22
2D Nanomaterials for Photocatalysis and Photoelectrocatalysis
Gubbala V. Ramesh1,, N. Mahendar Reddy1, Muvva D. Prasad2, D. Saritha1, and Kola Ramesh1
1Chaitanya Bharathi Institute of Technology, Department of Chemistry, Gandipet, Hyderabad, Telangana, 500075, India
2University of Hyderabad, UGC Networking Centre, School of Chemistry, Hyderabad, Telangana, 500046, India
22.1 Introduction
Two‐dimensional (2D) nanomaterials extracted enormous interest since graphene was first published in 2004 [1]. Thickness of the 2D nanomaterials is in the order of nanometers, i.e. single‐ or few atom thickness and the properties can be tuned finely by changing the thickness of the materials [2, 3]. These 2D nanomaterials were demonstrated exceptional properties in the fields of science and engineering such as physical, mechanical, electronic, and chemical properties that were different from their bulk counterparts [4]. Therefore, the potential uses of 2D nanomaterials are explored in a wide spectrum of applications such as electronics/optoelectronics, photocatalysis, photoelectrocatalysis, energy storage, and electrochemistry [5–8]. Researchers/scientists from all over the world inspired to investigate other 2D nanomaterials such as metal dichalcogenides (WS2, TaS2, MoS2, etc.), layered metal oxides (LDH), metal oxyhalides (MOX), hexagonal boron nitride (h‐BN), graphitic carbon nitride (g‐C3N4), Mxenes, metal–organic frameworks, polymers, and black phosphorus [4].
Over the past few years, considerable efforts have been made to explore and develop the reliable methods for the synthesis of 2D nanomaterials. Broadly, these routes can be classified into two ways such as chemical and physical methods for, e.g. wet‐chemical synthesis methods, [9, 10] chemical vapor deposition (CVD), [11, 12] chemical and electrochemical ion‐intercalation and exfoliation routes, [13, 14] liquid phase exfoliation in solution phase, [15, 16] and laser thinning technique [17, 18]. The exceptional properties of 2D nanomaterials come from the capability of the captivity of electrons in their interlayers and also the covalent bond between interplanes [19]. 2D nanoarchitecture possesses a high surface area that favors high catalytic activity.
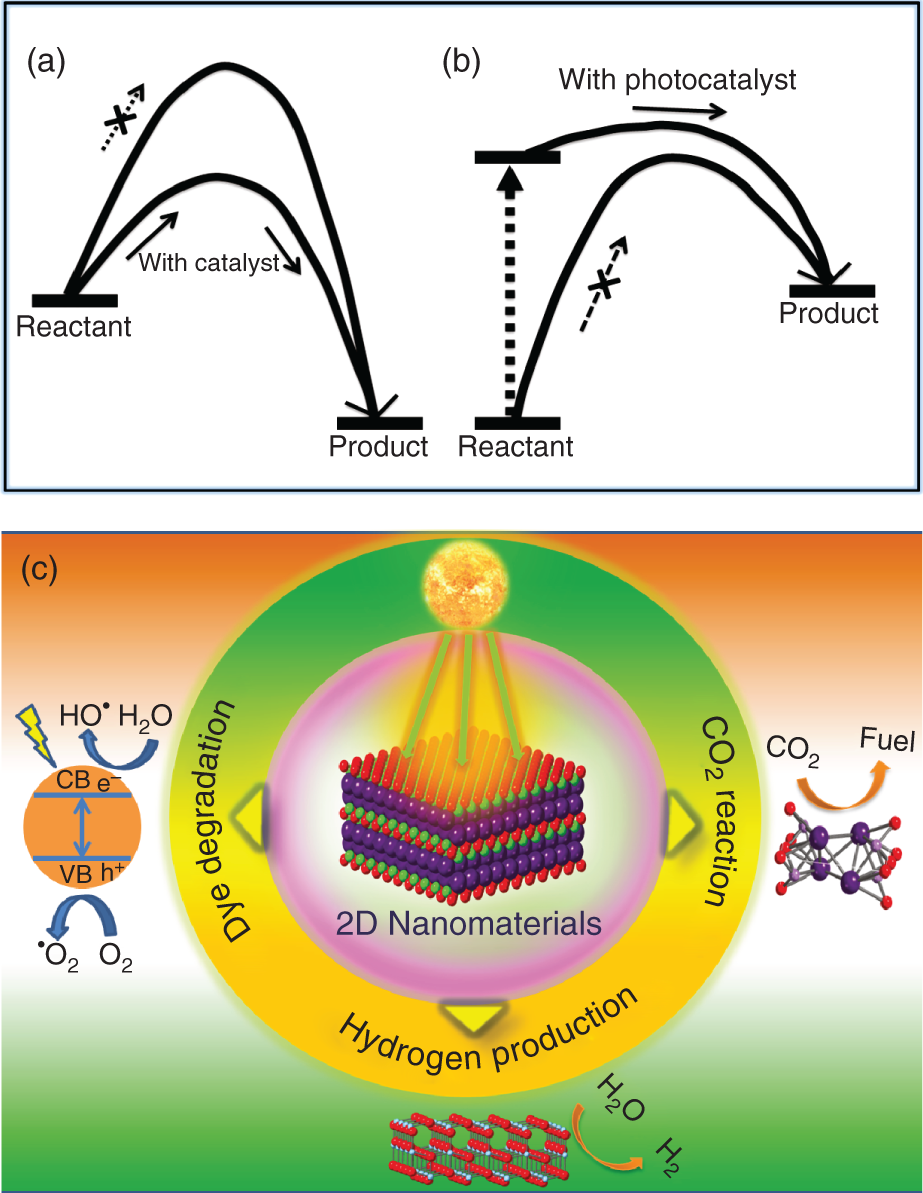
Figure 22.1 Catalytic reaction path in so called (a) downhill, (b) uphill reactions. (c) Schematic representation of different aspects of 2D nanomaterials.
The researchers put enormous effects to understand and develop the phenomena and practical applications in the two firmly related areas of photocatalysis [20, 21] and photoelectrochemistry [22–24] during the last few decades. Photocatalysis process is one of the most important phenomena to address global energy and environmental issues. Catalyst lowers the activation energies to advance the “downhill” chemical reactions, whereas it cannot promote “uphill” chemical reactions (Figure 22.1) [25]. These reactions can be triggered by giving external energy in the form of heat or light (i.e. photon energy). 2D nanomaterials are considered as the most prominent photocatalysts because of its capability of absorbing light and charge separation. On the other hand, the photoelectrochemical process occurs at semiconductor–electrolyte interfaces. When the semiconductor absorbs, it generates electron–hole pair at the interface between the electrolyte solution and semiconductor material, which leads to chemical reactions such as reduction and oxidation reactions. Various advanced catalysts based on 2D nanomaterials for photocatalysis and photoelectrocatalysis will be discussed in this chapter (Figure 22.1). The main topics discussed are photocatalytic/photoelectrochemical CO2 reduction, photocatalytic/photoelectrochemical hydrogen production, photocatalytic dye degradation, and also brief fundamental aspects of this research field.
22.2 Photocatalytic CO2 Reduction
Carbon dioxide (CO2) is one of the main gases responsible for the “green house effect.” Industrial Revolution and heavy consumption of fossil fuels have resulted in an enormous increase in the levels of CO2 gas in the atmosphere, which causes global warming and energy crisis [26]. The conversion of CO2 to valuable fuels and/or chemicals is the best way to address both the issues mentioned above. Using renewable energy sources like solar energy for catalytic conversion of CO2 is a favorable method. Several methods are used for this reaction such as photocatalytic, photoelectrochemical, photothermal, etc. Among them, the photocatalytic reduction has fascinated more [27, 28]. Many researchers have been working to develop novel and efficient catalysts for photocatalytic CO2 reduction. In particular, 2D nanomaterials have been attracted more and broadly used because of its high catalytic activity and stability.
When the light falls on a photocatalyst with energy greater than or equal to the bandgap energy, the electrons jump from valency band to conductions band, leaving holes in the VB. The photoexcited electrons in the conduction band (CB) react with H+ and CO2 molecules produce valuable hydrocarbon fuels and H2, whereas at valence band (VB), photooxidation occurs, and produces O2 from water molecules (Figure 22.2) [30, 31]. Since CO2 is thermodynamically stable and chemically inert molecule, the reduction process required high energy (highly endothermic). So finding and applying appropriate catalysts is the key challenge in the photocatalytic reduction of the CO2.
Graphene is a 2D nanomaterial with zero bandgap semiconductor having unique properties like high surface area, conductivity, and good flexibility [1, 32]. Liang et al. [33] reported novel graphene and titanium dioxide nanocomposite material for photoreduction of CO2, which shows a sevenfold enhancement as compared to TiO2 under visible light conditions. This finding triggered the research of graphene‐based composite materials for CO2 photoreduction. There are some interesting benefits arises as a result of coupling graphene, for example, stifling photogenerated transporter recombination, expanding the huge surface area, improving CO2 adsorption, upgrading photostability, uniform distribution of nanoparticles, and improving light absorption [34, 35]. Ong et al. synthesized graphene nanocomposite of nitrogen‐doped anatase TiO2 with 35% of {001} exposed facets (N–TiO2‐001/GR). It showed greatest activity of CO2 reduction to CH4 with the yield of 3.70 μmol/gcatalyst, which is 11‐fold higher than the TiO2 with {001} facet. The enhanced activity attributed to the presence of graphene which suppresses “electron–hole” recombination [36]. P‐25 TiO2 nanoparticles loaded smaller sized boron‐doped graphene nanosheets were first time reported by Mingyang Xing and coworkers for efficient CO2 photoreduction [29]. The reason behind this was the formation of TiOC bonds, which leads to the exceptional electron transporting property. The potential of boron‐doped graphene nanosheets (B‐GR) is transferring the holes from graphene to TiO2 and electrons from TiO2 to graphene (Figure 22.2). Tu fabricated molecular‐scale alternating titania (Ti0.91O2) nanosheets and graphene nanosheets for photocatalytic CO2 reduction into fuels (CO and CH4), which showed nine times improved activity. The activity due to extremely thin nature of TiO2 facilitates the moving of charge carries to surface of the catalyst and the life time of the charge carries increased because of the compact stacking between graphene and Ti0.91O2 [37].
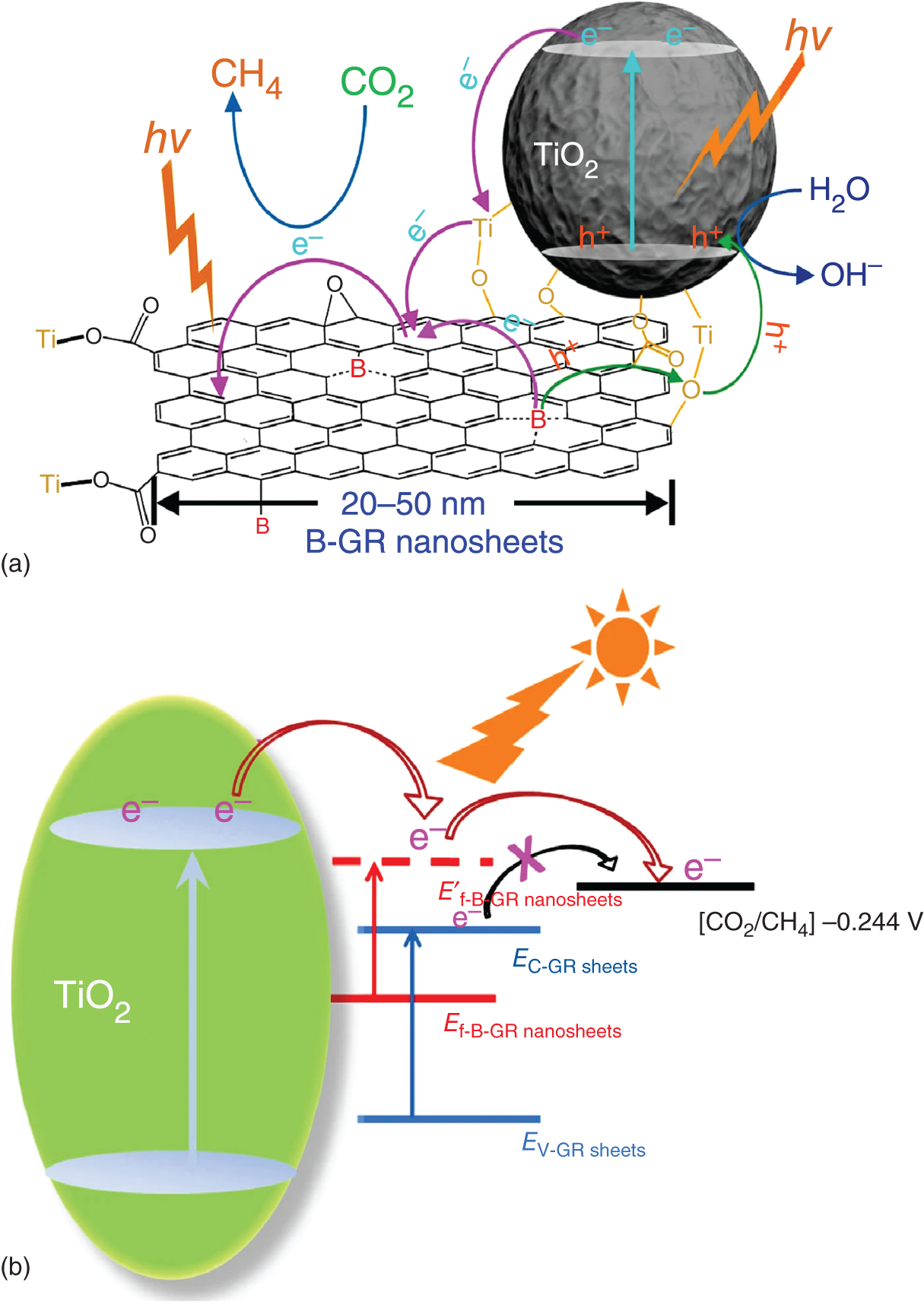
Figure 22.2 (a) Structural model of energy states. Schematic diagram of photo‐excited electrons and holes transfer among TiO2 nanoparticles and B‐GR nanosheets. (b) Schematic diagram of photo‐generated electrons transfer between TiO2 and graphene materials.
Source: Xing et al. [29].
Graphene oxide (GO) is a wide bandgap semiconductor which is a solution dispersible polyaromatic 2D carbon sheet. It can be synthesized from graphite by the modified Hummer's method [38]. Hsu et al. synthesized different GOs under various conditions and showed GO is an efficient low cost photocatalyst for CO2 reduction to methanol with a conversion rate of 0.172 mmol/(g cat h) on graphene oxide which is six times higher than the TiO2 under visible light. Some of the graphene‐based composite materials with metal oxides like zinc oxide (ZnO), [39, 40] copper oxide (Cu2O), [41, 42] bismuth vanedium oxide, [43] and tantalum oxide [44] have reported as a promising candidates for this reaction. Other than metal oxides, metal sulfide nanocomposites are also reported. Yu et al. successfully demonstrated reduced graphene oxide (rGO) – cadium sulfide (CdS) nanorod composite for the production of CH4 from photocatalytic reduction of CO2. They prepared rGO‐CdS nanocomposite via a single step microwave‐assisted hydrothermal process which showed 10 times more active than CdS nanorods. rGO acted as an efficient cocatalyst by adsorption and desorption of CO2 molecules and also pushed the electrons to the surface of the catalyst which enhances the catalytic activity of CO2 photoreduction to CH4 [45]. Over the past few years, a range of composite nanomaterials based on graphene has emerged such as metal complexes, [46, 47] perovskite quantum dots, [48] and bio‐enzyme [47, 49].
Graphitic carbon nitride (g‐C3N4) is another metal‐free 2D‐conjugated photocatalytic polymer material that has attracted much attention in recent years [50]. g‐C3N4 is a yellow colored semiconductor having a bandgap of 2.7–2.8 eV, and a suitable negative conduction band edge favors photocatalytic CO2 reduction to hydrocarbons [31]. After Wang's report on hydrogen generation under visible light, enormous research triggered to explore the potentiality of g‐C3N4. In spite of that, photocatalytic efficiency of g‐C3N4 is still low because of low visible light absorption (greater than 460 nm), low surface area, and high rate of photoexcited electron–hole recombination [51]. To conquer the above issues often g‐C3N4 is used as a combination with other materials. First Dong et al. reported successful conversion of CO2 to carbon monoxide (CO) in the presence of water vapor under visible light (λ > 420 nm) without any cocatalyst [52]. Photocatalytic activity and selectivity of the products depended on the precursor material used to synthesize g‐C3N4 [53]. The product (u‐g‐C3N4) obtained from the urea had a thinner mesoporous flake‐like structure with a large surface area, while the product (m‐g‐C3N4) derived from melamine had a nonporous bulk structure. u‐g‐C3N4 showed more photocatalytic CO2 reduction activity because of the high surface area and thinner in nature and gave a mixture of products containing methanol (CH3OH) and ethanol (C2H5OH), whereas m‐g‐C3N4 produced only C2H5OH. Selectivity of the products further can tune by varying band structure. g‐C3N4 with a bandgap of 2.77 eV produced acetaldehyde (CH3CHO), whereas CH4 was the main product when the bandgap was 2.97 eV [54]. Wang and coworkers first time used 200–420 nm UV light for CO2 photoreduction, surprisingly thiourea derived g‐C3N4 (TCN) showed less activity under visible light, but under UV light it performed better than urea derived g‐C3N4 and commercial TiO2 (P25) [55]. Nanocomposites of g‐C3N4 with metals, metal oxides, and metal complexes have been extensively studied. g‐C3N4–Pt nanocomposite were prepared by Yu et al. and showed Pt was an efficient cocatalyst to enhance the activity and selectivity of g‐C3N4 [56]. Several metal oxide/sulfide composites like g‐C3N4/ZnO, [57] gC3N4/WO3, [58], and g‐C3N4/CdS, [59] were reported as an efficient photocatalysts for CO2 reduction.
Pristine 2D metal oxide nanomaterials also have been widely investigated as photocatalysts for CO2 photoreduction. These oxide materials are more favorable because of nontoxic in nature, low cost, and extraordinary stability. Xu et al. synthesized single crystalline ultrathin {100} facet exposed anatase TiO2 nanosheets (2 nm thickness), which exhibited five times superior activity in both CO2 reduction and H2 evolution than the TiO2 cuboids [60]. Another, single crystalline nanosheets of WO3 with 4–5 nm in thickness were reported by Chen et al. for CO2 photoreduction to hydrocarbon fuels in the presence of water. The ultrathin nature of nanosheets facilitates the fast transfer of electron–hole pair onto the surface of the catalyst [61]. Various 2D metal chalcogenide, MOX, double‐layered hydroxides‐based photocatalysts are extensively studied for the CO2 photoreduction. 2D hybrid nanojunction of MoS2 and TiO2 nanosheets was reported by W. Tu et al. In situ growth of MoS2 nanosheets on TiO2 facilitated high interfacial area which resulted in enhanced high catalytic activity and selectivity of CO2 reduction to methanol under UV–visible light in aqueous solution [62]. Dai et al. investigated the catalytic activity of MoS2/Bi2WO6 hierarchical flower‐like composite material which was synthesized by impregnation followed by calcination method. Few layered MoS2 as a cocatalyst increased the absorption and charge separation leads to the enhanced CO2 photoreduction [63]. In the past few decades, bismuth oxyhalides, BiOX (X = Cl, Br, and I), gained much attention because of its unique layered structure, optical, and electronic properties. Ye et al. synthesized olive‐green colored layered BiOI with expanded spacing of the {001} facets which showed enhanced activity for CO2 reduction under visible/NIR light as compared with bulk BiOI [64].
22.3 Photoelectrocatalytic CO2 Reduction
Photoelectrocatalysis is a fascinating and efficient technique that elegantly amalgamates the photocatalysis and electrocatalysis to overwhelm the short comings of the both. The technique is based on the usage of semiconductor electrodes rather than normal conducting electrodes like in electrocatalysis, which are illuminated by light of equal or greater energy than the bandgap energy (hυ ≥ Eg) at the same time biased by a gradient potential. The photoelectrocatalysis offers higher efficiency than the photocatalysis due to the applied external bias voltage and can effectively separate the photogenerated electrons and holes, which are the decisive step in lowering the efficiency of photocatalysis [65].
According to the established protocols, it is known that the semiconductors possess two energy bands of electrons. These energy bands are known as valency and conduction bands, respectively. The two energy bands are separated an energy called as bandgap energy (Ebg). When these semiconductors are exposed to light with energy equal or more than the bandgap energy generates the electron/hole pair (e−/h+) by the excitation of an electron from the valence band (VB) to conduction band (CB). However, so formed electron/hole pair may recombine quickly and finds no use in chemical transformations or reactions. The photoelectrocatalysis shows an excellent solution to this problem and elegantly uses the so generated electron/hole pair in triggering the chemical reactions. Photoelectrocatalysis offers a solution to the said problem by separating the photogenerated e−/h+ pairs using the bias potential [66, 67].
A typical photoelectrochemical (PEC) system consists of two electrodes, and each of two compartments (two half cells) is separated by ion exchange membranes. Semiconductors may be used as either photocathode or photoanode. Semiconductors may also be used as both electrodes. The semiconductor may be connected to other conductive material and used as photoelectrode, the chemical reactions may occur either on the surface of semiconductor or photoelectrode. When the n‐type of semiconductors are attached to a photoelectrode the charges (i.e. electron/hole pair) will be generated by irradiating with light of proper energy. The hole (h+) formed is responsible for the oxidation of substrate. The electrons will be taken up by photoelectrode and transferred to the counter electrode through the outer circuit and consumed in the reduction of substrate on the surface of counter electrode. When p‐type semiconductors are used, then the above process will be reversed. Hence, this is an attractive, economical and environmentally clean technique finds applications in many fields such as CO2 reduction, water splitting, wastewater treatment, and fuel cell technology [68].
At present, the reduction of CO2 to appropriate carbonaceous materials is of paramount important endeavor for scientific spirit around the globe. Because this can deal with the essential problems connected with global warming and harvest suitable fuels for energy demands of world. Many methods were developed and applied for this purpose, among these photoelectrocatalysis stand‐out to be the best technique. Reduction of CO2 poses great challenge because the CO2 in stable molecule and its reduction is a thermodynamically unfavorable process.
The CO2 conversion takes place using photoelectrocatalysis. In this process, the reactions are driven by applied external potential and light irradiation. Both the oxidation of H2O and reduction CO2 occurs at anode and cathode, respectively. In terms of energy considerations, for successful reduction of CO2, the energy of photogenerated electrons (e−) and holes (h+) should be above the overpotential of H2O/O2 (0.82 V vs. NHE at pH = 7) and below the overpotential of CO2/HCOOH (−0.61 V vs. NHE at pH = 7 or CO2/CH3COOH/CH3OH), respectively [69, 70]. The semiconductors boasting band‐gap energy of at least 2.88 eV are key to accomplish both H2O oxidation and CO2 reduction. The product yield of CO2 conversion is governed by the choice of electrode materials and electrolytes [71].
Cheng et al. reported the PEC conversion CO2 into high‐value chemicals such as C2H5OH, HCOOH, and CH3COOH using Pt‐modified reduced graphene oxide () as cathode and Pt‐modified TiO2 nanotubes (Pt‐TNT) and photoanode (Figure 22.3). The achieved atomic carbon conversion rate is 1130 nmol/h cm2, and combined liquid product is 600 nmol/h cm2. Comparative study shows that the Pt‐rGO is better catalysts than that of Pt‐modified carbon nanotubes and platinum carbon as cathode catalysts [73].

Figure 22.3 Schematic diagram of the photoelectrochemical (PEC) system. Figure 22.7. Chemical generation rates and current efficiency under varying cathodes in a photoelectrocatalytic reactor with Pt‐TiO2 nanotubes photoanode: (a) chemical generation rates and (b) current efficiency.
Source: Cheng et al. [73].
Hasan et al. reported the synthesis of composites by doping the gallium (Ga) on reduced graphene oxide–titania (rGO–TiO2) by a sol–gel method and deposited on an ITO‐coated glass substrate using an electrophoretic deposition method. The recombination of electron–hole can be reduced significantly on the catalyst surface by using rGO with TiO2 while Ga doping aids in lowering the bandgap energy. It is observed that of the absorption range extended toward the visible region and CO2 adsorption increased on the catalyst surface due to both rGO and Ga resulting a high CO2 conversion. The products were largely formic acid and trace amounts of methanol. Maximum observed yield for a two‐hours reaction period was 178 ppm of formic acid. The Ga–rGO–TiO2 composite films are giving far better yields compared to the rGO–TiO2 composite and pure TiO2 film [74].
The same group reported the synthesis of crystalline Cu‐rGO–TiO2 nanoparticles for PEC reduction CO2. Light absorption was in the visible region and with bandgap of 2.98 eV. At 0.61 V bias potential under solar simulator irradiation during CO2 photoelectrocatalysis, a maximum 1.31 mA cm2 photocurrent density was observed. The reaction yielded formic acid (HCOOH) and methanol (CH3OH) as main products at rates of 255 and 189.06 μmol/h cm2, respectively. Initially, HCOOH was main product (maximum concentration of 157 ppm), but later it turned out to be an intermediate for the formation of methanol and higher hydrocarbons. It is observed that the Cu doping on the rGO–TiO2 is not only causing the product selectivity but also promoting formation of larger amounts of CH3OH (maximum 242 ppm) when the reaction time is more than three hours [75]. Jing et al. reported the preparation of Ti3C2/g‐C3N4 (TCCN) heterojunctions by overlaying the Ti3C2 sheet with g‐C3N4 and applied to PEC CO2 reduction. The system is showing the bandgap of 2.3 to 2.6 eV and the absorption of solar light and separation of electrons (e−) and holes (h+) increased due to the presence of Ti3+ species. Further, the heterojunctions of Ti3C2/g‐C3N4 were modified by various metals (i.e. Pd, Pt, and Au) and used as the photocathode in the M‐TCCN||BiVO4 cell for the reduction of CO2. The reduction products are formate (HCOO−) and methanol (CH3COOH), and the rate of production is 25.1 mM/h g [76].
Jing et al. reported an artificial photosynthetic process for the reduction of CO2 using PEC Pd/N‐TiO2/Ti3C2||BiVO4 system. First, a layered hybrid heterojunction of TiO2/Ti3C2 prepared using hydrothermal process and functionalized it with imine ligands and Pd nanoparticles. This PEC is evaluated for CO2 reduction to yield products such as formate, methanol, and ethanol and observed total evolution of hydrocarbons as 36.8 mM/h g [77]. Yin et al. reported the preparation and application of Cobalt‐doped MOS2 nanoparticles for PEC reduction of CO2. The Co‐doped MoS2 nanoparticles showing the valence band at 0.89 V and conduction band at −0.52 V, imparting the high catalytic efficiency. The chief product of the reduction is methanol and yields reaching 35 mmol/ l in 350 minutes [78].
22.4 Photocatalytic Hydrogen Production
Sudden technology evolution and population increase made a critical situation for the human to search for more sustainable/renewable energy sources. This not only reduces air pollution but also the greenhouse effect [79]. Among all types of renewable energy sources, hydrogen is considered to be important because it has high energy content per weight when compared with other fossil fuels and water is the only by‐product. Hydrogen as a fuel is an environmentally friendly, an ultraclean and promising alternative for addressing future human energy needs [80]. At present, hydrogen production from renewable energy resources has generally been seen as a viable approach that is efficient and environmentally safe. Production of hydrogen from water and sunlight will be one of the most desirable methods since both the water and sunlight is naturally abundant. Photocatalytic (PC) or PEC Water splitting is an excellent way to produce hydrogen with minimum pollution and good renewability [81]. Finding suitable, highly efficient and stable photo(electro)catalyst is the key challenge in PC and PEC processes, to get a high yield of hydrogen.
In 1972, Fujishima and Honda demonstrated the landmark breakthrough in photocatalytic water splitting reaction by using TiO2 photoelectrodes, thereafter a broad spectrum of photocatalysts have been tested for water splitting reaction. Many semiconductor materials such as metal based oxides/chalcogenides and metal free photocatalysts have been explored for these reactions. However, these photocatalysts are still facing considerable challenges such as wide bandgap [82], improper band positions [83], and photogenerated recombination [84]. To overcome these hurdles one of the best solutions is to use 2D materials because (i) ultrathin nature suppresses charge recombination (ii) bandgap can be regulated by optimizing the number of layers [85].
Even though 2D materials are considered to be promising photocatalysts for the production of hydrogen from solar water splitting, these materials facing some limitations such as stability in air or aqueous solutions, recombination of electron–hole pairs, and unfavorable increase in exciton binding energy. Briefly, 2D photocatalyst materials for overall water splitting should satisfy some certain requirements such as conduction band potential should be lower (more negative) than water reduction, valence band potential should be greater (more positive) than water oxidation and bandgap should be greater than 1.23 eV [86, 87]. However, it is very hard to satisfy all the requirements by pristine 2D materials; therefore, researchers started explored composite 2D materials to improve the efficiency and stability of photocatalysts in PC and PEC reactions. The interface between composite materials will play an important role in the activity and stability. Among many, some of the 2D photo(electro)catalysts are discussed here such as graphene‐based, metal oxides/chalcogenides, g‐C3N4, etc.
Apart from many advantages like conductivity, flexibility, and surface area; graphene can also play several roles in photocatalytic reactions such as electron acceptor [88], transport bridge for electron [89], and photosensitizer [90]. Electronic properties and structure of graphene can be tuned by not only doping of boron and nitrogen atoms but also doping patterns, which enhanced the photocatalytic H2 evolution. Jia and coworkers demonstrated that N‐doped graphene and CdS nanocomposite used as photocatalytic material for hydrogen evolution under visible light irradiation. They showed N‐graphene could be used as a charge collector and also it reduces photo corrosion of CdS [91]. For the first time, Xiang et al. reported graphene‐modified TiO2 nanosheets (exposed with [001] facets) by a two‐step microwave‐assisted hydrothermal method for photocatalytic H2 production. Graphene acted as an efficient cocatalyst to improve the photocatalytic activity of TiO2 nanosheets. The composite catalyst produced H2 at a rate of 736 μmol/h g with 3.1% quantum efficiency without Pt cocatalyst in an aqueous solution containing methanol as a sacrificial agent, which was 41 times greater than when compared with pure TiO2 nanosheets. The photoluminescence and photocurrent studies suggested the potential of graphene/graphene.– favors electron transfer which enhanced the reduction of H+ [92]. Some other semiconductor materials decorated/covered on graphene nanosheets were reported like CdS cluster, [93] Bi2WO6 [94], SiC [95], and MoS2‐graphene/ZnIn2S4 [89] for highly efficient photocatalytic H2 production.
GO nanosheets have been also investigated for this reaction. In 2012, J. Hou reported, visible‐light‐driven photocatalyst consists of GO nanosheets decorated with CdS@TaON core–shell showed higher production H2 (Figure 22.4). The highest activity came from the presence of CdS nanocrystals which altered the energy levels and graphene oxide acted as an electron collector which lengthened the lifetime of charge carriers of composite catalyst [96].

Figure 22.4 (a) Schematic illustration for the charge transfer and separation in the graphene‐modified TiO2 nanosheets system under UV light irradiation; (b) proposed mechanism for photocatalytic H2‐production under UV light irradiation.
Source: Xiang et al. [92].
Different thicknesses of g‐C3N4 nanosheets were reported for hydrogen evolution. Free‐standing 2 nm thick g‐C3N4 nanosheets extracted from bulk g‐C3N4 powder via a low cost and simple liquid exfoliation method showed high photocatalytic activity under visible light due to the suitable bandgap. The structural characteristics of these ultra‐thin g‐C3N4 nanosheets were homogeneous dispersion of carbon and nitrogen atoms, large surface area, and high aspect ratio [97]. In 2016, Han and coworkers investigated H2 production (λ > 420 nm) of different thicknesses of g‐C3N4 nanomeshes and also compared their results with other nanosheets. Atomically, thin g‐C3N4 porous nanomesh was synthesized by solvothermal exfoliation of bulk mesoporous g‐C3N4 which has extraordinary structural benefits such as electron transfer, and aligned energy levels. These mesoporous nanomeshes showed high H2 production rate of 8510 μmol/h g under visible light (λ > 420 nm) with a quantum efficiency of 5.1% at 420 nm [98]. She et al. reported highly crystalline 0.64 nm thick monolayer of oxygenated g‐C3N4 material with a H2 production rate of ∼44.37 mmol/g (∼8874.7 μmol/h g) and external quantum efficiency (EQE) of 13.7% (at 420 nm) [99]. A composite photocatalyst consisting of graphene and g‐C3N4 (graphene/g‐C3N4) was reported by Xiang which was synthesized by combined impregnation and chemical reduction method. A series of graphene/g‐C3N4 (Pt‐cocatalyst) were made to see the effect of graphene content on the photocatalytic H2 production. They found that the optimal content of graphene was 1.0 wt% to obtain maximum H2 production of 451 μmol/h g in methanol aqueous solution [100].
Apart from the pristine g‐C3N4, several composite photocatalysts with metal oxides and sulfides have been reported for the production of hydrogen. The hybrid catalyst of anatase TiO2 and g‐C3N4 nanosheets was synthesized by hydrothermal reaction followed by an air annealing. The face‐to‐face interface facilitated the light absorption and separation of electron–hole pair, which gave H2 evolution rate of 18 200 μmol/g h [101]. Synthesis and photocatalytic activity of Z‐scheme α‐Fe2O3/2D g‐C3N4 hybrid photocatalyst was reported by She et al. The α‐Fe2O3 serves as a catalyst to facilitate the development of nanosheets g‐C3N4 and further participates in a structure of the Z‐scheme that catalyzes water oxidation or sacrificial reagents. The engineered α‐Fe2O3/2D g‐C3N4 showed remarkable H2 evolution of 31 400 μmol/g h and a surprisingly huge EQE of 44.35% at a wavelength of 420 nm [102]. Another hybride photocatalyst consists of perovskite‐type 7 nm thick N‐doped La2Ti2O7 nanosheets with 2 nm thick g‐C3N4 layered composites exhibited high H2 production via water splitting [103]. Further, Hou et al. demonstrated that layered nanojunctions between MoS2 and mesoporous g‐C3N4 (mpg‐CN) were a good photocatalytic materials for H2 production [104]. 2D/2D hetrojunctions of g‐C3N4 nanosheets with ZnIn2S4 and CaIn2S4 reported separately and these photocatalysts exhibited high H2 evolution under visible light [105, 106].
Ide and coworkers reported 2D TiO2 nanosheets doped with rhodium (Rh) atoms. Doping of single Rh atoms in TiO2 nanosheets was confirmed by TEM studies. This single Rh atom acted as a reaction centers for photocatalytic hydrogen production, and it is further confirmed by first principle modeling calculations. The photocatalytic activity was 10 times higher than that of undoped TiO2 nanosheets [107]. Another, 2 nm thick black 2D TiO2 nanosheets were reported for photocatalytic hydrogen evolution reaction by Yan et al. They showed black TiO2 is more active than white TiO2 [108]. In addition to metal oxides, some of the metal chalcogenides such as MoS2 and its 2D nanocomposite materials are also extensively studied for photocatalytic hydrogen production. Pristine 2D single‐layered MoS2 nanosheets exhibited enhanced H2 evolution from the reduction of water. The reason behind the enhanced photoactivity of these nanosheets was the formation of functional hetero junctions developed between the various phases (2H semiconductor, 1T metallic, and 1T′ quasi‐metallic) of the chemically exfoliated MoS2 monolayer. The ratios of these three phases could be controlled systematically via thermal annealing. The best photocatalytic H2 evolution was observed when the heterojunction formed between 1T′‐2H phases of MoS2 nanosheets [109]. Yuan et al. constructed a 2D nano‐junction between (001) facets exposed anatase TiO2 nanosheets and layered MoS2 for enhanced solar H2 production. The optimum wt% of 2D MoS2 nanosheets was 0.5 which showed the highest H2 production rate of 2145 μmol/h g, and it was 36.4 times higher than that of pristine 2D TiO2 nanosheets. The quantum yield was reached up to 6.4% at 360 nm [110]. Iqbal et al. fabricated a few‐layered van der Waals heterostructures (vdWHs) of MoS2 and CdS nanosheets. MoS2/CdS vdWHs made by bubble exfoliation of MoS2 and further in situ stacking of CdS which showed H2 production rate of 140 mmol/g(CdS) h with a quantum yield of 66% at 420 nm [111]. Another 2D hybrid heterojunction photocatalyst containing CdS and MoS2 nanosheets was synthesized by the hydrothermal method followed by ultrasonic treatment. The hybrid photocatalyst showed H2 evolution activity of 1.75 mmol/g h under visible light from sulfide and sulfite containing aqueous solution. This was 2.03 times higher than pristine CdS nanosheets [112]. Xu et al. reported synthesis and visible‐light‐driven hydrogen evolution of ultrathin 4 nm thick CdS nanosheets [113]. Huang et al. generated BCN alloy by a novel and simple method of doping of carbon in h‐BN nanosheets. They demonstrated the efficient photocatalytic activity of water splitting and CO2 reduction [114].
22.5 Photoelectrocatalytic Hydrogen Production
Renewable hydrogen generation by PEC processes has been used as an appropriate strategy for addressing both energy and ecological issues. Both light and energy act together as guiding power for the production of charge carriers in PEC HER. The biggest feature of this process is the promise of cheap price and simplicity for the integrated collection of light energy and the water splitting into one device. The applying of the electric field resolves the limitation of poor charge separation performance in photocatalysis and light irradiation, which decreases the electrical energy usage in electrocatalysis. An optimal photoelectrocatalyst would have good photoabsorption properties in a broad range of the solar spectrum. In addition, it must work as an effective catalyst, with high charge mobility and appropriate Fermi level, and strong resistance to photo corrosion resistance. In PEC‐HER process, electrons and holes formed after irradiating with photons present in the light participates and produces O2 and H2 on the surface of the catalyst materials by the below‐mentioned procedure [115, 116].
The recombination of photoelectrons and holes in the PEC system is greatly suppressed by conducting electrode which collects the charge carriers. This results in an enhanced photocatalytic process [117]. Additionally, the external potential also applied to control the flow of electron or hole which further enhances the oxidation and reduction process. The minimum energy required for water splitting is 1.23 eV. Photogenerated electrons reduce the water to H2 at the conduction band, whereas the holes at the valence band oxidize water to O2 at the valence band. Owing to its unique properties and structure, rapidly growing 2D materials can provide significant opportunities for high‐efficiency hydrogen production.
Despite some development, the performance of PEC water splitting is still well below the requirement for real applications [118]. A hybrid method aimed at increasing the generation of photocarriers and promoting both sides of the reaction (photocathode and photoanode) must be followed to achieve the optimal, near‐zero bias PEC HER. p‐type silicon (p‐Si) is used as an electrode material which is a well‐known low bandgap (1.1 eV) semiconductor capable of absorbing a broad spectrum of UV–visible light for the formation of electron–hole pairs for use in different photovoltaic systems [119]. Sim et al. demonstrated that graphene monolayer on silicon photocathodes can be employed as a catalyst for solar‐driven HER. The catalytic activity of these photocathodes was enhanced by plasma treatment that induced abundant defects and doping of nitrogen atom in the graphene monolayer. N‐doped graphene exhibited extraordinary enhancement in the exchange current density and drives to a considerable anodic shift of the onset of photocurrent from the Si‐photocathode. In addition, this photocathode can be used in neutral water because graphene inhibited the oxidation of Si due to its passivation effect [120]. Alam et al. deposited hydrophobic rGO and hydrophilic GO on the p‐type Si wafer by plasma‐enhanced CVD. Both electrodes showed superior PEC H2 production compared with bare p‐Si electrode, especially hydrophobic rGO performed better than hydrophilic GO [121]. Furthermore; Meng et al. reported flexible and scalable synthesis of silicon nanowire arrays (SiNWs) decorated with electrochemically rGO photocathodes for PEC water splitting. They optimized rGO decoration on SiNWs which showed superior activity with a significant positive shift of onset potential and higher photocurrent density as compared with bare SiNWs FOR PEC H2 production (Figure 22.5) [122]. Carraro and coworkers reported the one‐pot aerosol synthesis of hierarchical nanocomposite of MoS2/crumpled graphene p–n nanojunctions in a short time with high yield for PEC H2 production. MoS2 nanoparticles were anchored by nitrogen dopants of crumpled graphene; further, crumpled structure limits the aggregation of MoS2 nanoparticles [123]. Shah and coworkers proposed a noble metal‐free photocathode for PEC H2 production which contains p‐type CuBi2O4 directly developed on top of FTO, overlaid with rGO. The developed composite photocathode showed a twofold higher current density than bare CuBi2O4 photocathode with an average faradaic efficiency of 91.7%. A major improvement in carrier density is suggested for improved charge injection as well as separation due to the effective transfer of charge from CuBi2O4 to rGO by desirable band alignment. Furthermore, the open‐circuit potential decay measurements (OCPDs) suggest improved charge separation with the incorporation of GO into CuBi2O4 [124].
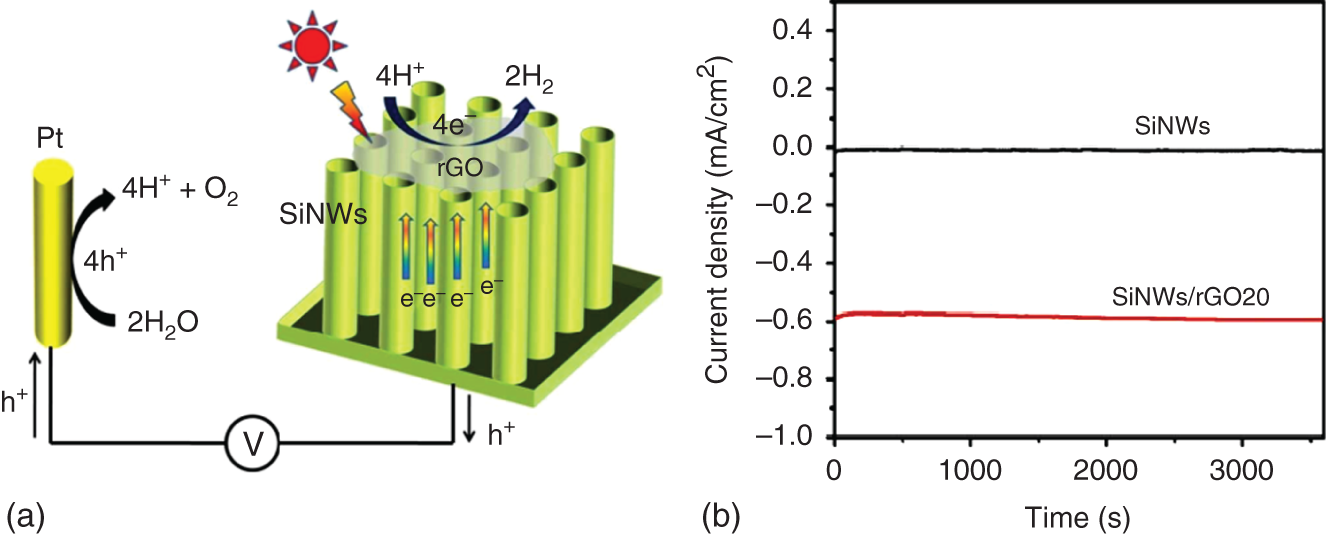
Figure 22.5 (a) Schematic illustration of PED performance enhancement in the rGO‐silicon system under illumination. (b) Amperometric (J–T) curves for of hydrogen generation on the SiNWs/rGO2O samples under illumination (100 mW/cm2) in a solution of 0.1 M H2SO4 and 0.5 M K2SO4 at a constant applied potential of 0 V vs. RHE.
Source: Meng et al. [122].
2D transition metal dichalcogenides (TMDs) are another class of materials wildly used in the PEC HER. TMDs such as WS2 and MoS2 have a wider bandgap (1.7–2 eV) and almost transparent when the thickness is as low as a few layers to a single layer. Therefore, the combination of 2D TMDs and p‐Si allows superior electron–hole pair separation and also forms p–n junctions which provide a number of active sites with proper energy potential for the HER. MoS2 thin films catalyst was synthesized by solution‐based thermolysis, further fabricated heterojunction photocathode consists of n‐MoS2/p‐Si by simple transfer method. The fabricated photocathode showed a high photocurrent density of 24.6 mA/cm2 at 0 V vs. RHE with reduced overpotential (0.79 V at 10 mA/cm2) [125]. Inspite of the high performance, the manufacture of p–n hetero junctions (n‐MoS2/p‐Si) is not readily reproducible since the transfer method is complex and time taking. To address this issue, Hasani et al. reported a novel, simple, reliable, and one‐step synthesis of 2D‐layered MoS2 directly on top of p‐Si substrate, which exhibited a benchmark current density of −13.5 ± 1 mA/cm2 at 0 V and an onset potential of +0.02 V [126]. WS2 is the another promising candidate for this reaction, 23‐nm thick WS2 thin film catalyst synthesized by the sulfurization of tungsten oxide films which were vacuum deposited. The photocathode made by a heterojunction of n‐type WS2 film and p‐type Si exhibited high current density and low onset potential when compared with the bare p‐Si and also showed long‐term stability over 10 hours. The reason behind the enhanced activity was the number of edge sites in the thin films [127]. Paul et al. demonstrated n‐doped MoS2/TiO2 (B) heterojunction photoelectrocatalyst which was selectively decorated with Pt nanoparticles at the edge of MoS2 exhibited strong enhancement of PEC hydrogen evolution under visible light [128].
22.6 Photocatalytic Dye Degradation
In recent decades, graphene‐based materials have been interesting as novel photocatalysts for the decontamination of pollutants [129]. Mainly in the photocatalytic system, rGO, or graphene oxide (GO) can change the band structure of photocatalysis and suppresses the recombination rate and enhances the photocatalytic performance under the light irradiation. The conduction band (CB) and valance band (VB) levels are larger than oxidation and reduction potentials of specific species involved in thermodynamic conditions for photocatalysis. 2D/2D (g‐C3N4/GO) hybrid composites with 1 : 9 weight ratio was synthesized via the hydrothermal method which enhances the photocatalytic reaction by removing the methyl orange (MO) more than 92% within four hours [130, 131]. The heterostructured catalyst can efficiently reduce the recombination of photoinduced charge carriers for degradation of tetracycline hydrochloride under the visible light irradiation is 94%. These heterostructured catalysts are prepared by coupling heterointerfaces between g‐C3N4 monolayer modified with oxygen and grapheme oxide [132].
The highly crystalline carbon materials like carbon dots (CDs), carbon sphere, and graphene quantum dots (GQDs) are hybridized with g‐C3N4 sheets. The degradation rate of Methylene blue (MB) for nitrogen‐doped carbon dots (NCDs) and g‐C3N4composite materials were 97% compared to 69% degradation rate of pristine g‐C3N4 within two hours [133, 134]. The 2D/2D composite materials like Ti3C2/g‐C3N4 was synthesized via evaporation‐induced self‐assembly approach, and it shows higher photocurrent compared with g‐C3N4, and photocatalytic performance was more than 2.2 times for ciprofloxacin degradation. These heterostructures of Ti3C2/porous g‐C3N4 were combined by vander Waals interactions. The simple vacuum filtration method effectively used for removal of phenol, and 98% degradation takes place because monolayers efficiently involved in migration of photo‐induced charge carriers to drive the photocatalytic activity [135–137].
2D sheets with metal oxide composites were developed and they exhibit higher photocatalytic activity for the removal of pharmaceuticals drugs and organic pollutants with a high separation rate of electron and holes. TiO2‐rGO nanocomposites show high performance in persulfate activation under visible‐light irradiation for the removal of bisphenol and phenols. Dominated {001} facets crystals of (TiO2‐100‐G) nanocomposites [138] were produced by controlling the surface facets of TiO2 grown on graphene oxide composite using F− and SO42−as capping agent [138]. The graphene‐supported TiO2 nanosheets with {001} facets are widely used as photocatalyst for dye degradation with high activity. Whereas g‐C3N4 nanoparticles were loaded on BOCl‐001 and BOCl‐010 facet engineering structures useful for dye degradation reactions [139]. The heterojunction photocatalyst of ng‐CN/BOCl‐010 exhibits high performance compared to ng‐CN/BOCl‐001 in the degradation of MO under the visible–light irradiation. The charge separation phenomenon in ngCN/BiOCl‐010 occurred along the BiOI in a short distance comparing to the {001} system (Figure 22.6). The photogenerated electrons from CB of n‐C3N4 to the CB of BiOCl in both composite materials driven by faceted‐oriented internal electric field in BiOCl depend on traveling the length of electron transfer [72] (Figure 22.7).
Some of the low‐bandgap semiconductors have been chosen for light response. Cadmium sulfide–graphene (CdS‐GR) nanocomposites are synthesized by a simple one‐step hydrothermal method. CdS‐GR nanocomposites are evaluated by selective oxidation of alcohols under the mild conditions. Graphene‐based semiconductor sows integrative effect for selective oxidation of alcohols to aldehydes in presence of visible light photocatalytic activity. The graphene and CdS influence hinders the recombination of electron and holes efficiently, the electron excited from the valence band (VB) of CdS NPs to its conduction band (CB) and leaving the hole in the VB to enhance the photocatalytic based on structure and morphology of the composites. The overall activity of CdS‐GR based on the amount from 1% to 5% weight ratio is added selectivity for oxidation of benzylic and allylic alcohols [140]. The important graphene‐based semiconductor oxide composite materials (GO‐BWO) show an increasing in transports properties of photocarriers. The graphene‐Bi2WO6 composite material was synthesized by hydrothermal reactions with the combination of GO. The electronic interaction and charge equilibration between GO and Bi2WO6 lead to decreasing in conduction band potential that leads to shifts the Fermi level. As a result, it has increased the migration efficiency of photo‐induced electrons and decreases the charge recombination reactions. There is negative shift in the Fermi level of GO‐BWO that enhanced the photocatalytic activity. The widely used Rhodamine B (RhB) dye of model pollutant was utilized to evaluate the photocatalytic activity showing amazing results that nearly 100% degradation after irradiation for eight minutes with G‐BWO. BWO and GO‐BWO were only completed 70% and 75% after irradiating for eight minutes [141].
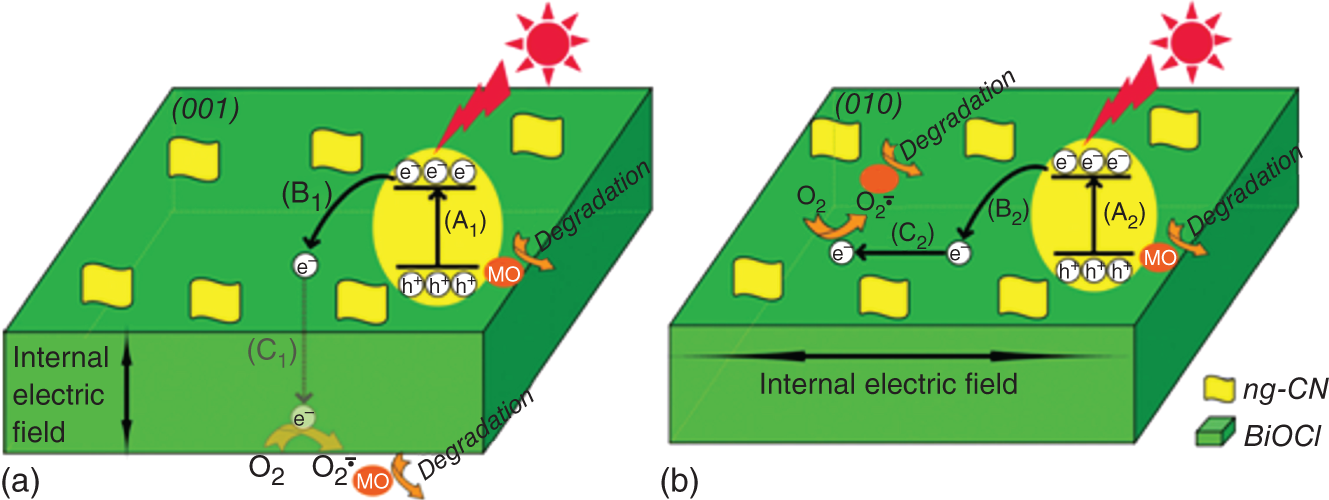
Figure 22.6 Proposed mechanism for photocatalytic reactions occurring on (a) ng‐CN/BOC‐001 and (b) ng‐CN/BOC‐010 heterojunction photocatalysts.
Source: Li et al. [72].
2D and plasmonic metal nanoparticles composite materials are interesting hybrid nanostructures with outstanding plasmonic behavior. These hybrid nanomaterials are having unique optical and physico‐chemical properties, including several plasmonic electrical effects that can improve the photogenerated electron–hole carriers in photocatalytic activity. Plasmonic metals such as Au, Ag, Cu, and Al with the 2D material possessing different functionalities into one hybrid nanostructure system [142]. In situ preparation of graphene oxide/Au nanoparticles (GO‐AuNPs) nanocomposites was synthesized by depositing the Au NPs on surface of rGO. The Au NPs effectively works to suppress the recombination of electrons and holes. The photocatalytic activity of Au‐rGO was evaluated using RhB under the visible light irradiations. The dye degradation was remarkably enhanced the rate constant, and the calculated constant was about 8.7 × 10−3 min−1. The rate constant for MB was 9.4 × 10−4 min−1, and the percentage of degradation was 88.66%. The potential of Au nanoparticles (−4.75 eV) direct electron transfer from RhB to graphene semiconductor. The dye degradation using Au‐rGO composite has high adsorption ability toward organic dyes and π–π interaction. The composite material effectively photosensitized the electron injection that will suppress the recombination rate. Au‐rGO composite material is good candidate for photodegradation of RhB dye pollutants in water [143]. Silver graphene oxide (Ag@GO) nanocomposite material shows high‐performance photocatalytic activity due to increased reactive surface area with superior charge separation. The charge carrier properties in graphene with conjugated system involves in electron drive and suppressing the recombination rate. Ag@GO composite used as visible light photocatalyst in oxidative coupling of benzylamines with molecular oxygen under the ambient conditions [144].
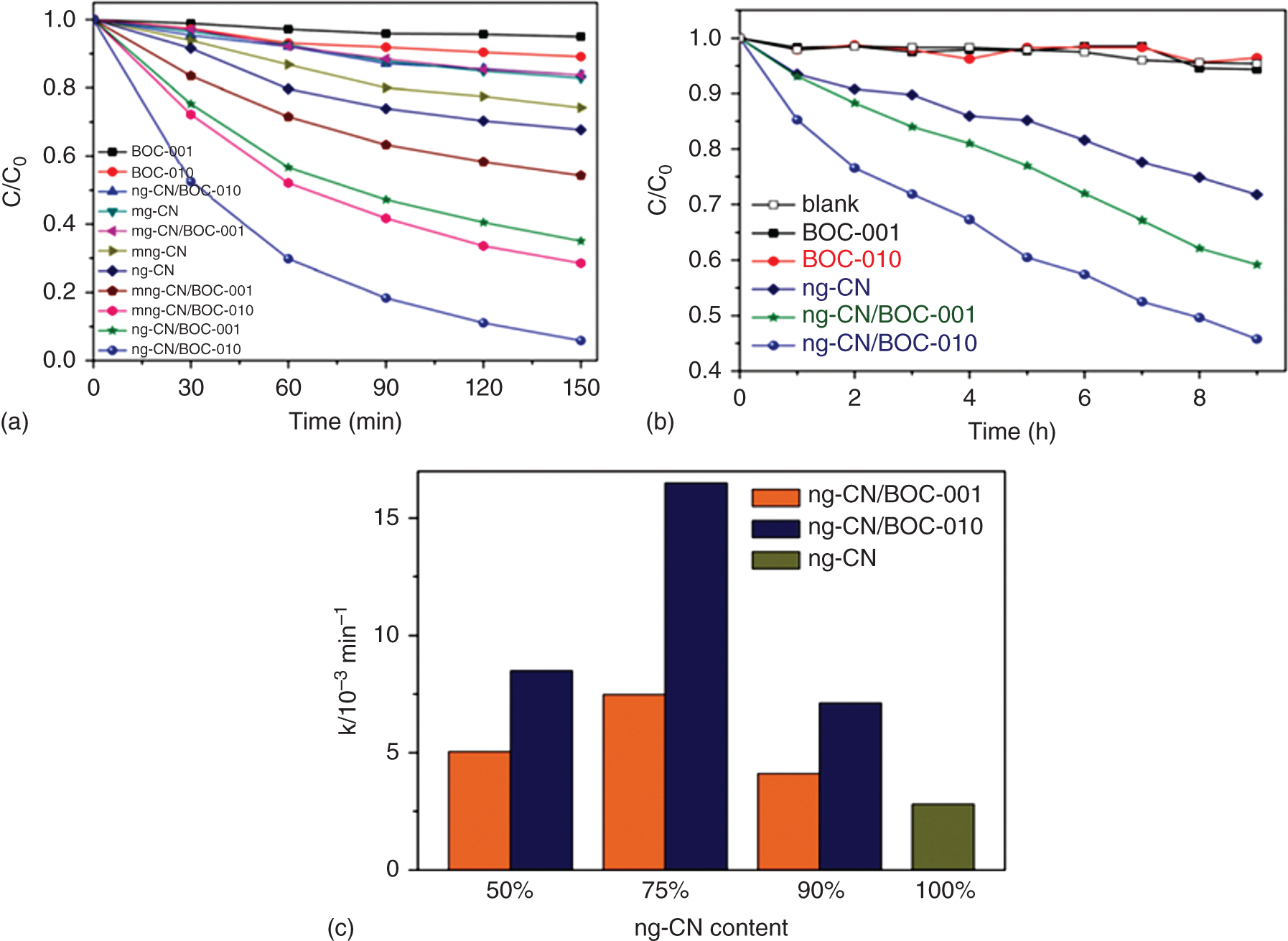
Figure 22.7 (a) Photocatalytic degradation of MO over samples under visible‐light irradiation. (b) Photocatalytic degradation of phenol over samples under visible‐light irradiation. (c) Visible‐light‐induced photocatalytic degradation of MO over samples containing different proportions of ng‐CN.
Source: Li et al. [72].
Bi‐based 2D‐layered photocatalyst based on thickness, facet, and defect engineering have been controlled by constructing the high‐performance photocatalyst on energy conversion. The photocatalytic degradation of RhB, methyl orange (MO), and phenol under the visible light irradiation is based on the thickness of layer structure bismuth oxyiodide (BiOI) square sheets. BiOI is an efficient visible‐light photocatalyst with exposed {001} facets and driven by self‐induced strong internal static electric field direction. BiOI square nanosheets reaches to 90% of RhB, 94% of MO, and only 70% of phenol degradation, respectively [145]. The 2D layer structures of bismuth oxyhalides (Cl, Br, and I), BiOCl microsized square nanosheets, with an average size of 3–5 μm and a thickness of 35 nm show good photocatalytic activity toward the decomposition of 97% of RhB under the UV–visible irradiation [146]. The highly reactive {001} facets exposed 2D BiOBr nanosheets selectively synthesized by a facile hydrothermal method. The facet oriented and low‐strain BiOBr improved the photocatalytic performance within 30 minutes of 100% degradation of RhB [147]. The overall catalytic activity depends on photoexcited charge separation and reduces the recombination rate. The monolayer of Bi2WO6 was synthesized by surfactant‐assisted self‐assembly strategy. In this system, the charge transfer mechanism is easy and highly active in surface species. Bi2WO6 is used as H2 evolution and shows high‐performance photocatalytic RhB activity and 98% degradation under visible‐light irradiation [148].
22.7 Conclusion
Research focus in photo(electro)catalysts of 2D nanomaterials is on the rise, encouraged by their unique properties like high surface area, chemical stability, large spectrum of light absorption, tunable redox capacity, and excellent compatibility with other materials. Endeavors in their structural architecture, synthetic discovery, band modulation, and mechanistic analysis have taken their photocatalytic performance to a stage much greater than those of other basic photocatalyst and also the rivals of several conventional photocatalysts. As a crucial note, while there is an outpouring of academic papers, fewer have been translated into successfully commercialized technologies because some 2D nanomaterials have low electrical conductivity, instability (chemical and electrochemical), electrode–electrode interfaces issues, and also difficulty in large scale synthesis. In this chapter, we have discussed about general introduction about the various 2D materials for various photo(electro)catalytic applications. The emphasis kept on the mechanism and activity of CO2 reduction, H2 production, and dye degradation reactions.
References
- 1 Novoselov, K.S., Geim, A.K., Morozov, S.V. et al. (2004). Electric field effect in atomically thin carbon films. Science 306 (5696): 666–669.
- 2 Yu, Z., Tetard, L., Zhai, L., and Thomas, J. (2015). Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 8 (3): 702–730.
- 3 Butler, S.Z., Hollen, S.M., Cao, L. et al. (2013). Progress, challenges, and opportunities in two‐dimensional materials beyond graphene. ACS Nano 7: 2898–2926.
- 4 Zhang, H. (2015). Ultrathin two‐dimensional nanomaterials. ACS Nano 9 (10): 9451–9469.
- 5 Geim, A.K. and Novoselov, K.S. (2007). The rise of graphene. Nat. Mater. 6: 183–191.
- 6 Novoselov, K.S., Falko, V.I., Colombo, L. et al. (2012). A roadmap for graphene. Nature 490: 192–200.
- 7 Wang, Q.H., Kalantar‐Zadeh, K., Kis, A. et al. (2012). Electronics and optoelectronics of two‐dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7: 699–712.
- 8 Tan, C.L., Liu, Z.D., Huang, W., and Zhang, H. (2015). Non‐volatile resistive memory devices based on solution‐processed ultrathin two dimensional nanomaterials. Chem. Soc. Rev. 44: 2615–2628.
- 9 Choucair, M., Thordarson, P., and Stride, J.A. (2009). Gram‐scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 4: 30–33.
- 10 Mahler, B., Hoepfner, V., Liao, K., and Ozin, G.A. (2014). Colloidal synthesis of 1T‐WS2 and 2H‐WS2 nanosheets: applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136: 14121–14127.
- 11 Ji, Q., Zhang, Y., Zhang, Y., and Liu, Z. (2015). Chemical vapour deposition of group‐VIB metal dichalcogenide monolayers: engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 44: 2587–2602.
- 12 Bae, S., Kim, H., Lee, Y. et al. (2010). Roll‐to‐roll production of 30‐inch graphene films for transparent electrodes. Nat. Nanotechnol. 5: 574–578.
- 13 Viculis, L.M., Mack, J.J., Mayer, O.M. et al. (2005). Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 15: 974–978.
- 14 Zeng, Z.Y., Sun, T., Zhu, J.X. et al. (2012). An effective method for the fabrication of few‐layer‐thick inorganic nanosheets. Angew. Chem. Int. Ed. 51: 9052–9056.
- 15 Paton, K.R., Varrla, E., Backes, C. et al. (2014). Scalable production of large quantities of defect‐free few‐ layer graphene by shear exfoliation in liquids. Nat. Mater. 13: 624–630.
- 16 Brent, J.R., Savjani, N., Lewis, E.A. et al. (2014). Production of few‐layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 50: 13338–13341.
- 17 Kollipara, P.S., Li, J., and Zheng, Y. (2020). Optical patterning of two‐dimensional materials. Research: 6581250. https://doi.org/10.34133/2020/6581250.
- 18 Castellanos‐Gomez, A., Barkelid, M., Goossens, A.M. et al. (2012). Laser‐thinning of MoS2: on demand generation of a single‐layer semiconductor. Nano Lett. 12 (6): 3187–3192.
- 19 Lee, C., Wei, X.D., Kysar, J.W., and Hone, J. (2008). Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321: 385–388.
- 20 Sun, X., Huang, H., Zhao, Q. et al. (2020). Thin‐layered photocatalysts. Adv. Funct. Mater. 30 (22): 1910005. (1–43)., https://doi.org/10.1002/adfm.201910005.
- 21 Sun, X., Zhang, X., and Xie, Y. (2020). Surface defects in two‐dimensional photocatalysts for efficient organic synthesis. Matter 2 (4): 842–861.
- 22 Wu, X.J., Huang, X., Qi, X.Y. et al. (2014). Copper‐based ternary and quaternary semiconductor nanoplates: templated synthesis, characterization, and photoelectrochemical properties. Angew. Chem. Int. Ed. 53: 8929–8933.
- 23 Saraswat, S.K., Rodene, D.D., and Gupta, R.B. (2018). Recent advancements in semiconductor materials for photoelectrochemical water splitting for hydrogen production using visiblelight. Renew. Sust. Energ. Rev. 89 (C): 228–248.
- 24 Li, M., Zhao, R., Su, Y. et al. (2016). Hierarchically CuInS2 nanosheet‐constructed nanowire arrays for Photoelectrochemical water splitting. Adv. Mater. Interfaces 3 (20): 1600494. (1–10). https://doi.org/10.1002/admi.201600494.
- 25 Hisatomi, T., Takata, T., and Domen, K. (2017). Water splitting on particulate semiconducting photocatalysts under visible light. In: Nanotechnology in Catalysis: Applications in the Chemical Industry, Energy Development, and Environment Protection (eds. B. Sels and M. Van de Voorde), 851–871. Wiley‐VCH Verlag.
- 26 Grills, D.C. and Fujita, E. (2010). New directions for the photocatalytic reduction of CO2: supramolecular, scCO2 or biphasic ionic liquid‐scCO2 systems. J. Phys. Chem. Lett. 1: 2709–2718.
- 27 Chen, Y., Jia, G., Hu, Y. et al. (2017). Two‐dimensional nanomaterials for photocatalytic CO2 reduction to solar fuels. Sustainable Energy Fuels 1: 1875–1898.
- 28 Wang, C., Sun, Z., Zheng, Y., and Hu, Y.H. (2019). Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 7: 865.
- 29 Xing, M., Shen, F., Qiu, B., and Zhang, J. (2014). Highly‐dispersed boron‐doped graphene Nanosheets loaded with TiO2 nanoparticles for enhancing CO2 Photoreduction. Sci. Rep. 4: 6341.
- 30 Kandy, M.M. (2020). Carbon‐based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels. Sustainable Energy Fuels 4: 469–484.
- 31 Ong, W.J., Tan, L.L., Ng, Y.H. et al. (2016). Graphitic carbon nitride (g‐C3N4)‐based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 116: 7159–7329.
- 32 Xiang, Q., Yu, J., and Jaroniec, M. (2012). Graphene‐based semiconductor photocatalysts. Chem. Soc. Rev. 41: 782–796.
- 33 Liang, Y.T., Vijayan, B.K., Gray, K.A., and Hersam, M.C. (2011). Minimizing graphene defects enhances Titania nanocomposite‐based photocatalytic reduction of CO2 for improved solar fuel production. Nano Lett. 11: 2865–2870.
- 34 Low, J., Yu, J., and Ho, W. (2015). Graphene‐based photocatalysts for CO2 reduction to solar fuel. J. Phys. Chem. Lett. 6: 4244–4251.
- 35 Tan, C., Cao, X., Wu, X.J. et al. (2017). Recent advances in ultrathin two‐dimensional nanomaterials. Chem. Rev. 117: 6225–6331.
- 36 Ong, W.J., Tan, L.L., Chai, S.P. et al. (2014). Self‐assembly of nitrogen‐doped TiO2 with exposed {001} facets on a graphene scaffold as photo‐active hybrid nanostructures for reduction of carbon dioxide to methane. Nano Res. 7: 1528–1547.
- 37 Tu, W., Zhou, Y., Liu, Q. et al. (2012). Robust hollow spheres consisting of alternating titania nanosheets and graphene nanosheets with high photocatalytic activity for CO2 conversion into renewable fuels. Adv. Funct. Mater. 22: 1215–1221.
- 38 Hsu, H.C., Shown, I., Wei, H.Y. et al. (2013). Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 5: 262–268.
- 39 Li, X., Wang, Q., Zhao, Y. et al. (2013). Green synthesis and photo‐catalytic performances for ZnO‐reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 411: 69–75.
- 40 Zhang, L., Li, N., Jiu, H. et al. (2015). ZnO‐reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 41: 6256–6262.
- 41 An, X., Li, K., and Tang, J. (2014). Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 7: 1086–1093.
- 42 Wang, A., Li, X., Zhao, Y. et al. (2014). Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo‐catalytic performances. Powder Technol. 261: 42–48.
- 43 Wang, A., Shen, S., Zhao, Y., and Wu, W. (2015). Preparation and characterizations of BiVO4/reduced graphene oxide nanocomposites with higher visible light reduction activities. J. Colloid Interface Sci. 445: 330–336.
- 44 Lv, X.J., Fu, W.F., Hu, C.‐Y. et al. (2013). Photocatalytic reduction of CO2 with H2O over a graphene‐modified NiOx–Ta2O5 composite photocatalyst: coupling yields of methanol and hydrogen. RSC Adv. 3: 1753–1757.
- 45 Yu, J., Jin, J., Cheng, B., and Jaroniec, M. (2014). A noble metal‐free reduced graphene oxide–CdS nanorod composite for the enhanced visible‐light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2: 3407–3416.
- 46 Kumar, P., Kumar, A., Sreedhar, B. et al. (2014). Cobalt phthalocyanine immobilized on graphene oxide: an efficient visible‐active catalyst for the Photoreduction of carbon dioxide. Chem.‐Eur. J. 20: 6154–6161.
- 47 Kumar, P., Sain, B., and Jain, S.L. (2014). Photocatalytic reduction of carbon dioxide to methanol using a ruthenium trinuclear polyazine complex immobilized on graphene oxide under visible light irradiation. J. Mater. Chem. A 2: 11246–11253.
- 48 Xu, Y.F., Yang, M.Z., Chen, B.X. et al. (2017). A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 139: 5660–5663.
- 49 Yadav, R.K., Baeg, J.O., Kumar, A. et al. (2014). Graphene‐BODIPY as a photocatalyst in the photocatalytic–biocatalytic coupled system for solar fuel production from CO2. J. Mater. Chem. A 2: 5068–5076.
- 50 Zheng, Y., Lin, L., Wang, B., and Wang, X. (2015). Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 54: 12868–12884.
- 51 Wang, X., Maeda, K., Thomas, A. et al. (2009). A metal‐free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8: 76–80.
- 52 Dong, G. and Zhang, L. (2012). Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 22: 1160–1166.
- 53 Mao, J., Peng, T., Zhang, X. et al. (2013). Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Cat. Sci. Technol. 3: 1253–1260.
- 54 Niu, P., Yang, Y., Yu, J.C. et al. (2014). Switching the selectivity of the photoreduction reaction of carbon dioxide by controlling the band structure of a g‐C3N4 photocatalyst. Chem. Commun. 50: 10837–10840.
- 55 Wang, H., Sun, Z., Li, Q. et al. (2016). Surprisingly advanced CO2 photocatalytic conversion over thiourea derived g‐C3N4 with water vapor while introducing 200–420 nm UV light. J. CO2 Util. 14: 143–151.
- 56 Yu, J., Wang, K., Xiao, W., and Cheng, B. (2014). Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g‐C3N4–Pt nanocomposite photocatalysts. Phys. Chem. Chem. Phys. 16: 11492–11501.
- 57 He, Y., Wang, Y., Zhang, L. et al. (2015). High‐efficiency conversion of CO2 to fuel over ZnO/g‐C3N4 photocatalyst. Appl Catal B 168–169: 1–8.
- 58 Ohno, T., Murakami, N., Koyanagi, T., and Yang, Y. (2014). Photocatalytic reduction of CO2 over a hybrid photocatalyst composed of WO3 and graphitic carbon nitride (g‐C3N4) under visible light. J. CO2 Util. 6: 17–25.
- 59 Yang, X., Xin, W., Yin, X., and Shao, X. (2016). Enhancement of photocatalytic activity in reducing CO2 over CdS/g‐C3N4 composite catalysts under UV light irradiation. Chem. Phys. Lett. 651: 127–132.
- 60 Xu, H., Ouyang, S., Li, P. et al. (2013). High‐active anatase TiO2 nanosheets exposed with 95% {100} facets toward efficient H2 evolution and CO2 photoreduction. ACS Appl. Mater. Interfaces 5: 1348–1354.
- 61 Chen, X., Zhou, Y., Liu, Q. et al. (2012). ACS Appl. Ultrathin, single‐crystal WO3 nanosheets by two‐dimensional oriented attachment toward enhanced Photocatalystic reduction of CO2 into hydrocarbon fuels under visible light. Mater. Interface 4: 3372–3377.
- 62 Tu, W., Yang, Y., Kuai, L. et al. (2017). Construction of unique two‐dimensional MoS2–TiO2 hybrid nanojunctions: MoS2 as a promising cost‐effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 9: 9065–9070.
- 63 Dai, W., Yu, J., Deng, Y. et al. (2017). Facile synthesis of MoS2/Bi2WO6 nanocomposites for enhanced CO2 photoreduction activity under visible light irradiation. Appl. Surf. Sci. 403: 230–239.
- 64 Ye, L., Wang, H., Jin, X. et al. (2016). Synthesis of olive‐ green few‐ layered BiOI for efficient photoreduction of CO2 into solar fuels under visible/near‐ infrared light. Sol. Energy Mater. Sol. Cells 144: 732–739.
- 65 Egerton, T.A. (2011). Does photoelectrocatalysis by TiO2 work? J. Chem. Technol. Biotechnol. 86: 1024.
- 66 Rajeshwar, K. (1995). Photoelectrochemistry and the environment. J. Appl. Electrochem. 25: 1067–1082.
- 67 Vinodgopal, K., Hotchandani, S., and Kamat, P.V. (1993). Electrochemically assisted photocatalysis: titania particulate film electrodes for photocatalytic degradation of 4‐chlorophenol. J. Phys. Chem. 97: 9040.
- 68 Wang, P., Wang, S., Wang, H. et al. (2018). RecentProgress on photo‐ Electrocatalytic reduction of carbon dioxide. Part. Part. Syst. Charact. 35 (1): 1700371.
- 69 Wang, L., Chen, W., Zhang, D. et al. (2019). Surface strategies for catalytic CO2 reduction: from twodimensionalmaterials to nanoclusters to single atoms. Chem. Soc. Rev. 48 (21): 5310–5349.
- 70 Xie, S., Zhang, Q., Liu, G., and Wang, Y. (2016). Photocatalytic andphotoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 52 (1): 35–59.
- 71 Pawar, A.U., Kim, C.W., Nguyen‐Le, M.T., and Kang, Y.S. (2019). General review on the components and parameters of photoelectrochemical system for CO2 reduction with in situ analysis. ACS Sustain. Chem. Eng. 7 (8): 7431–7455.
- 72 Li, Q., Zhao, X., Yang, J. et al. (2015). Exploring the effects of nanocrystal facet orientations in g‐C3N4/BiOCl heterostructures on photocatalytic performance. Nanoscale 7: 18971.
- 73 Cheng, J., Zhang, M., Wu, G. et al. (2014). Photoelectrocatalytic reduction of CO2 into chemicals using Pt‐modified reduced graphene oxide combined with Pt‐modified TiO2 nanotubes. Environ. Sci. Technol. 48 (12): 7076–7084.
- 74 Hasan, M.R., Abd Hamid, S.B., Basirun, W.J. et al. (2015). Ga doped rGO‐TiO2 composite on an ITO surface electrode for investigation of photoelectrocatalytic activity under visible light irradiation. New J. Chem. 39 (1): 369–376.
- 75 Hasan, R., Hamid, S.B.A., Basirun, W.J. et al. (2015). A sol–gel derived, copper‐doped, titanium dioxide–reduced graphene oxide nanocomposite electrode for the photoelectrocatalytic reduction of CO2 to methanol and formic acid. RSC Adv. 5: 77803.
- 76 Xu, Y., Wang, S., Yang, J. et al. (2018). Highly efficient photoelectrocatalytic reduction of CO2 on the Ti3C2/g‐C3N4 heterojunction with rich Ti3+ and pyri‐N species. J. Mater. Chem. A 6: 15213–15220.
- 77 Xu, Y., Wang, S., Yang, J. et al. (2018). In‐situ grown nanocrystal TiO2 on 2D Ti3C2 nanosheets forartificial photosynthesis of chemical fuels. Nano Energy 51: 442–450.
- 78 Peng, H., Lu, J., Wu, C. et al. (2015). Co‐doped MoS2 NPs with matched energy band and low over potential high efficiently convert CO2 to methanol. Appl. Surf. Sci. 353 (30): 1003–1012.
- 79 Dincer, I. (2000). Renewable energy and sustainable development: a crucial review. Renew. Sust. Energ. Rev. 4: 157–175.
- 80 Christoforidis, K.C. and Fornasiero, P. (2017). Photocatalytic hydrogen production: a rift into the future energy supply. ChemCatChem 9 (9): 1523–1544. https://doi.org/10.1002/cctc.201601659.
- 81 Osterloh, F.E. (2013). Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 42: 2294–2320.
- 82 Lee, Y.Y., Jung, H.S., and Kang, Y.T. (2017). A review: effect of nanostructures on photocatalytic CO2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 20: 163–177.
- 83 Hisatomi, T. and Domen, K. (2017). Progress in the demonstration and understanding of water splitting using particulate photocatalysts. Curr. Opin. Electrochem. 2: 148–154.
- 84 Hisatomi, T., Kubota, J., and Domen, K. (2014). Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43: 7520–7535.
- 85 Kouser, S., Thannikoth, A., Gupta, U. et al. (2015). 2D‐GaS as a photocatalyst for water splitting to produce H2. Small 11 (36): 4723–4730.
- 86 Li, Z., Luo, W., Zhang, M. et al. (2013). Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 6: 347–370.
- 87 Ager, J., Shaner, M., Walczak, K. et al. (2015). Experimental demonstrations of spontaneous, solar‐driven photoelectrochemical water splitting. Energy Environ. Sci. 8: 2811–2824.
- 88 Pu, C., Wan, J., Liu, E. et al. (2017). Two‐dimensional porous architecture of protonated GCN and reduced graphene oxide via electrostatic self‐assembly strategy for high photocatalytic hydrogen evolution under visible light. Appl. Surf. Sci. 399: 139–150.
- 89 Yuan, Y.J., Tu, J.R., Ye, Z.J. et al. (2016). MoS2‐graphene/ZnIn2S4 hierarchical microarchitectures with an electron transport bridge between light‐harvesting semiconductor and cocatalyst: a highly efficient photocatalyst for solar hydrogen generation. Appl Catal B 188: 13–22.
- 90 Min, S., Hou, J., Lei, Y. et al. (2017). Facile one‐step hydrothermal synthesis toward strongly coupled TiO2/graphene quantum dots photocatalysts for efficient hydrogen evolution. Appl. Surf. Sci. 396: 1375–1382.
- 91 Jia, L., Wang, D.‐H., Huang, Y.‐X. et al. (2011). Highly durable N‐doped graphene/CdS nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J. Phys. Chem. C 115 (23): 11466–11473.
- 92 Xiang, Q., Yu, J., and Jaroniec, M. (2011). Enhanced photocatalytic H2‐production activity of graphene‐modified titaniananosheets. Nanoscale 3: 3670–3678.
- 93 Li, Q., Guo, B., Yu, J. et al. (2011). Highly efficient visible‐light‐driven photocatalytic hydrogen production of CdS‐cluster‐decorated graphene nanosheets. J. Am. Chem. Soc. 133 (28): 10878–10884.
- 94 Sun, Z., Guo, J., Zhu, S. et al. (2014). A high‐performance Bi2WO6–graphene photocatalyst for visible light‐induced H2 and O2 generation. Nanoscale 6: 2186–2193.
- 95 Zhou, X., Gao, Q., Li, X. et al. (2015). Ultra‐thin SiC layers covered graphene nanosheets as advanced photocatalysts for hydrogen evolution. J. Mater. Chem. A 3: 10999–11005.
- 96 Hou, J., Wang, Z., Kan, W. et al. (2012). Efficient visible‐light‐driven photocatalytic hydrogen production using CdS@TaON core–shell composites coupled with graphene oxide nanosheets. J. Mater. Chem. 22: 7291.
- 97 Yang, S., Gong, Y., Zhang, J. et al. (2013). Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 25: 2452–2456.
- 98 Han, Q., Wang, B., Gao, J. et al. (2016). Atomically thin mesoporous nanomesh of graphitic C3N4 for high‐efficiency photocatalytic hydrogen evolution. ACS Nano 10: 2745–2751.
- 99 She, X., Wu, J., Zhong, J. et al. (2016). Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 27: 138–146.
- 100 Xiang, Q., Yu, J., and Jaroniec, M. (2011). Preparation and enhanced visible‐light photocatalytic H2‐ production activity of graphene/C3N4 composites. J. Phys. Chem. C 115: 7355–7363.
- 101 Gu, W., Lu, F., Wang, C. et al. (2017). Face‐to‐Face interfacial assembly of ultrathin g‐C3N4 and anatase TiO2 nanosheets for enhanced solar photocatalytic activity. ACS Appl. Mater. Interfaces 9 (34): 8674–28684.
- 102 She, X., Wu, J., Xu, H. et al. (2017). High efficiency photocatalytic water splitting using 2D α‐ Fe2O3/g‐C3N4 Z‐scheme catalysts. Adv. Energy Mater.: 1700025. (1–7), https://doi.org/10.1002/aenm.201700025.
- 103 Cai, X., Zhang, J., Fujitsuka, M., and Majima, T. (2017). Graphitic‐C3N4 hybridized N‐doped La2Ti2O7 two‐dimensional layered composites as efficient visible‐light‐driven photocatalyst. Appl Catal B 202: 191–198.
- 104 Hou, Y., Laursen, A.B., Zhang, J. et al. (2013). Layered nanojunctions for hydrogen‐evolution catalysis. Angew. Chem. Int. Ed. 52: 1–6.
- 105 Lina, B., Lia, H., Ana, H. et al. (2018). Preparation of 2D/2D g‐C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well‐designed high‐speed charge transfer nanochannels towards highefficiency photocatalytic hydrogen evolution. Appl Catal B 220: 542–552.
- 106 Jiang, D., Li, J., Xing, C. et al. (2015). Two‐dimensional CaIn2S4/g‐C3N4 heterojunction nanocomposite with enhanced visible‐light photocatalytic activities: interfacial engineering and mechanism insight. ACS Appl. Mater. Interfaces 7 (34): 19234–19242.
- 107 Ida, S., Kim, N., Ertekin, E. et al. (2015). Photocatalytic reaction centers in two‐dimensional titanium oxide crystals. J. Am. Chem. Soc. 137 (1): 239–244.
- 108 Yan, B., Zhou, P., Xu, Q. et al. (2016). Engineering disorder into exotic electronic 2D TiO2 nanosheets for enhanced photocatalytic performance. RSC Adv. 6: 6133–6137.
- 109 Peng, R., Liang, L., Hood, Z.D. et al. (2016). In‐plane heterojunctions enable multiphasic two‐dimensional (2D) MoS2 nanosheets as efficient photocatalysts for hydrogen evolution from water reduction. ACS Catal. 6 (10): 6723–6729.
- 110 Yuan, Y.‐J., Ye, Z.‐J., Lu, H.‐W. et al. (2016). Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two‐dimensional nanojunctions for enhanced solar hydrogen generation. ACS Catal. 6 (2): 532–541.
- 111 Iqbal, S., Pan, Z., and Zhou, K. (2017). Enhanced photocatalytic hydrogen evolution from in situ formation of few‐layered MoS2/CdS nanosheet‐based van der Waals heterostructures. Nanoscale 9: 6638.
- 112 Song, M., Jun, X., Jiuqing, W. et al. (2017). Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible‐light photocatalytic H2 generation. Appl. Surf. Sci. 391, Part B: 580–591.
- 113 Xu, Y., Zhao, W., Xu, R. et al. (2013). Synthesis of ultrathin CdS nanosheets as efficient visible‐light‐driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 49: 9803–9805.
- 114 Huang, C., Chen, C., Zhang, M. et al. Carbon‐doped BN nanosheets for metal‐free photoredox catalysis. Nat. Commun. 6: 7698.
- 115 Yuan, Y.‐J., Lu, H.‐W., Yu, Z.‐T., and Zou, Z.‐G. (2015). Noble‐metal‐free molybdenum disulfide cocatalyst for photocatalytic hydrogen production. ChemSusChem 8: 4113–4127.
- 116 Fujishima, A. and Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature 238: 37–38.
- 117 Bessegato, G.G., Guaraldo, T.T., de Brito, J.F. et al. (2015). Achievements and trends in photoelectrocatalysis: from environmental to energy applications. Electrocatalysis 6: 415–441.
- 118 Ding, Q., Meng, F., English, C.R. et al. (2014). Efficient photoelectrochemical hydrogen generation using heterostructures of Si and chemically exfoliated metallic MoS2. J. Am. Chem. Soc. 136 (24): 8504–8507.
- 119 Ding, Q., Song, B., Xu, P., and Jin, S. (2016). Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds. Chem 1: 699–726.
- 120 Sim, U., Yang, T.‐Y., Moon, J. et al. (2013). N‐doped monolayer graphene catalyst on silicon photocathode for hydrogen production. Energy Environ. Sci. 6: 3658.
- 121 Alam, K., Sim, Y., Yu, J.‐H. et al. (2020). In‐situ deposition of graphene oxide catalyst for efficient Photoelectrochemical hydrogen evolution reaction using atmospheric plasma. Materials. 13: 12.
- 122 Meng, H., Fan, K., Low, J., and Yu, J. (2016). Electrochemically reduced graphene oxide on silicon nanowire arrays for enhanced photoelectrochemical hydrogen evolution. Dalton Trans. 45: 13717–13725.
- 123 Carraro, F., Calvillo, L., Cattelan, M. et al. (2015). Fast one‐pot synthesis of MoS2/crumpled graphene p–n nanonjunctions for enhanced photoelectrochemical hydrogen production. ACS Appl. Mater. Interfaces 7: 25685–25692.
- 124 Shah, A.K., Sahu, T.K., Banik, A. et al. Reduced graphene oxide modified CuBi2O4 as an efficient and noble metal free photocathode for superior photoelectrochemical hydrogen production. Sustain. Energy Fuels 3: 1554–1561.
- 125 Kwon, K.C., Choi, S., Hong, K. et al. (2016). Wafer‐scale transferable molybdenum disulfide thin‐film catalysts for photoelectrochemical hydrogen production. Energy Environ. Sci. 9: 2240–2248.
- 126 Hasani, A., Le, Q.V., Tekalgne, M. et al. (2019). Direct synthesis of two‐dimensional MoS2 on p‐type Si and application to solar hydrogen production. NPG Asia Materials 11: 47.
- 127 Kwon, K.C., Choi, S., Hong, K. et al. (2017). Tungsten disulfide thin film/p‐type Si heterojunction photocathode for efficient photochemical hydrogen production. MRS Communications 7 (2): 272–279.
- 128 Paul, K.K., Narayanaru, S., Biroju, R.K. et al. (2018). Strongly enhanced visible light photoelectrocatalytic hydrogen evolution reaction in an n‐doped MoS2/TiO2 (B) heterojunction by selective decoration of platinum nanoparticles at the MoS2 edge sites. J. Mater. Chem. A 6: 22681–22696.
- 129 Haque, F.J., Daeneke, T., Kalantar‐zadeh, K., and Ou, J.Z. (2018). Two‐dimensional transition metal oxide and chalcogenide‐based photocatalysts. Nano‐Micro Lett. 10: 23.
- 130 Tong, Z., Yang, D., Shi, J. et al. (2015). Three‐dimensional porous aerogel constructed by g‐C3N4 and graphene oxide nanosheets with excellent visible‐light photocatalytic performance. ACS Appl. Mater. Interfaces 7: 25693.
- 131 Wang, X., Wang, H., Yu, K., and Hu, X. (2018). Immobilization of 2D/2D structured g‐C3N4 nanosheets/reduced graphene oxide hybrids on 3D nickel foam and its photocatalytic performance. Mater. Res. Bull. 97: 306.
- 132 Qu, L.‐L., Zhu, J.‐J., Yadav, T.P. et al. (2018). Recyclable visible light‐driven O‐g‐C3N4/graphene oxide/N‐carbon nanotube membrane for efficient removal of organic pollutants. ACS Appl. Mater. Interfaces 10 (49): 42427–42435.
- 133 Zhang, G., Ji, Q., Wu, Z. et al. (2018). Facile “spot‐heating” synthesis of carbon dots/carbon nitride for solar hydrogen evolution synchronously with contaminant decomposition. Adv. Funct. Mater. 28: 1706462.
- 134 Allagui, L., Chouchene, B., Gries, T. et al. (2009). Core/shell rGO/BiOBr particles with visible photocatalytic activity towards water pollutants. Appl. Surf. Sci. 490: 580–591.
- 135 Xu, T., Wang, D., Dong, L. et al. (2019). Graphitic carbon nitride co‐modified by zinc phthalocyanine and graphene quantum dots for the efficient photocatalytic degradation of refractory contaminants. Appl Catal B 244: 96.
- 136 Liu, N., Lu, N., Su, Y. et al. (2020). Efficient day‐night photocatalysis performance of 2D/2D Ti3C2/porous g‐C3N4 nanolayers composite and its application in the degradation of organic pollutants. Chemosphere 246: 125760.
- 137 Liu, N., Lu, N., Su, Y. et al. (2019). Fabrication of g‐C3N4/Ti3C2 composite and its visible – light photocatalytic capability for ciprofloxacin degradation. 211: 782–789.
- 138 Liu, L., Liu, Z., Liu, A. et al. (2014). Engineering the TiO2‐graphene Interface to enhance photocatalytic H2 production. ChemSusChem 7: 618.
- 139 Jiang, J., Zhao, K., Xiao, X., and Zhang, L. (2012). Synthesis and facet‐dependent Photoreactivity of BiOCl single‐crystalline nanosheets. J. Am. Chem. Soc. 134: 4473.
- 140 Zhang, N., Zhang, Y., Pan, X. et al. (2011). Assembly of CdS nanoparticles on the two‐dimensional graphene scaffold as visible‐light‐driven photocatalyst for selective organic transformation under ambient conditions. J. Phys. Chem. C 115: 23501–23511.
- 141 Gao, E., Wang, W., Shang, M., and Xu, J. (2011). Synthesis and enhanced photocatalytic performance of graphene‐Bi2WO6 composite. Phys. Chem. Chem. Phys. 13: 2887–2893.
- 142 Li, X., Zhu, J., and Wei, B. (2016). Hybrid nanostructures of metal/two‐dimensional nanomaterials for plasmon‐enhanced applications. Chem. Soc. Rev. 45: 3145.
- 143 Xiong, Z., Zhang, L.L., Ma, J., and Zhao, X.S. (2010). Photocatalytic degradation of dyes over graphene‐gold nanocomposites under visible light irradiation. Chem. Commum. 46: 6099–6101.
- 144 Kumar, A., Sadanandhan, A.M., and Jain, S.J. (2019). Silver doped reduced graphene oxide as promising plasmonic photocatalyst for oxidative coupling of benzylamines under visible light irradiation. New J. Chem. 43: 9116–9122.
- 145 Mi, Y., Zhou, M., Wen, L. et al. (2014). A highly efficient visible – light driven photocatalyst: two dimensional square‐like bismuth oxyiodine nanosheets. Dalton Trans. 43: 9549.
- 146 Li, M., Zhang, J., Gao, H. et al. (2016). Microsized BiOCl Square nanosheets as ultraviolet photodetectors and photocatalysts. ACS Appl. Mater. Interfaces 8: 6662–6668.
- 147 Feng, H., Xu, Z., Wang, L. et al. (2015). Modulation of photocatalytic properties by strain in 2D BiOBr nanosheets. ACS Appl. Mater. Interfaces 7: 27592–27596.
- 148 Zhou, Y., Zhang, Y., Lin, M. et al. (2015). Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 6: 8340.
