17
Graphene and Its Analogous 2D‐Layered Materials for Flexible Persistent Energy Storage Devices in Consumer Electronics
Himadri Tanaya Das1,2, K. Hariprasad3, and T. E. Balaji4
1National Taipei University of Technology, Department of Chemical Engineering, No.1 Section 4, Roosevelt Rd., Taipei, 10607, Taiwan
2Utkal University, RUSA, Center of Excellence for Advanced Materials and Applications, Vanivihar, Bhubaneswar, 751004, India
3Institute of Aeronautical Engineering, Department of Physics, Dundigal, Hyderabad, 500043, India
4Bishop Heber College, PG & Research Department of Chemistry, Tiruchirappalli, 620017, India
17.1 Introduction
Nowadays, energy has become an essential part of our day‐to‐day life, things that surround us like laptops, mobile phones, televisions, etc., have made our modern life flexible and luxurious as aided with small compact energy storage devices (ESDs) [1]. Our current energy needs are satisfied by consuming more amount of nonrenewable resources like coal, natural gas, and fossil fuels. In order to fulfill our energy needs, these sources have highly depleted that reversibly led to environmental pollution. In this modern era, there is rapid mobility of ESDs toward compact wearable and flexible electronic gadgets like foldable mobile phones, tablets, and watches [2]. So, unavoidable energy‐based lifestyle demands more advance energy storage technologies. The main drawbacks currently we are facing are the energy source used in displays, sensors, and mobile phone (lithium ion batteries [LiBs]) are not flexible. Many researchers have been tremendously working to find an energy source for these electronics and these are expected to hold some characteristics like economically viable, flexible, strong, and eco‐friendly [3]. Mostly, there is demand of high energy and power density ESDs for potential electronics. Electrochemical energy is the cleanest form of energy in the energy portfolio due to its low cost, high efficiency, eco‐friendliness, and it is a renewable source of energy [4]. Some of the devices working under the principle of electrochemical energy are supercapacitors, batteries, fuel cells, and solar cells. The supercapacitors (SCs) have high energy density than the conventional capacitors and lower energy density when compared with the fuel cells and batteries, in fact supercapacitors act as a bridge between capacitors with high power density and batteries with high energy density. Batteries also show good electrochemical performance, but they suffer from poor cyclic stability and less power density.
Supercapacitors were developed in the early 1960s, still in the run of progress. Currently, available SCs have energy density less than 10 Wh/kg, which when compared to batteries it is three to five times lower [5]. Flexible supercapacitors (s) are the future of energy source for flexible and wearable electronic devices, many articles are being reported in the recent five years, and it has become a hot topic in research.
Figure 17.1 illustrates that flexible electronic devices are becoming an unavoidable part in our day‐to‐day life. For the development of flexible ESDs, there is need of flexible electrodes for ESDs. The novel material investigated by Geim and Novoselov in 2004, graphene has become the material of choice by many researchers and industrialist for flexible ESDs due to its extraordinary theoretical specific surface area and theoretical specific capacitance of 2630 m2/g and 550 F/g, respectively [7]. The 2D graphene sheets are widely investigated as electrode material for supercapacitor applications. Further, to improve the performance of electrode material the functionalization of graphene, nanocomposites with graphene, and three‐dimensional (3D) architectures of graphene have been cultured [8]. Such approaches to develop graphene‐based flexible electrodes material not only boost the intrinsic properties of graphene but also it provides a high specific surface area, excellent mechanical strength, and excellent electrical conductivity. In this chapter, the various graphene electrodes along with its nanocomposites are well discussed. The graphene introduced with other 2D‐analogous has come out as potential candidate for flexible electrodes has been confirmed by different researches. The technologies to develop the flexible devices have been focused extensively.
17.2 Brief Sketch of the Types of SC and Its Working Mechanism
The reason for supercapacitors dominating the conventional capacitors in terms of capacitance value is because of the highly reversible ion absorption mechanism [9]. Charge storage in electrodes follows the electrostatic attraction between the ions of the electrolyte and the charges present at the electrode surface which permits the formation of oppositely charged layers at the electrode/electrolyte interface. Helmholtz in 1953 described the charge separation at the electrode/electrolyte interface using the double‐layer capacitance [10];
where εr is the relative permittivity of electrolyte, ε0 is the vacuum permittivity, d is the relative thickness of the double layer, and A is the surface area of the electrode. From this, we can understand that capacitance is mainly dependent on the electrode surface area, the separation distance between the two electrodes (thickness), and on the properties of the electrolyte. Supercapacitors have two electrodes separated by an ion‐permeable membrane that acts as a separator and immersed in an electrolyte. During charging state, the cations and anions from the bulk move toward the negative and positive electrodes, respectively, to form an electrical double layer at the electrode/electrolyte interfaces [11].
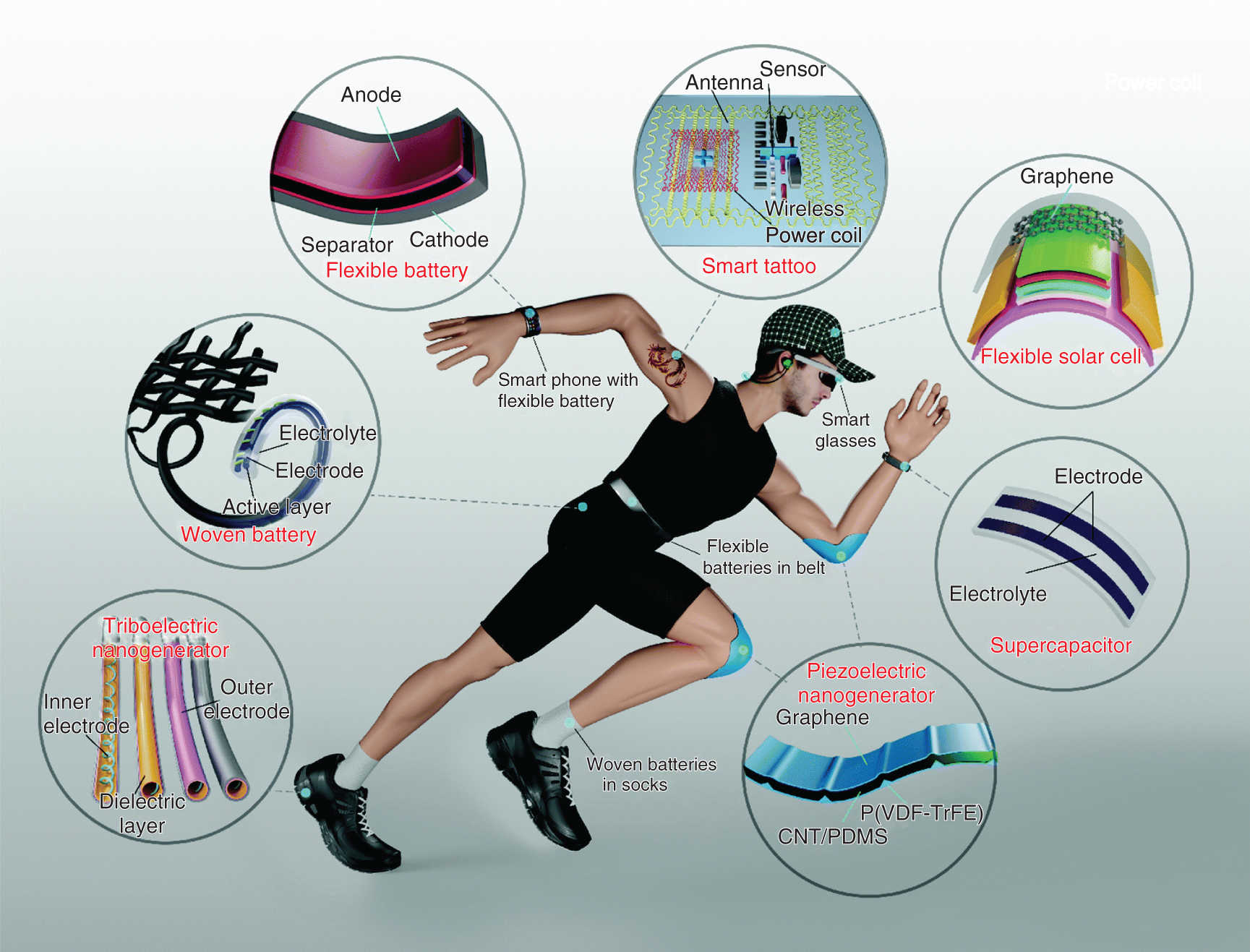
Figure 17.1 Role of flexible electronics in our day‐to‐day life.
Source: Adapted from Yi et al. [6]. Copyrights RSC, 2018.
In general supercapacitors stores charge by electric double‐layer formation or by surface charge transfer reactions so termed as electric double‐layered capacitors (EDLCs) or pseudocapacitors (PCs), as shown in Figure 17.2 [12]. It has been noticed that carbon‐based materials undergo electrical double layer (EDL) process of charge storage, whereas some metal oxides or chalcogenides such as RuO2, MnO2, WO3, or MoS2, etc. store charge via pseudocapacitive process [13]. PCs store electrons through reverse faradaic reactions occurring on the electrode/electrolyte interfaces [14]. Due to the structural instability associated with conducting polymers, PCs show short cyclic stability, low electrical conductivity, and very high rigidity of metal oxides results in low power density and poor flexibility [15]. PCs limits itself even columbic efficiency while EDLCs exhibit nearly 100% efficiency with long‐term cycling stability. The carbon‐based materials have been reported to deliver high energy and power density [16]. In order to achieve high electrochemical performance, high mechanical stability, long cycle‐life, and flexible behavior make carbon‐based materials as the best material for supercapacitor applications. Owing to the mechanical and structural stability, graphene has been exceptional material of choice for FSCs. Commercially, graphene‐based devices for various electronics dominate the supercapacitor market.
Researchers have been tremendously working on to increase the energy performance of SCs and to develop flexible SCs. As carbon‐based electrodes like carbon nanotubes (CNTs), graphene, and activated carbon (AC) are well‐known EDLCs, store charge via acquiring charge accumulation at the electrode/electrolyte interface via electrostatic interaction [16]. Carbon‐based electrode materials are economically viable, have high surface area for charge storage, and environmentally favored than the high cost and heavy metal oxides like RuO2 and RhO2 also these metal oxides are relatively harmful to the environment as well as human beings [17]. Among the carbon‐based electrode materials, CNTs and graphene are the most widely explored materials, CNTs have high electrical resistivity, and the pore structure is also complicated which resulted in slow ion transport. Graphene on the other hand is a single layer (2D) allotrope of carbon with carbon atoms connected in a honeycomb‐like structure that outperforms all other carbon‐based materials due to its high thermal stability, large surface area, and low electrical resistance, also graphene is the strongest material among any carbon‐based materials [18]. For these properties, graphene has triggered a “gold rush” in almost all fields of scientific research.
17.3 Evolution of Electrode Materials for Flexible Supercapacitors
Figure 17.3 shows the evolution of flexible electronics from concepts to consumable products, the trends in flexible electronics are growing every year. FSCs are an ultrafast rechargeable electrochemical ESD with several advantages like flexibility, high charge storage capacity, better cyclic stability, and high‐power output without sacrificing electrochemical performance [20]. In common, the three major structures of FSCs include fiber‐like, paper‐like, and foam‐like structures. Fiber‐like FSCs can be prepared using some of the precursors like metal wire, carbon fiber, polymer fiber, and composite fiber some of the examples of fibrous FSCs are ZnO nanofibers, CNT, MnO2@PPy [21]. Paper/fabric like supercapacitors are most favorable for supercapacitor applications due to the natural abundance, highly flexible, and eco‐friendly in nature. CNTs, graphene, and poly(3,4‐ethylenedioxythiophene) (PEDOT) are used in the paper‐like FSCs. The drawbacks of paper‐like and fiber‐like supercapacitors are the limited energy outputs. To address this issue, foam like electrode material was used which is a 3D porous structure that enhances the specific surface area and hierarchical porous. Commonly 3D materials are prepared by using carbon or carbon/polymers.
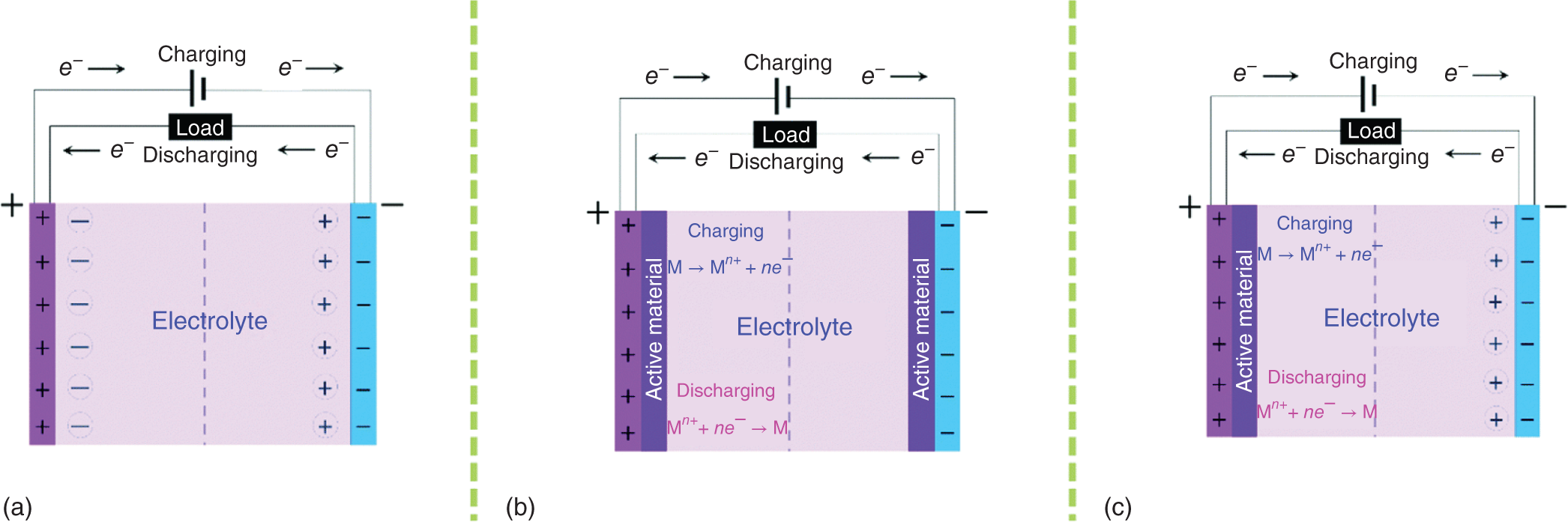
Figure 17.2 Types of supercapacitors and their charge storage mechanism. (a) EDLC, (b) pseudocapacitor, and (c) hybrid supercapacitor.
Source: Yi et al. [6].
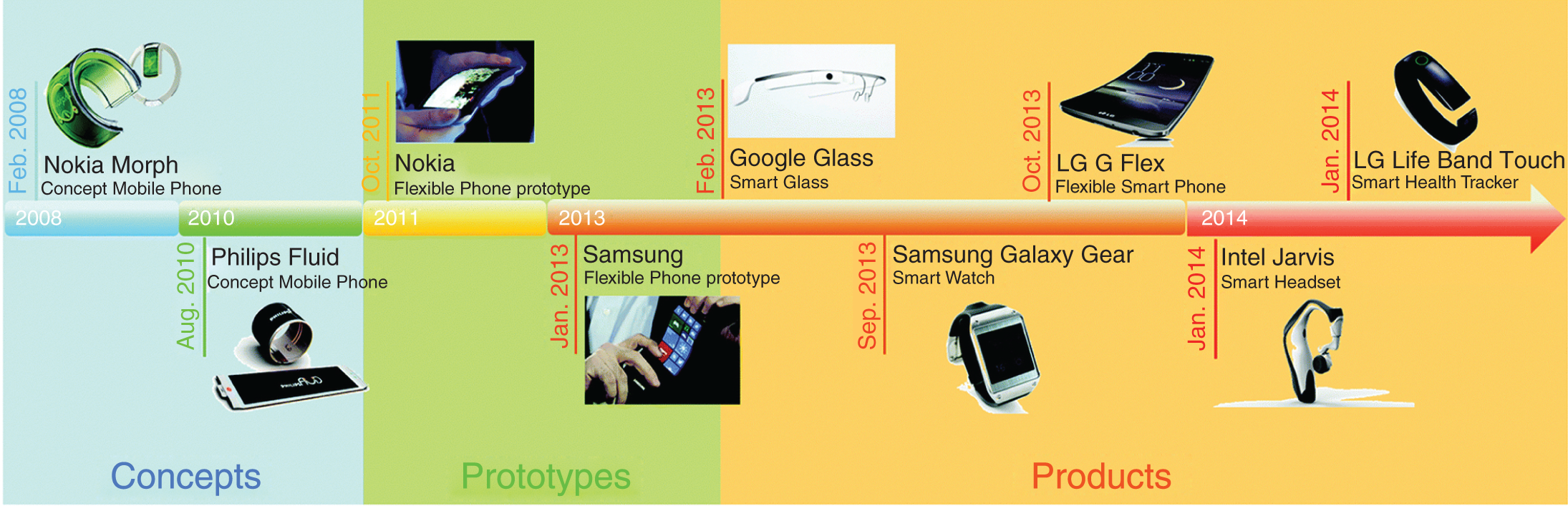
Figure 17.3 Evolution of flexible electronics from concepts to prototypes.
Source: Shao et al. [19].
Most of the conventional supercapacitors come with rigid electrode materials that are not flexible. The challenge put upon to the researchers is that they have to find a novel electrode material that not only shows better electrochemical performance but also remains stable even on bending, folding, or rolling [7]. Planar supercapacitors emerged as a better choice with decreasing the thickness along a vertical plane. 2D electrode materials such as graphene, and its composites are most favored for the application in FSCs owing to the properties like high specific surface area, numerous active sites for electrochemical reactions which enhances the capacity and power density, less thickness, highly flexible, and lightweight yet durable [6]. Since carbon materials are much lighter than the conducting polymers, and metal oxides, carbonaceous electrode material are preferred as electrode materials for FSCs. Among all other carbon‐based materials, graphene is a promising electrode material due to its better physical and electrochemical characteristics like maximum strength, thin single layered structure, and good cyclic stability and high specific capacitance due to the enhanced specific surface area. Also, the high electrical conductivity of graphene eliminates the need for any additives which enables the graphene with elevated energy density and cyclic stability.
Various approaches like reduction of graphene, chemical vapor deposition, and a ball mining method have been investigated for enhancing the electrochemical performance of graphene. Graphene has proved itself as a best material for supercapacitor application as approached in Figure 17.4, further its usage in the FSCs also turned out good results. Vivekchand et al. reported graphene material prepared by thermal treatment, and it showed a maximum specific capacitance of 117 F/g with H2SO4 electrolyte [23]. Further, Ruoff's group developed highly conductive and porous graphene oxide (GO) by chemical activation method, and the as‐synthesized material exhibited a specific surface area of 2400 m2/g with a higher energy density and specific capacitance of 26 Wh/kg and 120 F/g [24]. C.Z. Yuan et al. reported 3D reduced graphene oxide (rGO) that shows a maximum specific capacitance of 206 F/g [25]. This is higher than the 2D graphene the specific capacitance value due to the high specific surface area and low electrochemical resistance. Similarly, Yu and his group investigated the performance of graphene for FSC application, the synthesis of graphene followed a modified Hummer's method, and the obtained graphene showed ultrathin film‐like structure which exhibited a specific capacitance of 111 F/g at a scan rate of 10 mV/s for the 25 nm ultrathin films [26]. X. Fan et al. adopted chemical vapor deposition of graphene on Cu network which made the material both transparent and flexible electrode for supercapacitor [27]. The graphene network was covered by Cu network. This showed a good optical property of 86% transmittance at 550 nm wavelength and the specific surface capacitance was 4.2 μF/cm2. There are many morphologies obtained for CNTs like yarns, nanoribbons, and sheets. In order to explore other morphologies for graphene, Sun et al. developed graphene nanoribbons by a modified wet spinning method. The nanoribbon morphology was most suited for FSC due to its very thin nature and even after stretching; its cycling stability remains unchanged. The electrochemical studies outcomes a specific capacitance of 82.8 F/g at 2 mV/s [28]. In this basis, it is pertinent to develop the graphene‐based FSCs. Further, the graphene was made composite with various nanomaterials to boost the performance of the device.
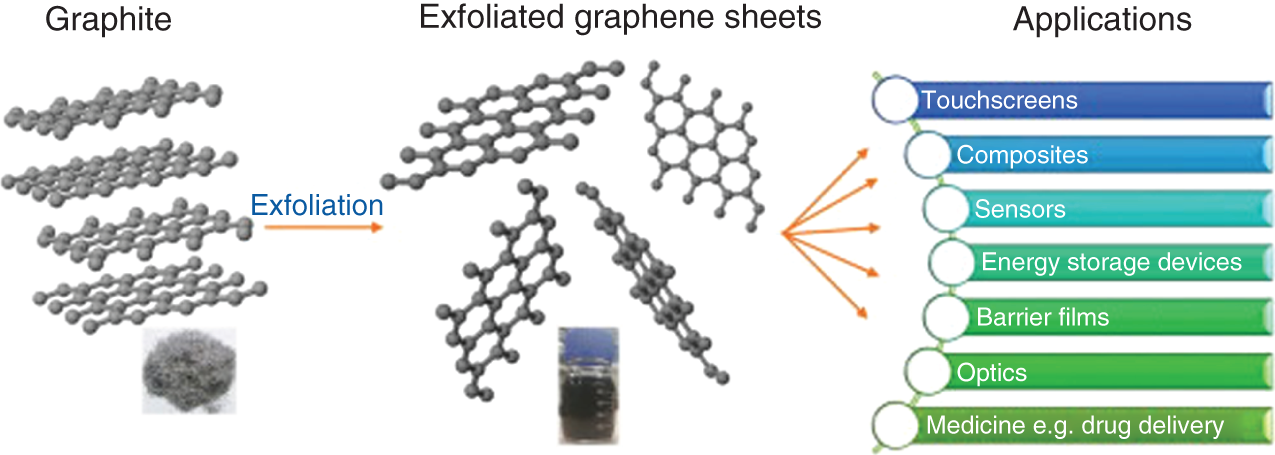
Figure 17.4 Schematic representation of application of graphene.
Source: Phiri et al. [22].
17.4 Developing Graphene Electrodes with Different Nanocomposites
17.4.1 Other Carbon‐Based Nanomaterials with Graphene
Graphene is also found as composite with other carbon‐based materials such as CNT or amorphous carbon, etc. [19] Both the CNTs and graphene show high power delivering rate capabilities and both synergistic effect can improve the capacitance, so in that order it can be combined as electrode material in FSCs. Cheng et al. proposed a new method for the synthesis of 1‐D CNT with 2‐D graphene [29]. The as‐synthesized material with fiber‐like morphology exhibited a high specific capacitance of 200 F/g and tested for flexibility by flat‐to‐bending cycles repeatedly. Initially, the capacitance was reduced in the initial stages due to the internal resistance, but gradually the capacitance retained after 200 bending cycles. This proves that the material is suitable for the application in flexible devices. However, combining CNTs and graphene is challenging and high price and complex preparation procedure of CNTs restrict its usage with graphene.
Wang et al. solved this issues by replacing CNTs by carbon black (CB), where the CB has good electrochemical properties at low price [30]. So, conductive CB composite with graphene was used for fabricating graphene hybrid films. As shown in Figure 17.5, the synthesis approach followed vacuum filtration process for generating rGO with CB. Along with the flexibility for the as‐synthesized rGO‐CB electrode, the restacking of rGO layers is also prevented. The CB improves the electrolyte ions diffusion when used as supercapacitor electrodes. The drawbacks in CB nanocomposites are agglomeration of CB takes place on increasing the CB concentration this might reduce the specific capacitance. The electrochemical performance of the fabricated solid‐state supercapacitor of rGO‐CB electrodes shows a high specific capacitance of 112 F/g with a good cyclic stability of 94% retention rate after 3000 cycles in normal state and 5000 cycles in bent state.
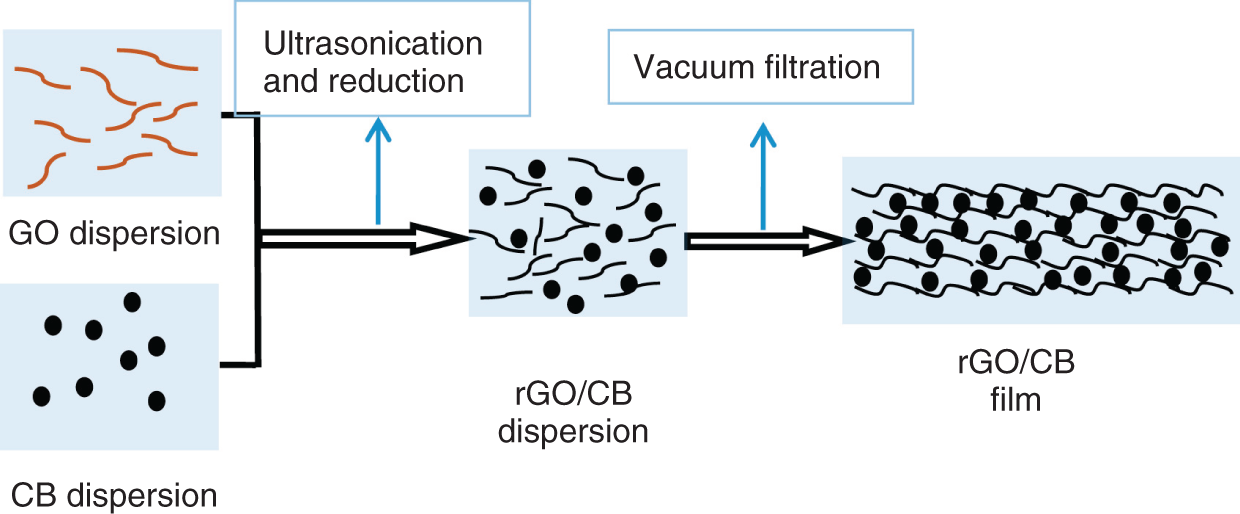
Figure 17.5 Schematic representation of synthesis of rGO‐CB.
Source: Wang et al. [30].
Even though rGO composite with CB reduced the restacking of layers but the agglomeration limits the CB usage in electrochemical applications as previously reported [31]. Interestingly, many carbon materials have been derived from renewable biomass based on human hairs, animal bones, food waste, and filter papers, etc. The biomass‐based carbon electrode is low cost, easily processable, highly porous, and good chemical conductivity. These features led to better supercapacitor properties. Huang et al. combined these biomass derived carbon material (BC) as carbon precursor to incorporate heteroatoms into carbon framework. The one‐step carbonization treatment fabricates the 3D N‐CNFs and reduces GO simultaneously. 3D N‐CNFs are deposited into the BC via vacuum filtration technique. The electrochemical studies revealed the high areal capacitance of 920 mF/cm2 at 2 mA/cm2 with a good cyclic stability of 99.6% retention after 10 000 cycles. The assembled asymmetric supercapacitor shows a maximum energy density of 0.29 mWh/cm2 at a power density of 1.5 mW/cm2 [32]. Similarly, Gong et al. reported the synthesis of carbon nanofiber (CNF)/rGO/CNT aerogel electrodes; the as‐synthesized electrode was tested for applicability to supercapacitors. The electrochemical studies proved the material with a high specific capacitance of 252 F/g with a good cyclic retention rate of 99.5% even after 1000 charge–discharge cycles. The electrode showed a maximum energy density of 8.1 mWh/kg and a power density of 2.7 W/kg [33]. The graphene nanocomposites with carbon improves the electrochemical performances but, its short fall in fabricating flexibility graphene‐based supercapacitors. As carbon materials got agglomerate during charging and discharging. So there is a search of proper composites with graphene for flexible electrodes.
17.4.2 Using Organic Composites with Graphene
Organic molecules like those that cellulose, benz[a]anthracene‐7,12‐quinone (BAQ), 2,5‐dihydroxy‐p‐benzoquinone (DHBQ), etc. has been used as a nanocomposite with graphene as a carbon support as well as a gelling agent for the graphene electrodes thus providing strength to graphene electrodes in FSCs [34]. Liu et al. incorporated paper as a substrate since the paper contains cellulose fibers (CFs), the natural features of CF make the substrate exceptional than the flat plastic substrates due to the presence of more functional groups present in CFs that binds with active electrode materials. The rough and porous structure makes the electron transport kinetics faster, proving CF as good substrate for FSCs. The low cost, recyclability, and environment friendly make the device effective. In this work, “dipping and drying” strategy is followed to coat the GO onto the surface of CFs in the paper followed by a hydrothermal process to assemble GO sheets into porous rGO. The morphological and flexibility tests show that the nanostructured rGO architecture in rGO/CF can be rolled up, bent, or twisted easily even it can be folded without causing any severe damage to the morphology. The as‐synthesized material was tested for applicability as electrode material, the results show that the material achieves a higher specific capacitance of 464 F/g, and the electrochemical properties of the material remained unchanged even at a very high bending state [35]. Cheng et al. developed a binder‐free graphene cellulose paper material for FSC application, and it showed excellent properties like large capacitance, low electrical resistance, and high strength the graphene nanosheets bonded with the CFs forming an interwoven structure. The functional groups present in the cellulose strongly binds with the graphene nanosheets that consequences in the distribution of electrolyte cellulose through the microporous structure of graphene and provides long‐term stability. The maximum value for the gravimetric capacitance is 120 F/g at a scan rate of 1 mV/s. The material showed a mechanical flexibility of only 6% decrease in electrical conductivity after 1000 cycles [36]. Thus, organic compounds were found to be effective nanocomposite with graphene, but the standardization of its synthesis is quite difficult.
17.4.3 Conductive Polymer with Graphene
On the better performance of graphene with organic polymer like cellulose, it invited researchers to work with graphene nanocomposite with various conductive polymers such as PEDOT, polyaniline (PANI), or PPy, etc. [37] Conducting polymers were widely applied in FSCs to bring the synergistic effect (electrical double‐layer behavior with pseudocapacitive behavior) of the electrode performance during charge storage. The problem with this is that the copolymerization of graphene suffers from polymeric aggregation and an elevation in electrode resistance due to poor interconnections. Shi et al. combined a conductive polymer, polyaniline with graphene through interfacial polymerization of aniline followed by ultrasonication of graphene with the synthesized polymer. The as‐synthesized nanofilms show high flexibility and high electrical conductivity than PANI. The active material shows a high specific capacitance of 210 F/g [38]. The most common method used in the preparation of PANI is in ‐situ chemical oxidative polymerization. There are several disadvantages seen in this method like, time consuming and complex procedure. To overcome this disadvantage, Zhu et al. proposed in situ electro‐polymerization method for the synthesis of graphene woven fabric (GWF) and polyaniline complexes, graphene was synthesized by chemical vapour deposition (CVD) method [39]. The applicability as electrode for supercapacitor revealed that the introduction of PANI material elevates the capacitance value from 2 to 23 mF/cm2, nearly 12 times higher than the free rGO. Even it showed a bended (112%) and curled (118%) rise in the areal capacitance increased that proved that the material is good for the applicability to FSCs [40].
Even though PANI showed high electrochemical performance, polypyrrole is less carcinogenic and its monomers are easily soluble in water. Yu et al. worked on the synthesis and electrochemical behavior of flexible polypyrrole/graphene through pulse electrodeposition, it showed better results due to the pyrrole monomer being diffused into the graphene, it results in uniform deposition of tiny polypyrroles through the porous graphene film, and it increases the exposed polypyrrole surface area on the graphene sheets. From this method flexible, uniform graphene/polypyrrole material was synthesized, and it delivered a specific capacitance of 237 F/g with a maximum energy density of 32.9 Wh/kg [41]. Even though the polymers, PPy is uniformly distributed the restacking of graphene nanosheets still prevailed that disturbed the compact structure and affect flexibility. There are two ways to overcome this problem, integrating porous graphene nanosheets into papers or sandwiching the fiber like nanomaterial between the graphene nanosheets. Lie et al. adopted the sandwiching of polypyrrole between graphene nanosheets. PPy was synthesized using surfactants to get uniform thickness and PPy/graphene is prepared by a simple vacuum filtration method followed by HI reduction. The PPy nanofibers are evenly distributed between graphene nanosheets and this reduced the restacking of graphene nanosheets. The as‐synthesized material showed excellent mechanical properties and the material showed a high specific capacitance of 345 F/g with a good cyclic stability of 93.6% retention after 1000 cycles [29]. Among these graphene mixed conducting polymers shows high specific capacitance but not achieved to graphene's theoretical value of 550 F/g calculated for single‐layer graphene [7]. Restacking of graphene sheets due to the strong sheet‐to‐sheet van der Waals interactions is considered as an obstacle in the synthesis and application process. An effort to avoid the restacking of the graphene sheets was put forward by Kaner et al. used a commercial light‐scribble digital video disk (DVD) optical drive to do the laser reduction of graphene oxide to graphene, this all solid‐state approach was expected to avoid the restacking of graphene sheets. Also, using this approach, the use of binder can be neglected. The resulting material showed an electrical conductivity of 1738 S/m (with 1520 m2/g surface area) also the material showed only 1% change in electrical reduction after performing 1000 bending cycles. The electrochemical performance of the graphene shows a specific capacitance of 265 F/g good charge discharge stability of 96.5% retention rate after 10 000 cycles also the material showed a high energy density and power density of 1.6 mWh/cm3 and 20 W/cm3. To avoid the agglomeration and restacking of graphene recently in the year 2020, N.M. Huang et al. reported the simultaneous exfoliation and reduction of graphene‐based materials by rapid microwave irradiation and compared it with graphene/PPy. The as‐synthesized graphene oxide shows porous structure and loose stacked layers of microwaved graphene. The G/PPy material was used as electrode for FSCs and the reported a high specific capacitance value of 137.2 F/g with a good cyclic stability of 89.8% retention rate after 1000 cycles. The device achieved a high energy density of 41.71 Wh/kg [42]. The composites with the organic or conducting polymer are compatible or the electrode fabrication in the FSCs, but they exhibited low specific capacitance. So for enhance the charge storage capacity, the researchers focused on metal oxides/hydroxides.
17.4.4 Combining Graphene with Other Metal Oxides/Hydroxides
Li et al. reported NiCo2O4/graphene synthesized by facile template‐free method. An asymmetric supercapacitor was assembled with the as‐synthesized material, and the device exhibited a maximum specific capacitance of 71.32 F/g with a good cyclic stability of 96.8% maintained after 5000 cycles. The device achieved a maximum energy density of 60.9 Wh/kg at a power density of 11.36 kW/kg [43]. Jin et al. worked on combining metal hydroxide with graphene, Co–Al layered and graphene are fabricated layer by layer. The electrode material was tested for the applicability to supercapacitors, and the results show a high specific capacitance of 880 F/g with no decrease in performance even after 2000 cycles [44].
Xie et al. synthesized β‐Ni(OH)2/graphene by solvothermal method as shown in Figure 17.6(i). The atomic force microscopy (AFM) image shows a thickness of ∼10 nm. However, the Ni(OH)2 arranged layer by layer by the stacking of graphene remains a challenge. The electrochemical studies show that the material has a good reversibility from cyclic voltammetry (CV) (Figure 17.6(ii)), and it exhibited high specific capacitance of 660.8 F/cm2 with a negligible capacitance degradation after 2000 cycles [45].
17.4.5 Combining Graphene with Other 2D‐Layered Materials
This layered materials store charge in between the 2‐D layers. The 2‐D materials are arranged in a layer‐by‐layer structure as shown in Figure 17.7. To prevent agglomeration as well as restacking of graphene layers, researchers have been gathered to find out various 2D‐layered materials to support graphene electrodes chemically and mechanically in FSCs. Lamberti et al. reported a one pot synthesis of MoS2‐decorated laser‐induced graphene. The MoS2 material showed a specific capacitance of 16 mF/cm2 with a good cyclic stability of no drop in capacitance up to 2000 cycles [46]. Gogotsi et al. investigated MXene/graphene as supercapacitor electrode material. Electrostatically assembled negatively charged MXene with positively charged rGO, minimized the aggregation and restacking issues. The material exhibited a higher electrical conductivity of 2261 S/cm and a high volumetric capacitance of 1040 cm−3 with no capacity degradation after 20 000 cycles. The assembled symmetric supercapacitor showed a high volumetric energy density of 32.6 Wh/l and an high power density of 74.4 kW/l [47].
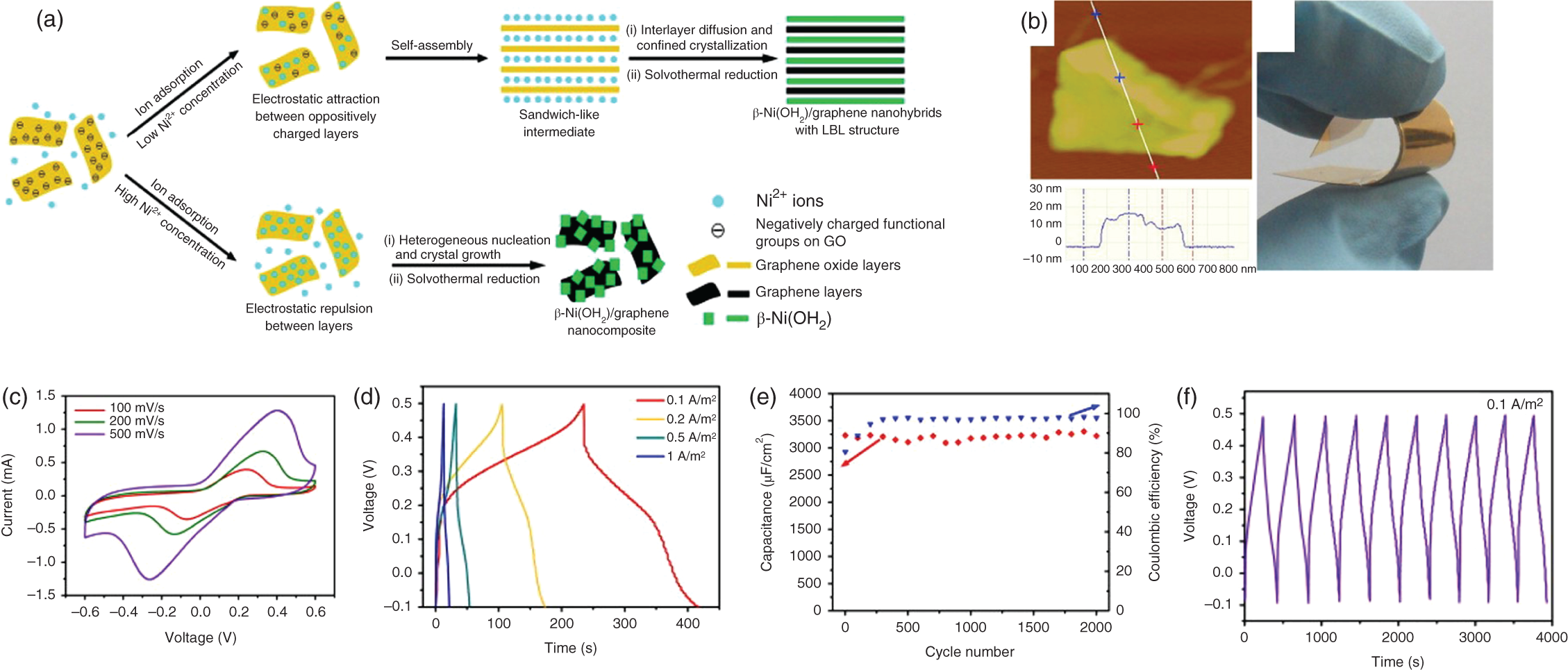
Figure 17.6 (a) Synthesis of Ni(OH)2/graphene nanocomposites, (b) The AFM image and photo showing flexibility, (c) Cyclic voltammetry curves. (d) Charge/discharge profile. (e) Cycle life for 2000 cycles (f) Few cycles of charge/discharge for NiOH2/Graphene electrode.
Source: Xie et al. [45].
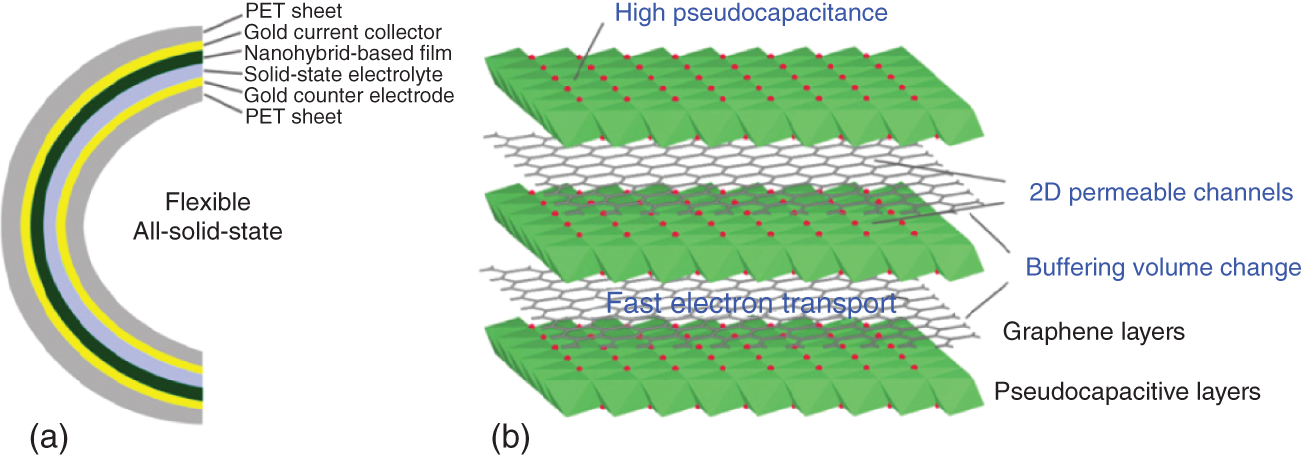
Figure 17.7 (a) Schematic representation of flexible all‐solid‐state thin‐film supercapacitors and (b) advantages of layer‐by‐layer of graphene and pseudocapacitive layers in electrochemical performance.
Source: Xie et al. [45].
Similarly, Min et al. reported tungsten disulfide/graphene, tungsten disulfide is prepared by solvent exfoliation and then mixed with graphene nanosheets. The as‐prepared material showed a high specific capacitance of 210.6 mF/cm2 with a good cyclic stability of 86.8% retention after 5000 cycles. The assembled device shows an energy density of 23.1 Wh/kg at a power density of 83.2 W/kg [48]. Similarly, Yang et al. proposed the synthesis of MXene/graphene‐based electrode material for supercapacitor application. The electrochemical studies show a high specific capacitance of 494.4 F/g with excellent cyclic stability over 3000 cycles. The assembled device showed high‐energy density of 13.03 mWh/cm3 at a power density of 0.59 W/cm3 [49].
17.5 Novel Technologies to Develop Flexible Graphene‐Based Supercapacitors
Some novel techniques have been developed in the recent times that can make the fabrication of flexible electrode materials easier. Some of the techniques like inkjet printing, roll to roll printing, and 3D printing are widely used to develop flexible electrodes of different design and shape and size. Printed flexible electrodes have become a trending topic due to advantages like very less usage of nanomaterials and an added advantage of the capability for integrating with rapidly emerging printed electronics. Lee et al. used graphene as ink for inkjet printing of flexible graphene‐based supercapacitor. Kapton was used as substrate, and the electrochemical properties of the material were investigated, and it shows a high specific capacitance of 192 F/g with a high energy density of 5.5 Wh/kg and a power density of 19 kW/kg [50]. Even though this method of synthesis did not match up with the theoretical values, but it has prevented the agglomeration of graphene sheets due to the all solid‐state approach. Followed by this approach, Liu et al. developed another strategy for the fabrication of FSCs using scalable, facile, and cost‐effective method.
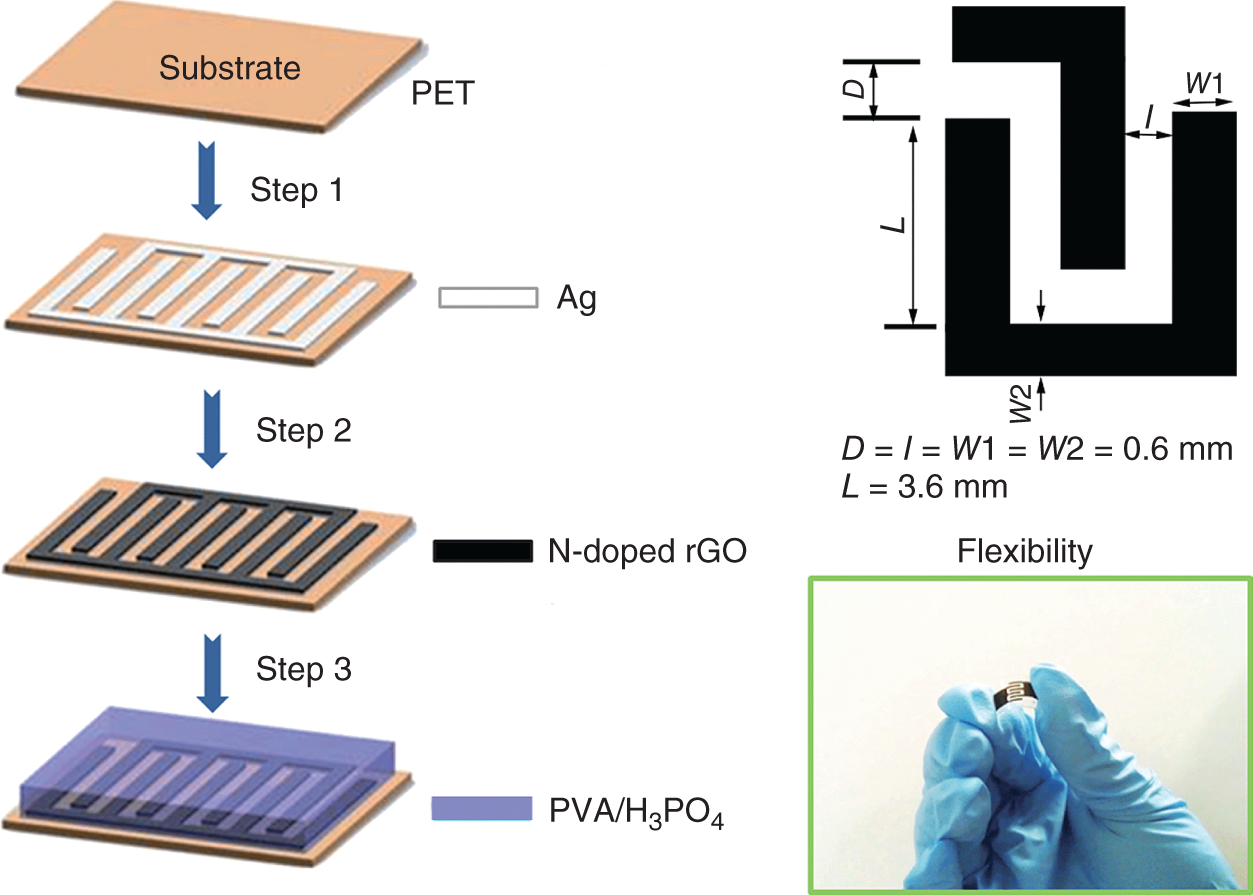
Figure 17.8 Schematic representation of the fabrication process of N‐doped P‐rGO.
Source: Adapted from Liu et al. [51]. Copyrights RSC, 2014.
This method follows the screen printing of the flexible all solid‐state supercapacitor using N‐doped reduced graphene as electrode material. The synthesis of N‐doped reduced graphene oxide follows a three‐step synthesis that includes a partial reduced graphene (P‐rGO) synthesis by a modified Hummers method followed by the formation of N‐doped P‐rGO intermediate and then finally redispersing it to obtain N‐doped rGO. Figure 17.8 shows the fabrication process of N‐doped P‐rGO. The mechanical stability of the fabricated electrode was tested by bending 180°, and the results showed that there is no significant change in the performance. The electrochemical studies shown the electrode materials achieved a maximum specific areal capacitance of 3.4 mF/cm2 with a good cyclic retention rate of 98.4% after 2000 cycles [51]. Similarly, one more printing technology was investigated by Hong et al., i.e. roll‐to‐roll printing of graphene–graphite carbon‐based supercapacitor, viz commercially available standard laser printer. The printed electrode material was tested for supercapacitor application, and it shows a high specific capacity of 100 mAh/g with a good cyclic stability of 55% at a current density of 5 A/g [52]. Besides these above reported literature, many more works have been explored as given in Table 17.1. Table 17.1 gives a clear view on the implementation of graphene nanocomposites for electrodes for the FSCs.
17.6 Conclusion
2D graphene nanosheets have been outraging all other carbon‐based materials, currently due to its own special properties like flexible nature, high mechanical strength, low internal resistance, and high specific surface area. Even though graphene showed higher electrochemical properties (theoretical capacitance value of 550 F/g) but not practically obtained yet due to the agglomeration and restacking of graphene nanosheets. These two issues are not very resolved as of now, but many researches have taken steps to increase its morphology through some modifications in synthesis methodology or composition. Where many technologies has come out to fabricated graphene electrodes for FSCs such as solid‐state, printing, or gel‐composite approach. Also, by incorporating these types of methods, the use of binder can be neglected that increases the specific capacitance of the graphene. By doing some modifications in the synthesis method and controlling the morphology, we may be able to meet with the theoretical value and by doing so we can fully incorporate free graphene without any dopants for the application FSCs since doping will result in decrease in stability of graphene.
Table 17.1 Table represents the graphene‐based electrodes for flexible supercapacitors.
| Material | Electrolyte | Specific capacitance | Cycle number | Cyclic retention (%) | Current density | Energy density | Power density | References |
|---|---|---|---|---|---|---|---|---|
| rGO | 1 M H2SO4 | 71.0 mF/cm2 | 5 000 | 96.7 | 10 mA/cm2 | 8.4 μWh/cm2 | 4900 μW/cm2 | [53] |
| Graphene/PANI | Polyvinyl alcohol (PVA)‐H3PO4 | 23 mF/cm2 | 2 000 | 100 | 0.1 mA/cm2 | 1.4 mWh/m2 | 0.03 mW/cm2 | [40] |
| rGO/MnO2/PANI | 1 M Na2SO4 | 636.5 F/g | 10 000 | 85 | 20 A/g | NA | NA | [54] |
| BiFeO3/graphene | 1 M Na2SO4 | 9 mF/cm2 | 5 000 | 95 | NA | 0.6 Wh/kg | 3.5 kW/kg | [55] |
| rGO/MoO3 | H2SO4/PVA | 617 F/g | 6 000 | 87.5 | 6 A/g | 55 Wh/kg | 400 W/kg | [56] |
| Graphene/polypyrrole | PVA‐H3PO4 | 363 F/cm3 | 12 000 | 98.6 | 1.0 mA/cm3 | NA | NA | [57] |
| Ni(OH)2/rGO/BC | 2 M KOH | 877.1 F/g | 15 000 | 93.6 | 50 mA/cm2 | NA | NA | [58] |
| V2O5/multi‐walled carbon nanotube (MWCNT)/graphene | 2 M KCl | 2590 F/g | 5 000 | 96 | 1 A/g | NA | NA | [59] |
| MnFe2O4/graphene | H2SO4/PVA | 300 F/g | 5 000 | 105 | 5 A/g | NA | NA | [60] |
| CNT/MnO2 | 1 M Na2SO4 | 488.6 F/g | 800 | 92.8 | 1 A/g | 24.8 Wh/kg | NA | [61] |
| MXene/rGO | H2SO4/PVA | 80 F/cm3 | 10 000 | 97 | NA | 8.6 mWh/kg | 0.2 W/cm3 | [62] |
| Au/graphene | H2SO4/PVA | 81.5 μF/cm2 | 1 800 | NA | NA | NA | NA | [63] |
| Ag/PANI/graphene | 1 M Na2SO4 | 828 F/g | 3 000 | 97.5 | 1.5 A/g | NA | NA | [64] |
| CNT/graphene | 1 M Na2SO4 | 200 F/g | 10 000 | NA | 100 μA/cm2 | NA | NA | [29] |
| CNTG/MG | PAAK/KCl | 220.8 F/g | 10 000 | 86 | 1 A/g | 32.7 Wh/kg | 9.0 kW/kg | [65] |
| Cellulose‐graphene | H2SO4/PVA | 207 F/g | 5 000 | 99.1 | 3.4 mA/cm2 | 20 μWh/cm2 | 15.5 mW/cm2 | [66] |
| rGO/MnO2 | NA | 217 F/g | 1 000 | 74 | 100 mA/g | 11.5 μWh/cm2 | 3.8 mW/cm2 | [67] |
| MnO2/N‐graphene | PVA‐LiCl | 305 F/g | 1 500 | 90 | 1 A/g | 3.5 mWh/cm3 | 0.019 W/cm3 | [68] |
| PPy/GO/MnO2 | 1 M Na2SO4 | NA | 1 000 | 96.58 | 1 A/g | 1.17 Wh/kg | 468.68 W/kg | [69] |
| rGO/PPy | H3PO4‐PVA | 345 F/g | 1 000 | 90.6 | 1 A/g | 20.6 Wh/kg | 1280 W/kg | [70] |
17.7 Future Aspects
Such flexible graphene‐based devices are growing in demand as ESD are shifting toward in wearable consumer electronics. Despite the rapid development of FSCs, numerous challenges remain to be tackled and taken into consideration to develop high‐performance and cost‐effective flexible devices. For example, although a graphene‐hydrogel based solid‐state supercapacitor has been successfully developed without the application of binders or additives, the need for a flexible substrate coated with a high‐conductivity component (Au‐coated) indirectly increased the production cost. So, apart from tailoring the flexibility of grapheme‐based electrodes, the future studies should also focus the electrochemical performances with cost‐effective approaches. The investigation on 2D‐materilas in flexible ESDs for their interesting properties is germane for researchers as well as industrialist.
References
- 1 Das, H.T., Saravanya, S., and Elumalai, P. (2018). Chemistry Select 3: 13275–13283.
- 2 Chen, T. and Dai, L. (2014). J. Mater. Chem. A 2: 10756–10775.
- 3 Das, H.T. (2019). ECS Meeting Abstracts, 281. IOP Publishing.
- 4 Das, H.T., Mahendraprabhu, K., Maiyalagan, T., and Elumalai, P. (2017). Sci. Rep. 7: 1–14.
- 5 Zhang, L.L., Zhou, R., and Zhao, X.S. (2010). J. Mater. Chem. 20: 5983–5992.
- 6 Yi, F., Ren, H., Shan, J. et al. (2018). Chem. Soc. Rev. 47: 3152–3188.
- 7 Liang, J., Jiang, C., and Wu, W. (2019). Nanoscale 11: 7041–7061.
- 8 Wang, Y., Shi, Z., Huang, Y. et al. (2009). J. Phys. Chem. C 113: 13103–13107.
- 9 Lee, H.Y. and Goodenough, J.B. (1999). J. Solid State Chem. 144: 220–223.
- 10 Conway, B.E., Bockris, J.O.M., and Ammar, I.A. (1951). Trans. Faraday Soc. 47: 756–766.
- 11 Grahame, D.C. (1947). Chem. Rev. 41: 441–501.
- 12 González, A., Goikolea, E., Barrena, J.A., and Mysyk, R. (2016). Renewable Sustainable Energy Rev. 58: 1189–1206.
- 13 Shuyan, G. and Hao, F. (2013). Chemistry 3.
- 14 Lu, Q., Chen, J.G., and Xiao, J.Q. (2013). Angew. Chemie Int. Ed. 52: 1882–1889.
- 15 Liu, L., Zhao, H., and Lei, Y. (2019). Small Methods 3: 1800341.
- 16 Zhang, L. and Zhao, X.S. (2009). Chem. Soc. Rev. 38: 2520–2531.
- 17 Jeng, H.A. and Swanson, J. (2006). J. Environ. Sci. Health, Part A Toxic/Hazard. Subst. Environ. Eng. 41: 2699–2711.
- 18 Liu, C., Yu, Z., Neff, D. et al. (2010). Nano Lett. 10: 4863–4868.
- 19 Ke, Q. and Wang, J. (2016). J. Materiomics 2: 37–54.
- 20 Peng, X., Peng, L., Wu, C., and Xie, Y. (2014). Chem. Soc. Rev. 43: 3303–3323.
- 21 Cherusseri, J. and Kar, K.K. (2016). J. Mater. Chem. A 4: 9910–9922.
- 22 Mahmood, N., Zhang, C., Yin, H., and Hou, Y. (2014). J. Mater. Chem. A 2: 15–32.
- 23 Vivekchand, S.R.C., Rout, C.S., Subrahmanyam, K.S. et al. (2008). J. Chem. Sci. 120: 9–13.
- 24 Zhang, L.L., Zhao, X., Stoller, M.D. et al. (2012). Nano Lett. 12: 1806–1812.
- 25 Yuan, C.Z., Zhou, L., and Hou, L.R. (2014). Mater. Lett. 124: 253–255.
- 26 Yu, A., Roes, I., Davies, A., and Chen, Z. (2010). Appl. Phys. Lett. 96: 253105.
- 27 Fan, X., Chen, T., and Dai, L. (2014). RSC Adv. 4: 36996–37002.
- 28 Sun, J., Li, Y., Peng, Q. et al. (2013). ACS Nano 7: 10225–10232.
- 29 Cheng, H., Dong, Z., Hu, C. et al. (2013). Nanoscale 5: 3428–3434.
- 30 Wang, Y., Chen, J., Cao, J. et al. (2014). J. Power Sources 271: 269–277.
- 31 Mishra, A.K. and Ramaprabhu, S. (2011). J. Phys. Chem. C 115: 14006–14013.
- 32 Ma, L., Liu, R., Niu, H. et al. (2016). ACS Appl. Mater. Interfaces 8: 33608–33618.
- 33 Zheng, Q., Cai, Z., Ma, Z., and Gong, S. (2015). ACS Appl. Mater. Interfaces 7: 3263–3271.
- 34 Xing, J., Tao, P., Wu, Z. et al. (2019). Carbohydr. Polym. 207: 447–459.
- 35 Liu, L., Niu, Z., Zhang, L. et al. (2014). Adv. Mater. 26: 4855–4862.
- 36 Weng, Z., Su, Y., Wang, D.W. et al. (2011). Adv. Energy Mater. 1: 917–922.
- 37 Basnayaka, P.A. and Ram, M.K. (2017). Conducting Polymer Hybrids, 165–192. Springer.
- 38 Wu, Q., Xu, Y., Yao, Z. et al. (2010). ACS Nano 4: 1963–1970.
- 39 Lee, J.‐S., Kim, S.‐I., Yoon, J.‐C., and Jang, J.‐H. (2013). ACS Nano 7: 6047–6055.
- 40 Zang, X., Li, X., Zhu, M. et al. (2015). Nanoscale 7: 7318–7322.
- 41 Davies, A., Audette, P., Farrow, B. et al. (2011). J. Phys. Chem. C 115: 17612–17620.
- 42 Hamra, A.A.B., Lim, H.N., Huang, N.M. et al. (2020). J. Mol. Struct. 1220: 128710.
- 43 Gao, Z., Yang, W., Wang, J. et al. (2015). Nano Energy 13: 306–317.
- 44 Dong, X., Wang, L., Wang, D. et al. (2012). Langmuir 28: 293–298.
- 45 Xie, J., Sun, X., Zhang, N. et al. (2013). Nano Energy 2: 65–74.
- 46 Clerici, F., Fontana, M., Bianco, S. et al. (2016). ACS Appl. Mater. Interfaces 8: 10459–10465.
- 47 Yan, J., Ren, C.E., Maleski, K. et al. (2017). Adv. Funct. Mater.: 27. https://doi.org/10.1002/adfm.201701264.
- 48 Li, J., Liao, K., Wang, X. et al. (2017). Adv. Mater. Interfaces 4: 1700419.
- 49 Yang, Q., Xu, Z., Fang, B. et al. (2017). J. Mater. Chem. A 5: 22113–22119.
- 50 Ervin, M.H., Le, L.T., and Lee, W.Y. (2014). Electrochim. Acta 147: 610–616.
- 51 Liu, S., Xie, J., Li, H. et al. (2014). J. Mater. Chem. A 2: 18125–18131.
- 52 Kang, S., Lim, K., Park, H. et al. (2018). ACS Appl. Mater. Interfaces 10: 1033–1038.
- 53 Xiong, Z., Liao, C., Han, W., and Wang, X. (2015). Adv. Mater. 27: 4469–4475.
- 54 Li, H., He, Y., Pavlinek, V. et al. (2015). J. Mater. Chem. A 3: 17165–17171.
- 55 Soam, A., Kumar, R., Mahender, C. et al. (2020). J. Alloys Compd. 813: 152145.
- 56 Cao, X., Zheng, B., Shi, W. et al. (2015). Adv. Mater. 27: 4695–4701.
- 57 Wu, X. and Lian, M. (2017). J. Power Sources 362: 184–191.
- 58 Ma, L., Liu, R., Liu, L. et al. (2016). J. Power Sources 335: 76–83.
- 59 Shakir, I., Ali, Z., Bae, J. et al. (2014). Nanoscale 6: 4125–4130.
- 60 Cai, W., Lai, T., Dai, W., and Ye, J. (2014). J. Power Sources 255: 170–178.
- 61 Jin, Y., Chen, H., Chen, M. et al. (2013). ACS Appl. Mater. Interfaces 5: 3408–3416.
- 62 Couly, C., Alhabeb, M., Van Aken, K.L. et al. (2018). Adv. Electron. Mater. 4 https://doi.org/10.1002/aelm.201700339.
- 63 Chen, Y., Fu, X.Y., Yue, Y.Y. et al. (2019). Appl. Surf. Sci. 467, 468: 104–111.
- 64 Sawangphruk, M., Suksomboon, M., Kongsupornsak, K. et al. (2013). J. Mater. Chem. A 1: 9630–9636.
- 65 Gao, H., Xiao, F., Ching, C.B., and Duan, H. (2012). ACS Appl. Mater. Interfaces 4: 7020–7026.
- 66 Gao, K., Shao, Z., Li, J. et al. (2013). J. Mater. Chem. A 1: 63–67.
- 67 Sumboja, A., Foo, C.Y., Wang, X., and Lee, P.S. (2013). Adv. Mater. 25: 2809–2815.
- 68 Liu, Y., Miao, X., Fang, J. et al. (2016). ACS Appl. Mater. Interfaces 8: 5251–5260.
- 69 Ng, C.H., Lim, H.N., Lim, Y.S. et al. (2015). Int. J. Energy Res. 39: 344–355.
- 70 Li, S., Zhao, C., Shu, K. et al. (2014). Carbon 79: 554–562.
