3
2D Nanomaterials for Biomedical Applications
Poliraju Kalluru1 and Raviraj Vankayala2
1Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4, Canada
2Department of Bioscience and Bioengineering, Indian Institute of Technology Jodhpur, Karwar, Rajasthan, 342037, India
3.1 Introduction
Over the past few decades, nanomaterials have played a vital role in the development of scientific and technological disciplines. Among various nanomaterials, two‐dimensional (2D) nanomaterials have attracted significant attention in the field of biomedicine [1]. For instance, graphene oxide (GO)/reduced graphene oxide (rGO), transition metal chalcogenides, MXenes, black phosphorous (BP), boron nitride, and carbon nitride‐layered nanostructures have some unique features such as high surface area, conductivity, mechanical property, and hydrophilic surface functionality which would enrich the biocompatibility of the 2D nanomaterials in their respective biomedical applications [2–6]. In this book chapter, we aim to describe the importance of the 2D nanomaterials for bioimaging, phototherapy (photothermal and photodynamic), drug/gene delivery, biosensors, antibacterial activity, tissue engineering, and regenerative medicine and their potential considerations for future clinical translation will be elaborated.
3.1.1 Photothermal and Photodynamic Therapy
In general, the 2D nanomaterials exhibit broad absorption ranging from visible to near‐infrared (NIR) region due to their intrinsic few nanolayered conjugation structures. To this end, several methodologies were attempted to obtain few layered 2D nanosheets (NSs) of various nanomaterials [7]. In this regard, Hummers process is well known to exfoliate the bulk 2D graphite flakes into nanosized GO sheets and the GO has lower molar absorption at NIR wavelength region. To improve the molar absorption at NIR wavelength, the as‐synthesized nanographene oxide (nGO) further reduced using strong reducing agents or through thermal heating in order to ameliorate the absorption coefficient value at NIR wavelength. Hongjie Dai and his coworkers demonstrated that rGO sheets have high NIR absorption and the amphiphilic surface functionality can be modified with polyethylene glycol (PEG) and RGD peptide to improve the cellular uptake of U87MG cancer cells and further to mediate the photodestruction of cancer cells upon 808 nm laser irradiation. This is primarily due to the absorption of the excited state energy by nano‐rGO, which can then be converted to thermal heat [8]. In order to overcome the blood brain barrier (BBB) and also to eradicate the brain tumors, Jingjing and coworkers have reported that the organic porphyrin was immobilized onto the nano‐GO surface to improve the molar absorption at NIR wavelength and further conjugated with tripeptide L‐arginyl‐glycyl‐L‐aspartic (RGD) peptide to overcome the BBB to target the brain tumor [9].
Zhang and coworkers have reported that Chlorine e6 (Ce6)‐loaded PEGylated GO exhibits the enhancement of singlet oxygen (1O2) generation in twofold as compared with Ce6 alone upon 660 nm laser irradiation and further illuminated with 808 nm laser to mediate photothermal effect induced by the enhancement of the photodynamic therapy (PDT) to achieve superior cellular deaths upon double laser illumination [10]. The rGO was decorated with Ru complex and engulfed with PEG to mediate the combination of photothermal therapy (PTT) and PDT, which can kill cancer cells under 450 nm (PDT) and 808 nm (PTT) laser irradiation. The rGO–Ru–PEG nanoplatform induced the apoptosis by the generation of reactive oxygen species (ROS) and further ameliorated the cathepsin for the apoptotic signal pathway under light illumination, and the A549 tumor‐bearing nude mice have shown considerable tumor suppression with combined PDT/PTT therapy upon 450 and 808 nm laser irradiations (Figure 3.1a) [11]. The 2D BP NSs were utilized as PTT/PDT agents [15], and the exfoliated BP NSs were exhibited with high singlet oxygen quantum yield of ∼0.91 singlet oxygen phosphorescence emission that was observed at below 1270 nm wavelength as compared with Rose Bengal; it is due to surface environment of BP NSs. The high singlet oxygen quantum yield BP NSs were employed as photodynamic therapeutic agent for the suppression of breast cancer tumor growth upon 660 nm laser irradiation (Figure 3.1b) [12]. Treating deep tissue buried tumors is an unmet need in biomedical clinics. The BP NS was conjugated with photosensitizer (PS) (Ce6) to generate the singlet oxygen upon visible light irradiation, and the BP NSs have high absorbance at visible to NIR region, and upon 660 nm laser irradiation, high photothermal conversion was observed and the synergistic effect induced the effective killing of cancer cells [16]. Xiaochen and coworkers demonstrated that BP NSs were coated with polydopamine and covalently conjugated with PSs (Ce6) and TPP (triphenylphosphonium) to induce the cancer cell killing. The combined PTT and PDT have shown considerable tumor inhibition of in vivo results [17]. To demonstrate, the BP NSs were employed as chelating agents to capture the toxic metal ions at neuronal environment and the BP NSs overcome the BBB upon NIR laser irradiation to induce the BP NS uptake in brain neuronal cells to reduce the neurotoxicity from the toxic metal such as Cu2+ ion [18]. Jianlin and coworkers demonstrated that Nb2C NSs were coated with polyvinylpyrrolidone (PVP), in order to improve the biocompatibility and hydrophilicity to utilize for the in vitro and in vivo studies. The Nb2C–PVP NSs have shown broad NIR absorbance at longer wavelength; the first bio‐window (NIR‐I) has exhibited ∼36% photothermal conversion efficiency upon 808 nm laser irradiation, and simultaneously second bio‐window (NIR‐II) has shown ∼45% photothermal conversion efficiency upon 1064 nm laser irradiation; those features imparted the photothermal tumor ablation at NIR‐I and NIR‐II, and the tumor eradication was observed in mouse tumor xenografts by the respective phototherapy (Figure 3.1c) [13]. The aluminum oxoanion‐functionalized Ti3C2 NSs were employed as a photothermal therapeutic agent [19], and the multifunctional titanium carbide NSs were utilized as a PTT, PDT, and chemotherapeutic agent for the controlled release of drug by heat stimuli‐responsive polymer degradation upon 808 nm laser irradiation, and further more singlet oxygen generation was attributed during therapy in vitro and in vivo [20].
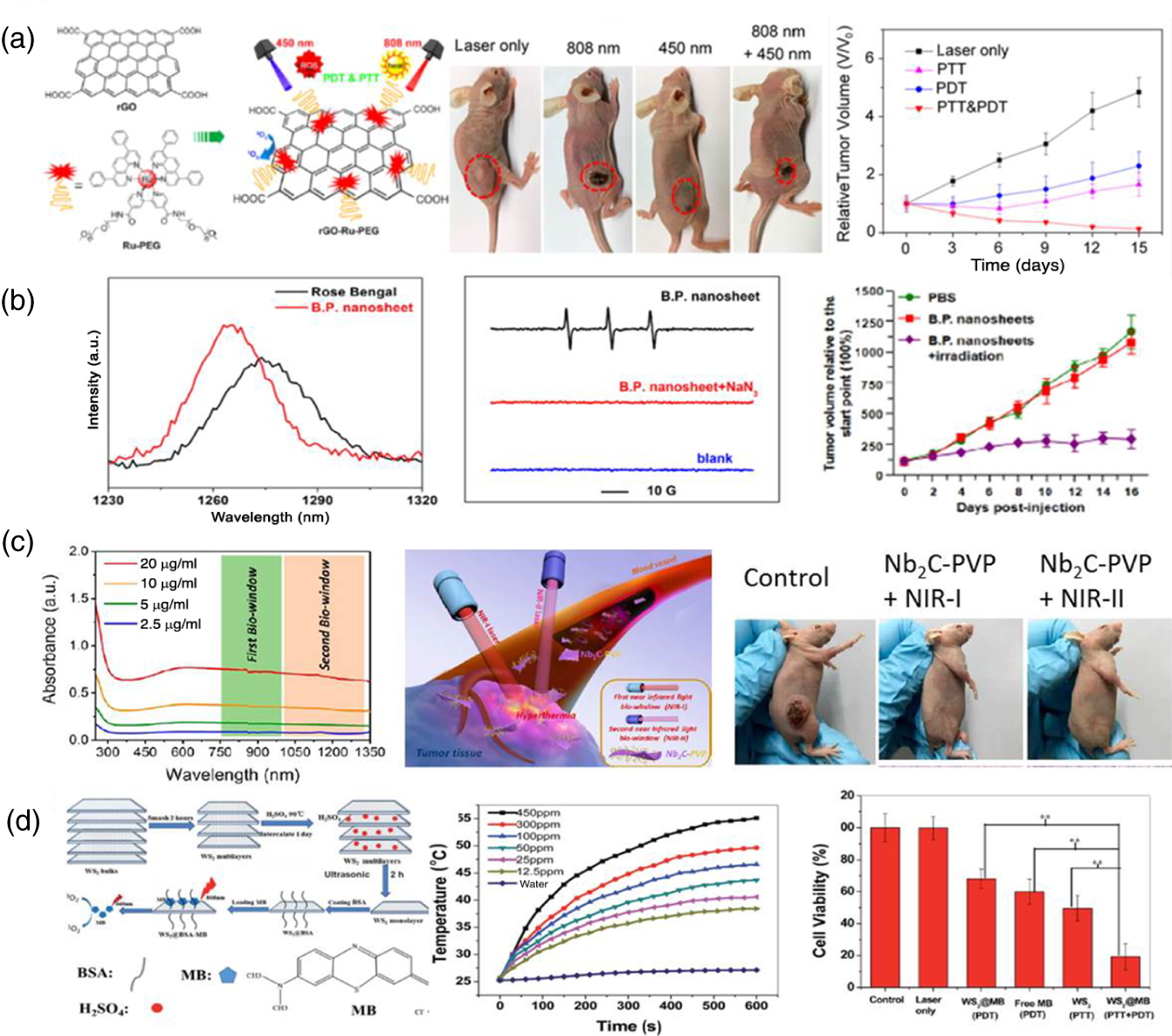
Figure 3.1 (a) rGO‐Ru‐PEG for PTT and PDT therapeutic effect by laser irradiation in order to suppress the tumor growth.
Source: Zhang et al. [11]. Reproduced with permission of American Chemical Society
. (b) Singlet oxygen phosphorescence emission spectra and the detection of singlet oxygen by EPR analysis and tumor growth inhibition was observed upon laser treatment by inoculate with BP nanosheets.
Source: Wang et al. [12]. Reproduced with permission of American Chemical Society.
(c) Nb2C–PVP employed as a NIR‐I and NIR‐II bio‐window PTT therapeutic agent.
Source: Lin et al. [13]. Reproduced with permission of American Chemical Society.
(d) WS2–BSA utilized as a PTT and PDT therapeutic agent and cell viability study.
Source: Yong et al. [14]. Reproduced with permission of Royal Society of Chemistry.
The hydrophilic WS2 NSs were wrapped with a layer of bovine serum albumin (BSA) protein coating, and it was featured with high surface area and NIR absorption to mediate photothermal and photodynamic therapeutic capabilities. Upon 808 nm photoexcitation, the BSA‐wrapped WS2 NSs were able to convert the excited state energy into heat. Furthermore, the methylene blue PS was physically adsorbed onto the surface of BSA‐wrapped WS2 NSs for the subsequent generation of singlet oxygen upon 660 nm light irradiation to mediate the combination of PTT and PDT synergistic therapy (Figure 3.1d) [14]. The PVP assisted exfoliation of MoSe2 NSs in which the PVP was employed as a stabilizer in aqueous environment. The hydrophilic PVP–MoSe2 NSs were treated with HeLa cells, and upon 808 nm laser irradiation, it has induced the killing of cancer cells by PTT [21]. The functionalized hexagonal boron nitride NSs (h‐BN NSs) were conjugated with DNA oligonucleotide and Cu(II) phthalocyanine. It was utilized as a photodynamic therapeutic agent to completely eliminate the early‐stage breast cancer tumor at lower dose of CuPc@HG@BN (25 μg/ml) NSs upon 655 nm laser irradiation [22]. Overall, it is clear that 2D nanoplatform was mainly attributed to the broad absorption at visible to NIR wavelengths, and this phenomenon has proven that upon photoexcitation, 2D NSs absorb the incident light and converted to either heat or via energy transfer process by the PS to enhance the generation of singlet oxygen (ROS).
3.1.2 Bioimaging and Drug/Gene Delivery
Generally, a bulk 2D material does not exhibit good molar absorptivity in the visible to NIR region due to its stacked layer structure. The exfoliated 2D NSs possess high molar absorption coefficients and enhanced optical properties owing to their few layers of thickness. The nanosized GO/rGO has appeared with absorption at visible to NIR wavelength due to π–π electron conjugation of aromatic sp2 carbon atoms [23]. It is well known that GO itself is an fluorescent material and, on the other hand, GO and rGO can also act as fluorescence quenchers upon interactions with organic dyes due to fluorescence resonance energy transfer (FRET) or non‐radiative dipole–dipole coupling takes place between fluorescent probe and GO/rGO NSs [24]. In order to improve the performance of the graphene nanomaterials in biomedical applications, the GO and rGO surface was modified with PEG, polyethylene imide (PEI), and antibody [25]. The nanoGO was conjugated with PEG to improve the circulation time in order to reach the target site, and GO–PEG was intravenously injected to the mice; further deep tissue penetration was monitored using two‐photon luminescence imaging in mice brain up to 300 μm depth limit. The advantages of the two‐photon imaging are its superior tissue penetration depths as compared with one‐photon imaging, reduced scattering, and minimal autofluorescence [26]. The tumor‐targeted radioactive molecule labeled on GO NS platform has utilized to detect the 4T1 breast tumors using positron emission tomography (PET) imaging to visualize the accumulation of radioactive GO NSs at the tumor site [27]. The unique feature of GO NSs is that the long‐chain sp2 carbon atoms conjugation can facilitate better interaction with aromatic structured drugs and the π–π stacking interactions between GO sp2 carbon surfaces and drug aromatic rings to form the π–π stable complex formation in order to avoid the covalent conjugations [28]. The negatively charged GO NSs wrapped with positively charged PEI polymer have employed as plasmid DNA carriers and attained very high intracellular gene transfection efficiency in HeLa cells [29]. The nGO was effectively uptaken by NIH‐3T3 cells, and the internalization of nGO was quantitatively evaluated through label‐free digital holographic imaging technique to save the time and hazardous sampling techniques (Figure 3.2A) [30].
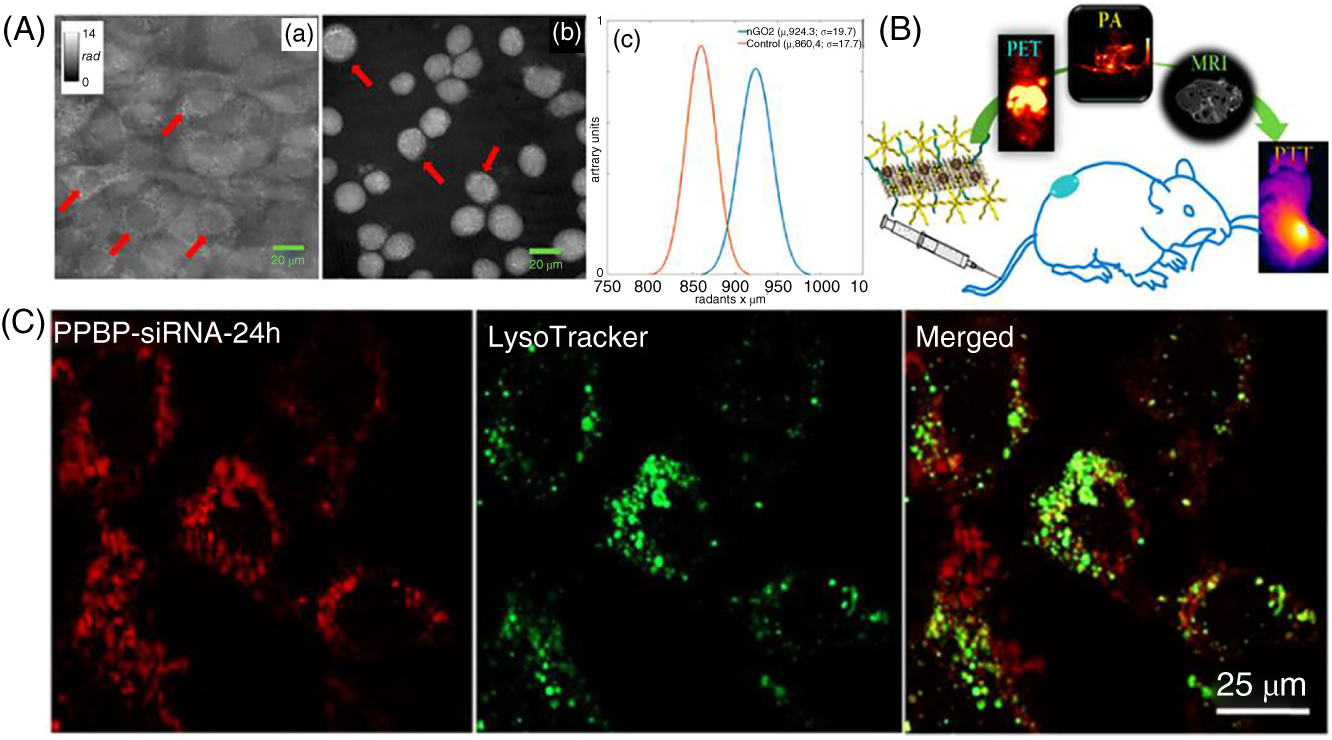
Figure 3.2 (A) Holographic imaging technique for the quantitative live cells estimation.
Source: Mugnano et al. [30]. Reproduced with permission of American Chemical Society
. (B) MoS2 as a triple model imaging agent.
Source: Liu et al. [31]. Reproduced with permission of American Chemical Society
. (C) Cellular uptake and endosome escape investigation while delivered the Cy7‐labeled free siRNA or PPBP–siRNA.
Source: Chen et al. [32]. Reproduced with permission of American Chemical Society
.
In situ growth of superparamagnetic Ti3C2 NSs was demonstrated as a theranostic agent, and the magnetic Ti3C2–Fe3O4 composite material was demonstrated as the T2‐weighted MR imaging contrast agent to monitor the 4T1 breast cancer tumors in nude mice model [33]. The ultrathin MXene NSs showed moderate absorption at NIR wavelengths which makes them suitable for biomedical applications. The titanium carbide NS surface was modified with soybean phospholipid (SP) in order to improve its stability under physiological conditions. Further, an anticancer drug (Doxorubicin) was loaded to obtain Dox@Ti3C2‐SP and was appeared with high amount of drug released in 4T1 cells upon NIR laser irradiation, and the synergistic effect has shown effective killing of cancer cells. The Ti3C2–SP endows with high photothermal conversion ability leading to high thermal energy which favors to the enhanced photoacoustic (PA) contrast which demonstrated in 4T1 tumor‐bearing mice model and the high‐intensity PA imaging signals at four hours time point upon intravenous injection of TiC3C2–SP [34]. The lipoic acid–PEG‐conjugated WS2 NSs were employed as theranostic agents, and the WS2–PEG was injected intravenously and intratumoral to the 4T1 tumor‐bearing mice to compare the targeting ability. The WS2‐PEG‐injected mice were further attributed to the enhancement of photoacoustic tomography (PAT) and X‐ray computed tomography (CT) imaging at tumor site [35]. The Sb2Se3 NSs were modified with PVP in order to improve the biocompatibility and physiological stability. It was demonstrated as an inefficient PA image‐guided agent upon 808 nm excitation wavelength to monitor the accumulation of Sb2Se3–PVP at tumor environment, and the increased PA single intensity was observed after intravenously injected Sb2Se3–PVP NSs at six hours time point [36]. The chitosan‐decorated MoS2 NSs have further loaded with doxorubicin and utilized as a drug delivery vehicle for the release of drug upon 808 nm laser irradiation and the heat stimuli‐controlled drug release upon NIR light illumination. Generally, molybdenum metal would absorp the X‐ray source, this feature has been utilized to monitor the CT imaging upon X‐ray illumination, and MoS2 NSs have shown better CT contrast imaging ability in phantom mode [37]. The MoS2 nanoplatform was utilized as a triple model imaging agent, and for that, iron oxide nanoparticles were self‐assembled onto MoS2 NS defect sites and further functionalized with lipoic acid‐terminated PEG and further anchored with six‐arm PEG, and subsequently 64Cu isotope was labeled with chelating agents on the surface of the MoS2 nanoplatform. The triple functional MoS2 composite material was employed as a PAT, magnetic resonance, and positron emitting tomography imaging agent to visualize the 4T1 breast tumor‐bearing mice. The PA imaging obtained from the thermal energy which was elevated from the MoS2 NSs and the negative contrast magnetic resonance imaging from the super paramagnetic iron oxide nanocrystals and the 64Cu isotope have shown tumor contrast enhancement at three hours post injection of PET imaging, and it was due to the enhanced permeability and retention effect of cancer tumors (Figure 3.2B) [31].
The MoS2 was conjugated with hyaluronic acid (HA) and PEI and followed by the loading of anticancer drug (doxorubicin), and the nanocarrier was internalized with MCF‐7‐ADR cells to target the CD44 receptor which is overexpressed in drug‐resistant cancer cells; upon 808 nm laser irradiation, P‐glycoprotein (P‐gp) expression was downregulated in MCF‐7‐ADR cells; on the other hand, HA is degraded slowly by hyaluronidase at tumor microenvironment and as a result of that enhanced release of doxorubicin in cytoplasm which favors to greater extent of killing of drug‐resistant cancer cells. Later on, authors have demonstrated that HA‐ and PEI‐functionalized MoS2 NSs were labeled with 64Cu isotope in order to get the real‐time PET image of in vivo tumor [38]. The MoS2@CS@Dex nanocarrier employed as an intra‐articular injection to cure the osteoarthritis (OA) in controlled manner. It was fabricated by the functionalization of chitosan on the surface of MoS2 NSs and further coated with dexamethasone, and the fabricated nanocarrier was injected directly into the mouse joint cavity and the controlled release of drug delivered by remotely upon irradiation of NIR light. The therapeutic effect of MoS2@CS@Dex on the OA of mice joint cavity has exhibited anti‐inflammatory effect through downregulating the release of inflammatory cytokines such as TNF‐α and IL‐1β, and the expression of aggrecan, ADAMTS5, and MMP13 in the cartilage tissue immunofluorescence was observed in OA‐treated mice model [39]. The BP with P—P bonding and layer structure NSs was employed as a theranostic agent, and the PEG‐NH2‐functionalized BP NSs were loaded with Doxorubicin and Cy7 to target the tumor site. The BP NSs have shown therapeutic PTT effect upon NIR laser irradiation which induced the DOX release and inhibited the lysosomes and autophagy to kill the cancer cells; the NIR fluorescence accumulation was observed at the tumor site by PEGylated BP NSs which was loaded with Cy7; the strong fluorescence signals were attributed at one hour post injection, and after 12 hours post injection, Cy7 was cleared out from the mice body and then 24 hours later, dye accumulation was observed at only tumor site [40]. The degradation of BP NSs in cancer cells leads to the critical release of intracellular phosphate anions, and it induced the G2/M phase arrest which causes anti‐proliferation of cancer cells and biocompatible to the normal cells; the Doxorubicin‐conjugated BPNs are selective chemotherapeutic agents, and further suppression of tumor growth was observed in vivo mouse model study [41]. The biodegradable BP NSs were loaded with Cas9N3 ribonucleoprotein through electrostatic interactions to obtain a Cas9N3‐BP delivery platform, and it was targeted to the release of Cas9N3 complexes at the cytoplasm, and the uptake was happened through either endocytosis or direct membrane interaction pathway. The Cas9N3 complexes further directed by nuclear localization signals, and it promotes to the gene editing and gene silencing in cancer cells and tumor‐bearing mice [42]. The small‐interfering RNA (siRNA) human telomerase reverse transcriptase loaded onto BP NSs PEG and PEI composite nanomaterial such as PPBP, and it has shown better siRNA loading efficiency and with cell uptake. The PPBP released the siRNA at cytoplasm by the lysosomal escape and further achieved with effective gene silencing upon photothermal heat treatment which leads to the synergistic effect, suppression of tumor growth and metastasis was observed in mice model (Figure 3.2C) [32].
3.1.3 Biosensors
Graphene has a sp2 hybridized carbon atoms with repeated conjugated structure, and it has a lightest, strongest, and transparent conductive material. It is emerging to protect the public health from the water‐borne diseases and food poisoning. To address this issue, Arben Merkoci and coworkers have employed GO as a solid phage acceptor of energy transfer for biosensing and it is due to π–π stacking of GO and it has detected the pathogens at 5 CFU/ml in PBS and tap water [43] and the nanoGO has demonstrated that to detect the unknown proteins at lower concentrations (Figure 3.3A) [44]. The hydrophilic GO has incorporated to PEDOT:PSS polymer to fabricate the pressure‐resistive composite films as sensors, and those were utilized to monitor the rat intracranial brain pressure and understand the different types of music in hearing aids (Figure 3.3B) [45]. Owing to high electrical conductivity, high surface area, hydrophilic nature, and tunable structure of 2D MXene nanomaterials utilize for biosensing applications [48, 49]. The titanium carbide NSs were functionalized with peptide probe, and it was devised to employ the assay of post‐translational modification (PTM) enzymes activity by the carboxypeptidase Y (CPY)‐mediated cleavage; it would be a useful tool as clinical diagnosis, and the peptide nanoprobes could cognize the switch on fluorescent detection of PMT (Figure 3.3C) [46]. The Ti3C2Tx NSs were coated on flexible polyimide device to sense the volatile organic gases such as ethanol, methanol, and ammonia at room temperature [50]. The hemoglobin‐immobilized Nafion–Hb–Ti3C2–GC electrode was utilized to detect H2O2 by Amperometric analysis, and in this method, hemoglobin (Hb) acts as a fast electron transfer agent to reduce the H2O2 during the electrochemical process [51].

Figure 3.3 (A) nGO utilized to detect the proteins.
Source: Chou et al. [44]. Reproduced with permission of American Chemical Society
. (B) PEDOT:PSS‐GO pressure sensor utilized for the real‐time intracranial surgery of rat and rat brain surface was monitored through R–P curve to investigate brain damage by the implanted device. Detection of sound vibrations by PEDOT:PSS‐GO device and detected different styles of music.
Source: Wang et al. [45]. Reproduced with permission of American Chemical Society
. (C) Titanium carbide as interface for the post‐translations modification enzymes activity detection assay.
Source: Wang et al. [46]. Reproduced with permission of American Chemical Society
. (D) Fluorescence quenching mechanism of 2D nanosheets and fluorescent probe (a) Detection of DNA. (b) Detection of Hg2+ metal ion.
Source: Wang et al. [47]. Reproduced with permission of American Chemical Society.
The WS2 NSs are a 2D layer structure, and it accommodated the space to assemble the bioprobes due to high surface area, hydrophilic surface functional groups, and the layered WS2 NSs were conjugated with the poly acrylic acid (PAA) and further the single‐strand DNA adsorbed on the NS surface, and it was detected through the fast quenching of fluorescent dye which is tagged to the ssDNA, and it has achieved to sensing of the particular protein and nucleic acid. The quenching phenomenon is occurred due to excited state energy transfer and static quenching interaction between a WS2 NSs and fluorophore (Figure 3.3D) [47], and the metal dichalcogenide NSs were constructed as Arg‐rich peptide probe [52]. The SnS nanoflakes were used as a NO2 sensing agent [53], and WS2 NS‐coated device has shown high sensitivity of NH3 detection in ppm level at room temperature [54]. The h‐BN NSs were chemical enzyme‐mimicked Pt NPs coated on the surface of h‐BNNs which was utilized for the oxidation of enzymatic peroxidase in the presence of hydrogen peroxide (H2O2), and the dopamine was employed as an inhibitor of oxidation which is ascribed by colorimetric detection of dopamine and it was sensed in micro molar level [55]. The fluorescence quenching mechanism was occurred by the photo‐induced electron transfer (PET) from the excited fluorophore via the electron transfer to the conduction band of the 2D nanoplatform. The fluorescence sensing strategy is a quick amplification tool to identify the DNA, toxic metal cations, proteins, and small molecules [47]. The BP NSs were employed as a biosensor for the detection of mRNA [56].
3.1.4 Antibacterial Activity
Graphene NSs have shown antibacterial activity, and the cytotoxicity is due to sharp edges of graphene NSs, which are caused by membrane stress by direct contact of bacteria. The four types of 2D graphene materials are GO, graphite oxide (GtO), rGO, and graphite (Gt), and out of these graphene materials, GO has more dispersion in aqueous medium due to strong hydrophilicity of GO and it favors to kill the highest bacteria as compared the other graphene materials. The graphite and rGO have shown higher oxidative conductivity as compared with GO and GtO, and it improved the oxidative stress which caused antimicrobial activity [57]. The antibacterial activity depends on the lateral size of the hydrophilic GO NSs, and the larger sheet would cover the bacteria cell surface and it might improve the antibacterial activity and the membrane rupture was observed on bacteria cell wall by AFM images with and without treatment of GO (Figure 3.4A) [58]. The GO NSs were conjugated with theantibacterial peptide G(IIKK)4I‐NH2 via layer‐by‐layer deposition. Further, sustained release of the peptide has facilitated the killing of the bacteria [61]. The wrinkled GO surface film has exhibited the appreciable amount of antibacterial property, and the wrinkled GO surface may trap the bacteria; it could create enough interaction space with cell membrane and due to mechanical stress induced the bacterial cell membrane damage [62], and the benzophenone‐grafted GO film has attributed as a chemically inert membrane surface to inactivate the bacteria [63].

Figure 3.4 (A) AFM images of with and without treatment of GO after two hours incubation.
Source: Liu et al. [58]. Reproduced with permission of American Chemical Society.
(B) Thiolated 1T and 2H‐MoS2 NSs UV–visible NIR spectra, (C) SEM images of thiolated 2H‐MoS2 NSs have shown antibacterial activity toward drug‐resistant MRSA bacteria (a, e) Control of MRSA. (b, f) MoS2‐L3 treated MRSA. (c, g) MoS2‐L7 treated MRSA and (d, h) MoS2‐L8 treated MRSA.
Source: Karunakaran et al. [59]. Reproduced with permission of American Chemical Society.
(D) LED light irradiation (E) SEM images of Pseudomonas aeruginosa and Staphylococcus aureus treated with smooth Au surface and electrophoretic deposited mesoporous g‐C3N4 surface (a) Rod shape P. aeruginosa and sphere shape S. Aureus on Au surface and (b) on EPD‐g‐C3N4 surface without irradiation. (c) Au surfaces with 90 min LED irradiation, (d) EPD‐g‐C3N4 surfaces with 90 min LED irradiation.
Source: Wang et al. [60]. Reproduced with permission of American Chemical Society.
Two‐dimensional MXenes played an attractive antibacterial activity, and the few layers titanium carbide colloids suspension has shown highest antibacterial behavior toward gram positive (Bacillus subtilis) and negative (Escherichia coli) bacteria at 200 μg/ml concentration for four hours interaction time as compared with GO, and it is due to direct contact of titanium carbide which can disrupt the bacteria cell membranes [64]. The colloidal Ti3C2Tx NSs with sharp edges which causes bacterial cell wall damage by the physical interaction between titanium carbide NSs and further more antibacterial activity depend on the size and time [65]. The WS2 NSs have shown antibacterial activity which is due to direct contact of WS2 NSs on the surface of bacteria membrane and further leads to the damage of bacteria membrane [66]. The MoS2 exists in two phases such as metallic (1T phase) and semiconducting (2H‐phase), and in this, 2H‐MoS2 has shown efficient antibacterial activity as compared with 1T‐MoS2 nanostructure. The thiol‐functionalized 2H‐MoS2 NSs were treated with drug‐resistant bacteria MRSA, showing efficient killing of MRSA bacteria, and it was due to release of ROS from the thiolated 2H‐MoS2 NSs (Figure 3.4B,C) [59].
The nitrogen plasma‐treated graphite carbide (N‐g‐C3N4) NSs were employed as bactericidal agents, and the antibacterial activity is more effective as compared with pristine graphite nitride (g‐C3N4), and it occurred due to improved hydrophilic surface environment of N‐g‐C3N4 sheets which favored to interact with bacteria cell wall and leads to high amount of pathogen death rate [67]. The porous and rough surface could accommodate more contact area to interact with bacteria in order to tear the cell wall; in addition to that upon 12 mW/cm2 light‐emitting diode, lamp irradiation improved the killing of pathogens more effectively and the g‐C3N4 exhibited as a photocatalytic bactericidal agent which generates the ROS upon light illumination (Figure 3.4D,E) [60]. The BN nanoflakes were mixed with low‐density polyethylene polymer to make the composite extruded biofilm sample, and it was evaluated to check the antibacterial activity [68] and the quaternary ammonium compound was grafted on h‐BN nanoplates and filled with polymer, and the quaternary functional group has exhibited the antibacterial activity [69]. A new solvent N,N‐dimethylpropyleneurea was introduced to exfoliate the BP NSs, and the NSs exhibited the antibacterial toward gram negative and gram positive bacteria such as E. coli and S. aureus species [70]. The ultrathin functionalized BP NSs are decorated with gold nanoparticles which have shown the enhanced antibacterial activity upon treatment of BPNs/Au NPs [71].
3.1.5 Tissue Engineering and Regenerative Medicine
2D nanomaterials have been exploited in the field of tissue engineering due to their surface, conductivity, and mechanical which alters to interact with polymers to form the biocompatible tissue scaffolds to improve the cell affinity [72]. The conducting rGO was incorporated with gelatin methacryloyl to form the composite rGO–GelMA scaffolds, and it exhibited better cell viability and proliferation of the cardiomyocytes which induces the cardiac tissue beating behavior and morphogenesis [73]. The combination of collagen and GO electrical conductive scaffolds utilized as a cardiac patch material to improve the signal propagation among the cardiac cells [74]. The double network tunable nanoengineered (NE) hydrogels were fabricated by the addition of BP NSs to create the mimicking of the extracellular matrix to enhance the tissue regeneration. The methacrylate‐decorated polymers were conjugated by chemical and physical interactions with gelatin and alginate; chitosan (polysaccharides) network polymers were cross‐linked through photo polymerization. The obtained double network further incorporated with BP NSs, and the obtained NE hydrogels enabled the mineralization which induce the CaP crystal formation toward tissue engineering and bone regeneration [75]. The 2D BP NSs were constructed into 3D printed scaffolds to the treatment of osteosarcoma, and the oxidized BP sheets favor to release PO43− anion ligands and further extract calcium (Ca2+) ions from physiological environment which leads to the calcium phosphate nanoparticle formation [76]. The polydopamine‐BP NS was incorporated into gelatin methacryloyl hydrogel scaffolds to fabricate the electrical conductive 3D scaffolds to improve the mesenchymal stem cell migration into 3D scaffolds [77]. The negatively charged GO NSs were engulfed with BP to form the GO@BP, and it was further coated onto the positively charged poly(propylene fumarate) 3D scaffolds. The surface area accommodated by GO NSs which increases the cell adhesive property and the BP would release the phosphate ions of 3D scaffolds to promote the osteogenesis cell proliferation and new bone formation [78].
MXenes are attributed with physiochemical and biocompatibility which favors the osteoinductivity and guided bone regeneration by MXene film in vivo bone tissue engineering [79]. The 2D Ti3C2 combined with bio‐glass (BG) material to construct the Ti3C2‐BG scaffolds accelerates the treatment of bone cancer killing and bone tissue regeneration. The bone tumor ablation was achieved by photothermal effect, and the bio‐glass has favored to induce the newborn bone tissue formation of the Ti3C2‐BG scaffolds [80]. Ultrathin Nb2C sheets are covered with S‐nitrosothiol‐grafted mesoporous silica in order to get the bioactive glass 3D printing porous scaffolds, and those were utilized for the destruction of bone tumors upon 1064 nm laser irradiation, and it induced optimal release of NO gas to increase the antitumor effect and subsequently bone tissue regeneration was observed in mice cranial defect site [81]. The MXene nanofiber composite was fabricated by the electrospinning method to develop the biocompatible and hydrophilic surface functional nanofiber network; smart biomaterial was utilized for the bone marrow‐derived mesenchymal stem cell tissue engineering and cell culture (Figure 3.5a [82]. The conductive Ti2C‐cryogel was employed as an engineering cardiac patch material toward the treatment of myocardial infarction. The titanium carbide NSs were mixed into the dopamine‐N,N‐methylene‐bisacrylamide, methacrylate‐gelatin, and poly(ethylene glycol) diacrylate mixture and then followed by the bath sonication, and the resultant composite gel was proceeded to treatment in myocardial infarction of rat model and the heart function was improved in myocardial infarction rat while introduced by Ti2C‐cryogel [84].
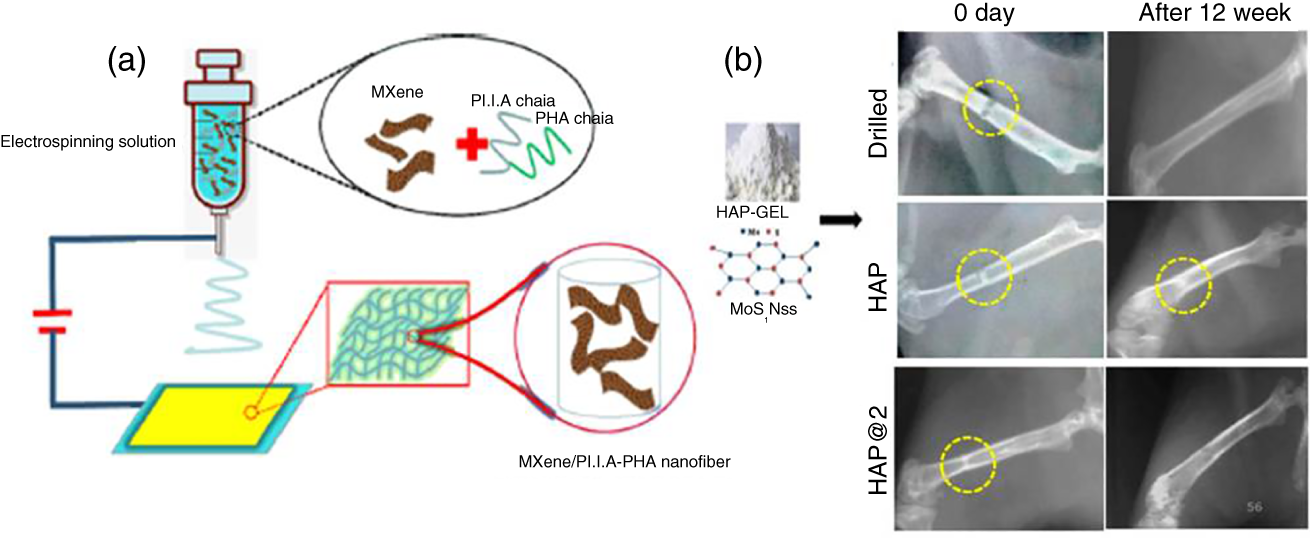
Figure 3.5 (a) Electrospinning method to fabricate the MXene composite.
Source: Huang et al. [82]. Reproduced with permission of American Chemical Society.
(b) The radiological images of HAP and HAP@2 scaffolds implanted in rat femur and bone regeneration monitored after 12 weeks.
Source: Yadav et al. [83]. Reproduced with permission of American Chemical Society.
The 2D MoS2 NSs were mixed with polyacrylonitrile to fabricate MoS2 composite nanofibers. The molybdenum disulfide fibers regulated the bone marrow mesenchymal stem cells proliferation, the alkaline phosphatase expression was increased by the increase of MoS2 concentration, and those findings give potential directions in development of tissue engineering and biomedical applications [85]. The MoS2 NSs combined with hydroxyapatite (HAP) gel to fabricate HAP@2 scaffolds and the implanted HAP@2 scaffold exhibited improved osteogenesis and bioresorbability as compared with HAP and drilled and the results were observed after 12 week post implantation (Figure 3.5b). Simultaneously, the HAP@2 scaffold increased cell adhesive and proliferations while treated with MG‐63 cells [83]. BN NSs combined with gelatin polymer to reinforce electrospun fiber mats were fabricated and further employed as bone tissue engineering application [86]. New bone formation takes place upon introduction with human bone marrow‐derived mesenchymal stem cells (hBMSCs), and it is a therapeutic approach to generate the bone formation. In order to improve the remarkable cell proliferation, osteogenic regulation is controlled by the runt‐related transcription factor (Runx 2) while imparted the C3N4 NSs were activated by red‐light illumination. The mice skull bone regeneration has occurred 91% within four weeks upon red light activation, and it happened due to deep tissue penetration of red light and the cell stimulation enhanced by the two‐photon excitation of C3N4 sheets near to the cells. The cytosolic Ca2+ ion accumulation occurred by the photo‐induced charge transfer, which promotes the synthesis of metabolic reactions and cell proliferation [87].
3.2 Conclusions
2D nanostructures have been extensively used in various biomedical applications, and specifically, they played a very prominent role in cancer therapy, biosensors, antimicrobial activity, tissue engineering, and regenerative medicine.
References
- 1 Chimene, D., Alge, D.L., and Gaharwar, A.K. (2015). Adv. Mater. 27: 7261–7284.
- 2 Chung, C., Kim, Y.‐K., Shin, D. et al. (2013). Acc. Chem. Res. 46 (10): 2211–2224.
- 3 Huang, K., Li, Z., Lin, J. et al. (2018). Chem. Soc. Rev. 47: 5109–5124.
- 4 Gogotsi, Y. and Anasor, B. (2019). ACS Nano. 13: 8491–8494.
- 5 Agarwal, V. and Chatterje, K. (2018). Nanoscale 10: 16365–16397.
- 6 Qu, G., Xia, T., Zhou, W. et al. (2020). Chem. Rev. 120: 2288–2346.
- 7 Liu, S., Pan, X., and Liu, H. (2020). Angew. Chem. Int. Ed. 59: 5890–5900.
- 8 Robinson, J.T., Tabakman, S.M., Liang, Y. et al. (2011). J. Am. Chem. Soc. 133: 6825–6831.
- 9 Su, S., Wang, J., Vargas, E. et al. (2016). ACS Biomater. Sci. Eng. 2: 1357–1366.
- 10 Tian, B., Wang, C., Zhang, S. et al. (2011). ACS Nano 5 (9): 7000–7009.
- 11 Zhang, D.‐Y., Zheng, Y., Tan, C.‐P. et al. (2017). ACS Appl. Mater. Interfaces 9: 6761–6771.
- 12 Wang, H., Yang, X., Shao, W. et al. (2015). J. Am. Chem. Soc. 137: 11376–11382.
- 13 Lin, H., Gao, S., Dai, C. et al. (2017). J. Am. Chem. Soc. 139: 16235–16247.
- 14 Yong, Y., Zhou, L., Gu, Z. et al. (2014). Nanoscale 6: 10394–10403.
- 15 Jana, D., Jia, S., Bindra, A.K. et al. (2020). ACS Appl. Mater. Interfaces 12: 18342–18351.
- 16 Yang, X., Wang, D., Shi, Y. et al. (2018). ACS Appl. Mater. Interfaces 10: 12431–12440.
- 17 Yang, X., Wang, D., Zhu, J. et al. (2019). Chem. Sci. 10: 3779–3785.
- 18 Chen, W., Ouyang, J., Yi, X. et al. (2018). Adv. Mater. 30: 1703458.
- 19 Xuan, J., Wang, Z., Chen, Y. et al. (2016). Angew. Chem. Int. Ed. 55: 14569–14574.
- 20 Liu, G., Zou, J., Tang, Q. et al. (2017). ACS Appl. Mater. Interfaces 9: 40077–40086.
- 21 Lei, Z., Zhu, W., Xu, S. et al. (2016). ACS Appl. Mater. Interfaces 8: 20900–20908.
- 22 Liu, J., Zheng, T., and Tian, Y. (2019). Angew. Chem. Int. Ed. 58: 775–7761.
- 23 Georgakilas, V., Tiwari, J.N., Kemp, K.C. et al. (2016). Chem. Rev. 116: 5464–5519.
- 24 Loh, K.P., Bao, Q., Eda, G., and Chhowalla, M. (2010). Nat. Chem. 2: 1015–1024.
- 25 Yang, K., Feng, L., Hong, H. et al. (2013). Nat. Protoc. 8 (12): 2393–2403.
- 26 Qian, J., Wang, D., Cai, F.‐H. et al. (2012). Angew. Chem. Int. Ed. 51: 10570–10575.
- 27 Hong, H., Yang, K., Zhang, Y. et al. (2012). ACS Nano 6: 2361–2370.
- 28 Bitounis, D., Boucetta, H.A., Hong, B.H. et al. (2013). Adv. Mater. 25: 2258–2268.
- 29 Feng, L., Zhanga, S., and Liu, Z. (2011). Nanoscale 3: 1252–1257.
- 30 Mugnano, M., Lama, G.C., Castaldo, R. et al. (2020). ACS Appl. Nano Mater. 3: 428–439.
- 31 Liu, T., Shi, S., Liang, C. et al. (2015). ACS Nano 9 (1): 950–960.
- 32 Chen, L., Chen, C., Chen, W. et al. (2018). ACS Appl. Mater. Interfaces 10: 21137–21148.
- 33 Liu, Z., Zhao, M., Lin, H. et al. (2018). J. Mater. Chem. B 6: 3541–3548.
- 34 Han, X., Huang, J., Lin, H. et al. (2018). Adv. Healthcare Mater. 7: 1701394.
- 35 Cheng, L., Liu, J., Gu, X. et al. (2014). Adv. Mater. 26: 1886–1893.
- 36 Zhou, Y., Feng, W., Qian, X. et al. (2019). ACS Appl. Mater. Interfaces 11: 19712–19723.
- 37 Yin, W., Yan, L., Yu, J. et al. (2014). ACS Nano 8 (7): 6922–6933.
- 38 Dong, X., Yin, W., Zhang, X. et al. (2018). ACS Appl. Mater. Interfaces 10: 4271–4284.
- 39 Zhao, Y., Wei, C., Chen, X. et al. (2019). ACS Appl. Mater. Interfaces 11: 11587–11601.
- 40 Tao, W., Zhu, X., Yu, X. et al. (2017). Adv. Mater. 29: 1603276.
- 41 Zhou, W., Pan, T., Cui, H. et al. (2019). Angew. Chem. Int. Ed. 58: 769–774.
- 42 Zhou, W., Cui, H., Ying, L., and Yu, X.‐F. (2018). Angew. Chem. Int. Ed. 57: 10268–10272.
- 43 Narváez, E.M., Hassan, A.‐R., and Merkoçi, A. (2013). Angew. Chem. Int. Ed. 52: 13779–13783.
- 44 Chou, S.S., De, M., Luo, J. et al. (2012). J. Am. Chem. Soc. 134: 16725–16733.
- 45 Wang, J.‐C., Karmakar, R.S., Lu, Y.‐J. et al. (2019). ACS Appl. Mater. Interfaces 11: 34305–34315.
- 46 Wang, S., Zeng, P., Zhu, X. et al. (2020). Anal. Chem. 92: 8819–8826.
- 47 Wang, Q., Wang, W., Lei, J. et al. (2013). Anal. Chem. 85: 12182–12188.
- 48 Lorencova, L., Gajdosova, V., Hroncekova, S. et al. (2019). Electroanalysis 31: 1833–1844.
- 49 Soleymaniha, M., Shahbazi, M.‐A., Rafieerad, A.R. et al. (2019). Adv. Healthcare Mater. 8: 1801137.
- 50 Lee, E., VahidMohammadi, A., Prorok, B.C. et al. (2017). ACS Appl. Mater. Interfaces 9: 37184–37190.
- 51 Wang, F., Yang, C.H., Duan, C.Y. et al. (2015). J. Electrochem. Soc. 162 (1): B16–B21.
- 52 Tapasztó, O., Balko, J., Puchy, V. et al. (2017). Sci. Rep. 7 (1): 10087.
- 53 Jannat, A., Haque, F., Xu, K. et al. (2019). ACS Appl. Mater. Interfaces 11: 42462–42468.
- 54 Brien, M.O., Lee, K., Morrish, R. et al. (2014). Chem. Phys. Lett. 615: 6–10.
- 55 Ivanova, M.N., Grayfer, E.D., Plotnikova, E.E. et al. (2019). ACS Appl. Mater. Interfaces 11: 22102–22112.
- 56 Zhou, J., Li, Z., Ying, M. et al. (2018). Nanoscale 10: 5060–5064.
- 57 Liu, S., Zeng, T.H., Hofmann, M. et al. (2011). ACS Nano 5 (9): 6971–6980.
- 58 Liu, S., Hu, M., Zeng, T.H. et al. (2012). Langmuir 28: 12364–12372.
- 59 Karunakaran, S., Pandit, S., Basu, B., and De, M. (2018). J. Am. Chem. Soc. 140: 12634–12644.
- 60 Wang, X., Lyu, C., Wu, S. et al. (2020). ACS Appl. Bio. Mater. 3: 2255–2262.
- 61 Cao, M., Zhao, W., Wang, L. et al. (2018). ACS Appl. Mater. Interfaces 10: 24937–24946.
- 62 Zou, F., Zhou, H., Jeong, D.Y. et al. (2017). ACS Appl. Mater. Interfaces 9: 1343–1351.
- 63 Kaneda, M., Lu, X., Cheng, W. et al. (2019). Environ. Sci. Technol. Lett. 6: 141–147.
- 64 Rasool, K., Helal, M., Ali, A. et al. (2016). ACS Nano 10: 3674–3684.
- 65 Shamsabadi, A.A., Gh, M.S., Anasori, B., and Soroush, M. (2018). ACS Sustainable Chem. Eng. 6: 16586–16596.
- 66 Liu, X., Duan, G., Li, W. et al. (2017). RSC Adv. 7: 37873–37880.
- 67 Cui, H., Gu, Z., Chen, X. et al. (2019). Nanoscale 11: 18416–18425.
- 68 Pandit, S., Gaska, K., Mokkapati, V.R.S.S. et al. (2019). RSC Adv. 9: 33454–33459.
- 69 Xiong, S.‐W., Zhang, P., Xia, Y. et al. (2019). Mater. Chem. Front. 3: 2455–2462.
- 70 Sun, Z., Zhang, Y., Yu, H. et al. (2018). Nanoscale 10: 12543–12553.
- 71 Wu, Q., Liang, M., Zhang, S. et al. (2018). Nanoscale 10: 10428–10435.
- 72 Menaa, F., Abdelghani, A., and Menaa, B. (2015). J. Tissue Eng. Regener. Med. 9: 1321–1338.
- 73 Shin, S.R., Zihlmann, C., Akbari, M. et al. (2016). Small 12 (27): 3677–3689.
- 74 Norahan, M.H., Amroon, M., Ghahremanzadeh, R. et al. (2019). J. Biomed. Mater. Res. A 107A (1): 204–219.
- 75 Wang, Z., Zhao, J., Tang, W. et al. (2019). Small 15: 1901560.
- 76 Yang, B., Yin, J., Chen, Y. et al. (2018). Adv. Mater. 30: 1705611.
- 77 Xu, C., Xu, Y., Yang, M. et al. (2020). Adv. Funct. Mater.: 2000177.
- 78 Liu, X., Miller, A.L., Park, S. et al. (2019). ACS Appl. Mater. Interfaces 11: 23558–23572.
- 79 Zhang, J., Fu, Y., and Mo, A. (2019). Int. J. Nanomed. 14: 10091–10103.
- 80 Pan, S., Yin, J., Yu, L. et al. (2020). Adv. Sci. 7: 1901511.
- 81 Yang, Q., Yin, H., Xu, T. et al. (2020). Small 16: 1906814.
- 82 Huang, R., Chen, X., Dong, Y. et al. (2020). ACS Appl. Bio. Mater. 3: 2125–2131.
- 83 Yadav, U., Mishra, H., Singh, V. et al. (2019). ACS Biomater. Sci. Eng. 5: 4511–4521.
- 84 Ye, G., Wen, Z., Wen, F. et al. (2020). Theranostics 10 (5): 2047–2066.
- 85 Taheri, N.S., Wang, Y., Berean, K. et al. (2018). ACS Appl. Nano Mater. 1: 337–343.
- 86 Nagarajan, S., Belaid, H., Bohatier, C.P. et al. (2017). ACS Appl. Mater. Interfaces 9: 33695–33706.
- 87 Tiwari, J.N., Seo, Y.‐K., Yoon, T. et al. (2017). ACS Nano 11: 742–751.
