016). Chem. Rev. 116: 2826–2885.
- 31 Xiang, H., Lin, H., Yu, L., and Chen, Y. (2019). ACS Nano 13: 2223–2235.
- 32 Liu, Y., Jiang, Y., Zhang, M. et al. (2018). Acc. Chem. Res. 51: 2502–2511.
- 33 Li, X., Kwon, N., Guo, T. et al. (2018). Angew. Chem. Int. Ed. 57: 11522–11531.
- 34 Yi, X., Chen, L., Zhong, X. et al. (2016). Nano Res. 9: 3267–3278.
- 35 Zhu, W., Dong, Z., Fu, T. et al. (2016). Adv. Funct. Mater. 26: 5490–5498.
- 36 Tian, B., Wang, C., Zhang, S. et al. (2011). ACS Nano 5: 7000–7009.
- 37 Liu, T., Wang, C., Cui, W. et al. (2014). Nanoscale 6: 11219–11225.
- 38 Yang, X., Wang, D., Shi, Y. et al. (2018). ACS Appl. Mater. Interfaces 10: 12431–12440.
- 39 Peng, M.‐Y., Zheng, D.‐W., Wang, S.‐B. et al. (2017). ACS Appl. Mater. Interfaces 9: 13965–13975.
- 40 Wei, J., Li, J., Sun, D. et al. (2018). Adv. Funct. Mater. 28: 1706310.
- 41 Delaney, G., Jacob, S., Featherstone, C., and Barton, M. (2005). Cancer 104: 1129–1137.
- 42 Begg, A.C., Stewart, F.A., and Vens, C. (2011). Nat. Rev. Cancer 11: 239–253.
- 43 Jackson, S.P. and Bartek, J. (2009). Nature 461: 1071–1078.
- 44 Kuthala, N., Vankayala, R., Li, Y.‐N. et al. (2017). Adv. Mater. 29: 1700850.
- 45 Song, G., Cheng, L., Chao, Y. et al. (2017). Adv. Mater. 29: 1700996.
- 46 Cheng, L., Yuan, C., Shen, S. et al. (2015). ACS Nano 9: 11090–11101.
- 47 Cheng, L., Shen, S., Shi, S. et al. (2016). Adv. Funct. Mater. 26: 2185–2197.
- 48 Tang, W., Dong, Z., Zhang, R. et al. (2019). ACS Nano 13: 284–294.
- 49 Wang, S., Li, X., Chen, Y. et al. (2015). Adv. Mater. 27: 2775–2782.
- 50 Wang, J., Tan, X., Pang, X. et al. (2016). ACS Appl. Mater. Interfaces 8: 24331–24338.
- 51 Cheng, Y., Yang, F., Xiang, G. et al. (2019). Nano Lett. 19: 1179–1189.
- 52 Studniarek, M., Cherifi‐Hertel, S., Urbain, E. et al. (2017). Adv. Funct. Mater. 27: 1700259.
- 53 Huang, H., He, L., Zhou, W. et al. (2018). Biomaterials 171: 12–22.
- 54 Du, J., Gu, Z., Yan, L. et al. (2017). Adv. Mater. 29: 1701268.
- 55 Chen, L., Zhong, X., Yi, X. et al. (2015). Biomaterials 66: 21–28.
- 56 Chao, Y., Wang, G., Liang, C. et al. (2016). Small 12: 3967–3975.
- 57 Riesz, P., Berdahl, D., and Christman, C.L. (1985). Environ. Health Perspect. 64: 233–252.
- 58 Rosenthal, I., Sostaric, J.Z., and Riesz, P. (2004). Ultrason. Sonochem. 11: 349–363.
- 59 Umemura, S.‐i., Yumita, N., Nishigaki, R., and Umemura, K. (1990). Jpn. J. Cancer Res. 81: 962–966.
- 60 Umemura, S.‐i., Kawabata, K.‐i., and Sasaki, K. (1997). J. Acoust. Soc. Am. 101: 569–577.
- 61 Yumita, N., Nishigaki, R., Umemura, K., and Umemura, S.‐i. (1989). Jpn. J. Cancer Res. 80: 219–222.
- 62 Kessel, D., Jeffers, R., Fowlkes, J.B., and Cain, C. (1994). Int. J. Radiat. Biol. 66: 221–228.
- 63 Xu, Z.‐Y., Li, X.‐Q., Chen, S. et al. (2012). Technol. Cancer Res. Treat. 11: 615–623.
- 64 Xu, Z.‐Y., Wang, K., Li, X.‐Q. et al. (2013). Ultrasonics 53: 232–238.
- 65 Yumita, N. and Umemura, S. (2003). Cancer Chemother. Pharmacol. 51: 174–178.
- 66 Umemura, S.‐i., Yumita, N., Umemura, K., and Nishigaki, R. (1999). Cancer Chemother. Pharmacol. 43: 389–393.
- 67 Sugita, N., Kawabata, K.‐i., Sasaki, K. et al. (2007). Bioconjugate Chem. 18: 866–873.
- 68 Qian, X., Zheng, Y., and Chen, Y. (2016). Adv. Mater. 28: 8097–8129.
- 69 Paris, J.L., Mannaris, C., Cabañas, M.V. et al. (2018). Chem. Eng. J. 340: 2–8.
- 70 Wang, Y., Liu, J., Ma, X., and Liang, X.‐J. (2018). Nano Res. 11: 2932–2950.
- 71 Lin, X., Qiu, Y., Song, L. et al. (2019). Nanoscale Horiz. 4: 747–756.
- 72 Di, J., Yu, J., Wang, Q. et al. (2017). Nano Res. 10: 1393–1402.
- 73 Gong, F., Cheng, L., Yang, N. et al. (2019). Adv. Mater. 31: e1900730.
- 74 Han, X., Huang, J., Jing, X. et al. (2018). ACS Nano 12: 4545–4555.
- 75 He, Y., Wan, J., Yang, Y. et al. (2019). Adv. Healthcare Mater. 8: e1801254.
- 76 Dai, C., Zhang, S., Liu, Z. et al. (2017). ACS Nano 11: 9467–9480.
- 77 Gao, F., He, G., Yin, H. et al. (2019). Nanoscale 11: 2374–2384.
- 78 Surendran, S.P., Moon, M.J., Park, R., and Jeong, Y.Y. (2018). Int. J. Mol. Sci. 19.
- 79 Shao, K., Singha, S., Clemente‐Casares, X. et al. (2015). ACS Nano 9: 16–30.
- 80 Jo, S.D., Nam, G.‐H., Kwak, G. et al. (2017). Nano Today 17: 23–37.
- 81 Sau, S., Alsaab, H.O., Bhise, K. et al. (2018). J. Controlled Release 274: 24–34.
- 82 Grimaldi, A.M., Incoronato, M., Salvatore, M., and Soricelli, A. (2017). Nanomedicine 12: 2349–2365.
- 83 Park, Y.‐M., Lee, S.J., Kim, Y.S. et al. (2013). Immune Netw. 13: 177–183.
- 84 Yoon, H.Y., Selvan, S.T., Yang, Y. et al. (2018). Biomaterials 178: 597–607.
- 85 Saeed, M., Gao, J., Shi, Y. et al. (2019). Theranostics 9: 7981–8000.
- 86 Velpurisiva, P., Gad, A., Piel, B. et al. (2017). J. Biomed. 2: 64–77.
- 87 Gao, S., Yang, D., Fang, Y. et al. (2019). Theranostics 9: 126–151.
- 88 Fan, Q., Chen, Z., Wang, C., and Liu, Z. (2018). Adv. Funct. Mater. 28: 1802540.
- 89 Liang, X., Ye, X., Wang, C. et al. (2019). J. Controlled Release 296: 150–161.
- 90 Yan, M., Liu, Y., Zhu, X. et al. (2019). ACS Appl. Mater. Interfaces 11: 1876–1885.
- 91 Tao, Y., Ju, E., Ren, J., and Qu, X. (2014). Biomaterials 35: 9963–9971.
- 92 Han, Q., Wang, X., Jia, X. et al. (2017). Nanoscale 9: 5927–5934.
