7
Recent Trends in Graphene – Latex Nanocomposites
Anand Krishnamoorthy
Amal Jyothi College of Engineering, Department of Basic Sciences, Kanjirapally, 686518, India
7.1 Introduction
Graphene‐polymer nanocomposites are widely studied by researchers. But relatively fewer attempts are made with polymer lattices. Furthermore, latex route preparation of graphene nanocomposites offer several advantages such as ease of processing, improved compatibilization between polymer and filler, and excellent wet gel strength, compared to conventional nanocomposites. Therefore, latex‐based graphene nanocomposites can be explored for potential applications such as energy storage, improved barrier properties, etc. Most recent advancements happening in latex‐graphene nanocomposites across the world are discussed in this chapter.
7.2 Polymer Lattices – An Overview
Latex is defined as a stable colloidal dispersion of polymer particles in aqueous medium. It is a complex emulsion that consists of protein, resins, sugars, etc. In latex, there are two phases namely dispersed phase and dispersion medium. The dispersed phase consists of small particles having diameter less than 5 μm. Each small particle encloses a large number of polymeric rubber molecules. Latex is usually found in nature as milky fluid and can be exuded from the plant/tree by controlled wounding. “Hevea brasiliensis” is the important source of natural rubber and more than 97% of natural rubber is produced from this tree, commonly known as rubber tree.
Centrifuged natural rubber latex (NRL) concentrate, obtained by centrifuging the field latex, is at present considered as the most versatile raw material for the production of a wide spectrum of products such as examination and surgical gloves, condoms, catheters, balloons, mattresses, extruded threads, adhesives, etc. Great emphasis is given to produce quality centrifuged natural rubber concentrate as the processing and end properties of finished latex goods are highly dependent on the quality of the latex.
The special types and grades of latex concentrates having commercial importance are given below:
- ➢ Pre‐vulcanized latex (PVL) concentrates
- ➢ Radiation vulcanized latex
- ➢ Hevea plus MG‐modified latex concentrates
- ➢ Freeze resistant latex
- ➢ Other specialty lattices
PVL is the most popular class of NRL widely used for the preparation of dipped latex goods. PVL is cross‐linked latex in which the rubber particles are chemically cross‐linked so that upon drying the latex, a vulcanized film is obtained and which does not require further heating. The preparation of PVL involves the addition of cross‐linking agent (sulfur) and accelerator along with other compounding ingredients into NRL and stirring at a standard temperature for a specific period of time. It is then cooled and matured for few days before taking them for product manufacturing.
Radiation vulcanized latex, known as radiation vulcanized natural rubber latex (RVNRL) is manufactured by mixing the latex with co‐cross‐linking agent n‐butyl acrylate and then subjected to γ‐radiation. i.e. Cross‐linking is brought by gamma radiation and the properties are different from conventional sulfur PVL. The advantages of RVNRL are absence of nitrosamines, low cytotoxicity, low protein allergy, no contamination of effluent with zinc oxide, etc. Therefore, they are potential candidates for the production of high‐demand products such as medical tubes, condoms, catheters, endoscopic balloons, baby teats, dental dams, etc. [1].
Hevea plus MG latex is prepared by grafting polymethyl methacrylate chains into natural polymer. The resultant latex is coagulated and then made into a crepe. Two grades of this type of rubber are available and are named as MG 30 and MG 49. The former contains 30% methacrylate whereas the latter contains 49%. They are used in adhesives for bonding rubber to plastics.
Latex concentrates undergoes colloidal destabilization at extremely low temperatures (below 0 °C). Under this circumstances, latex experiences very high viscosity and low mechanical stability. Hence they cannot be used in areas which experiences very low temperature and the continuous freezing resulting in coagulation and which on thawing become a gel. The freeze–thaw stabilized latex concentrates can be made by adding 0.2% of latex weight of sodium salicylate and 0.04–0.05% by latex weight of lauric acid to latex concentrates. The addition of these ingredients enhances the stability of latex during freezing and regains its fluidity. A variety of other grades of latex concentrates with specific characteristics are also developed. This includes antioxidant modified rubber (provides very good oxidation protection), positized latex by adding cationic surfactant (for textile applications), bromo trichloromethane‐modified rubber (offers good flame‐resistant properties) and deprotenized dipped latex goods (for use in electrical sector).
Besides the above, other varieties of modified natural rubber is also available as they are not important compared to the above and hence not discussed here.
Synthetic latex or emulsion polymers are considered as superior counterparts of natural rubber lattices as they offers improved stability and superior performance and hence widely used in a variety of applications. Synthetic lattices are man‐made polymers prepared using petrochemicals. Synthetic lattices were first developed during the period of World War II to alleviate the shortage in natural rubber production. Acrylic, styrene‐acrylic, and styre‐butadiene latex have huge demand in paints and coatings industry as they can deliver excellent strength, flexibility, and improved water resistance property. Polyurethane is a flexible elastic polymer used in a variety of applications such as coatings, adhesives, and medical devices, and synthetic polyisoprene latex is a stereoregular polymer with structure and properties similar to that of natural polyisoprene. Styrene‐vinylpyridine‐butadiene terpolymer lattices are developed to be used as rubber to textile adhesives. A text book “Polymer Lattices Science and Technology,” Vol. 2 by Blackley is a standard reference book widely used by polymer researchers which explains the principles and production of various types of lattices [2].
7.3 Graphene – Background
Graphene is an advanced functional material obtained from nonpetroleum allotrope of carbon, natural graphite. The natural graphite is abundant in the earth. The University of Manchester scientists, namely, Andre Geim and Konstantin Novoselov, were awarded Nobel Prize in Physics in 2010 for their experimentation resulting in the invention of the wonderful material graphene. The amazing properties such as high tensile strength (130 GPa), Young's modulus (1100 GPa), electrical conductivity (108 S/m), and thermal conductivity (5300 W/m K) make graphene a potential material for demanding applications [3]. Graphene is a 2‐D material consists of hexagonal arrangements of carbon atoms. Graphene possesses very high melting point and exceptional strength due to regular arrangement of carbon atoms via strong covalent bonds. It is also electrically conductive because of the presence of delocalized electrons, which are highly mobile. Graphene is perceived as the strongest material in the universe. It is harder than diamond but more elastic; tougher than steel yet lighter than aluminum. Considering these extraordinary features, synthesis of high‐purity graphene and their use for potential applications attracted significant interest in the global research community. The discovery of the most powerful and versatile material, graphene has brought revolutionary changes in all domains changing from high‐capacity batteries to super small computer.
The applications of graphene and grapheme‐related composite materials are shown below [4] (Figure 7.1).

Figure 7.1 Industrial applications of graphene based materials.
Source: Dasari et al. [4]. Reproduced with permission from Elsevier.
7.4 Preparation and Functionalization of Graphene
Just like other nanomaterials, graphene can be synthesized by top‐down and bottom‐up methods. The top‐down method includes chemical synthesis, chemical exfoliation, and mechanical exfoliation. The bottom‐up approach includes pyrolysis, epitaxial growth, chemical vapor deposition (CVD), etc. Other than the above, arc‐discharge of graphite, electron beam irradiation of poly(methyl methacrylate) (PMMA) nanofibers, thermal fusion of PAH's, and conversion of nanodiamond are also reported. The detailed synthesis procedures of graphene are reviewed by Bhuyan et al. [5]. There are several challenges involved starting from graphene synthesis to the end‐use applications. Large volume production with low cost is one of the main challenges in front of the industry. However, active research is going in this direction to find mass production of graphene which will reduce the price significantly. But the primary challenge in front of the research community is to produce pure (defect‐free) graphene. The spectacular properties of graphene particularly the heat conductivity, optical transparency, carrier mobility, etc. can be achieved in its pure form. Focus on industrial‐scale production of graphene and future demanding applications is well documented [6].
Graphene‐based polymer composites are usually fabricated by solution mixing. Homogeneous dispersion of graphene in polymer matrix and improved polymer–filler interaction is essential in preparation and processing of graphene‐polymer nanocomposites. Due to high surface area, graphene has a tendency for stacking/agglomeration, which impedes the end properties of the composite. Functionalization of graphene by physical or chemical means is considered as ideal method for the fabrication of graphene‐polymer nanocomposites [7]. Graphene oxide (GO) was successfully synthesized from pure graphite and functionalized with dodecyl amine and then reduced using hydrazine hydrate [7]. Noncovalently functionalized reduced graphene oxide (rGO) reinforced poly(vinyl alcohol) (PVA) nanocomposites were prepared by solution mixing [8]. The researchers prevented the agglomeration of graphene sheets using surface modifying agent poly(sodium 4‐styrenesulfonate) (PSS). scanning electron microscopy (SEM) analysis showed good dispersion of graphene in PVA matrix. Cheng et al. reported a simple preparation of functionalized graphene by ball milling of graphite in presence of an aryl diazonium salt [9]. The efficient exfoliation of graphite was achieved through the tandem surface reaction of graphite with aryl diazonium salt and mechanical shear by ball milling. They claim this chemical–mechanical procedure as industrially compatible and mass volume route for the production of graphene.
Graphene exhibits limited flame retardancy effect in polymer matrix due to thermal barrier effect. Therefore, a facile and effective approach for the preparation of graphene‐polymer nanocomposites is made by decorating graphene with phosphorus and nitrogen. Because phosphorus and nitrogen containing compounds are considered as highly efficient halogen free flame retardants. Supermolecular aggregates of piperazine (PiP) and phytic acid (PA) were self‐assembled onto the GO surface in water and fabricated functionalized GO (PPGO) [10]. The induction of organic moieties on the surface of GO improved the adhesion between PPGO and epoxy resin (EP). The flame resistance of EP/PPGO composites exhibited 42% decrease in peak heat release rate and 22% reduction in total heat release compared with pure EP. The tribological properties of nanolubricants dispersed with dodecylamine functionalized graphene (DAG) in commercial engine oil are investigated [11]. Detailed tribological performance of DAG‐loaded nanolubricants was evaluated and compared with pure engine oil lubricant. It was summarized that there was improvement of antifriction and antiwear properties of steel–steel tribo‐pair in the presence of lubricating commercial engine oil by dispersing minimal quantity of functionalized GO. Muthoosamy and Manickam reviewed the fundamental concepts of ultrasonochemistry for the synthesis of graphene, its dispersion, exfoliation, as well as its functionalization [12]. Amino‐functionalized GO decorated with Fe3O4 nanoparticles were prepared and studied the Cr (VI) removal ability in aqueous solution [13]. A one‐pot processing technique was used to produce (3‐mercaptopropyl)trimethoxysilane (MPS) functionalized graphene/PU sponge (FGN/PU sponge) with excellent superhydrophobicity [14]. In short, it can be seen that functionalized graphene exhibits enhanced dispersibility and improved performance and hence opens up new pathways for graphene‐polymer nanocomposites for advanced applications.
7.5 Graphene – Latex Nanocomposites: Preparation Properties and Applications
Latex co‐coagulation method involves the mixing of latex with other polymers or fillers and the mixtures will be coprecipitated by adding suitable coagulants. Graphene/polyurethane latex nanocomposites were prepared by cocoagulation method [15]. The negatively charged aqueous suspension was mixed with positively charged cationic waterborne polyurethane followed by chemical reduction of the mixture in dimethylformamide (DMF) solution. The authors claim that the graphene nanoplatelets were well dispersed in the polymer matrix because of the electrostatic interaction between filler and polymer. The fabricated nanocomposite showed a significant improvement in electrical and thermal conductivity, 9 orders of magnitude and by 6.1 times, respectively, compared with the pure cationic waterborne polyurethane.
It has been explained in previous session that the nature of the surfactant and its concentration are critical which determines the stability of the dispersion. Graphene nanoplatelets were dispersed in natural rubber matrix and studied the effect of single, double, and triple chain anionic surfactants on dispersion stability and electrical conductivity of natural rubber‐graphene nanocomposites. A clear correlation between the number of aromatic moieties of a given surfactant and its role is stabilizing graphene in NRL is deducted by electrical conductivity and zeta potential measurements [16] (Table 7.1).
Table 7.1 Zeta potential of graphene dispersions in various surfactants.
| Surfactant | Number of aromatic ring(s) | ζ‐potential (mV) | Electrical conductivity enhancement |
|---|---|---|---|
| SDS | N/A | −43 ± 4 | 1.17 × 106 |
| SDBS | 1 | −40 ± 8 | 2.36 × 108 |
| DC3Ph2 | 2 | −69 ± 7 | 6.21 × 108 |
| TC3Ph3 | 3 | −95 ± 6 | 1.47 × 109 |
Source: Mohamed et al. Reproduced with permission from Elsevier [16].
Sodium 1,4‐bis(neopentyloxy)‐3‐(neopentyloxycarbonyl)‐1,4‐dioxobutane‐2‐sulfonate (TC14), a custom‐made surfactant, was used to assist the dispersion of GO/NRL nanocomposites by one‐step method [17] (Figure 7.2). The authors claim that the addition of custom‐made surfactant TC14 results in the improved interaction between graphene (GO) and NRL and was evident from significant improvement in electrical properties. The electrical and physical properties of latex composites having SDS dispersed GO nanoparticles were also presented.
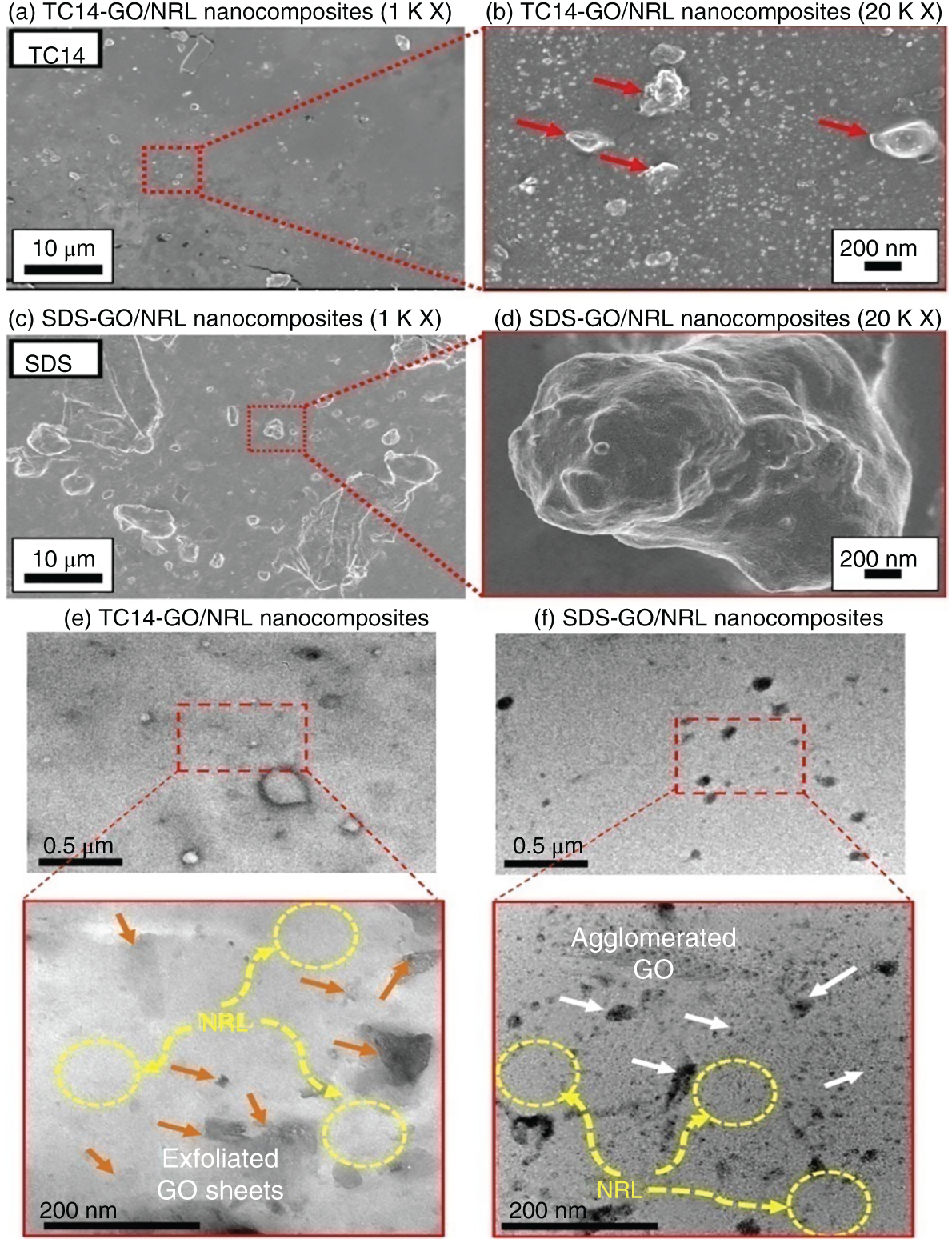
Figure 7.2 FESEM images of (a, b) TC14‐GO/NRL nanocomposite and (c, d) SDS‐GO/NRL nanocomposite. HRTEM images of (e) TC14‐GO/NRL nanocomposites and (f) SDS‐GO/NRL nanocomposites.
Source: Suriani et al. [17]. Reproduced with permission from Elsevier.
A facile one step method for the fabrication of GO/NRL nanocomposites by electrochemical exfoliation method is reported [18] (Figure 7.3). The results showed a higher specific capacitance of 103.7 F/g for one‐step process compared to 32.6 F/g via two‐step route.
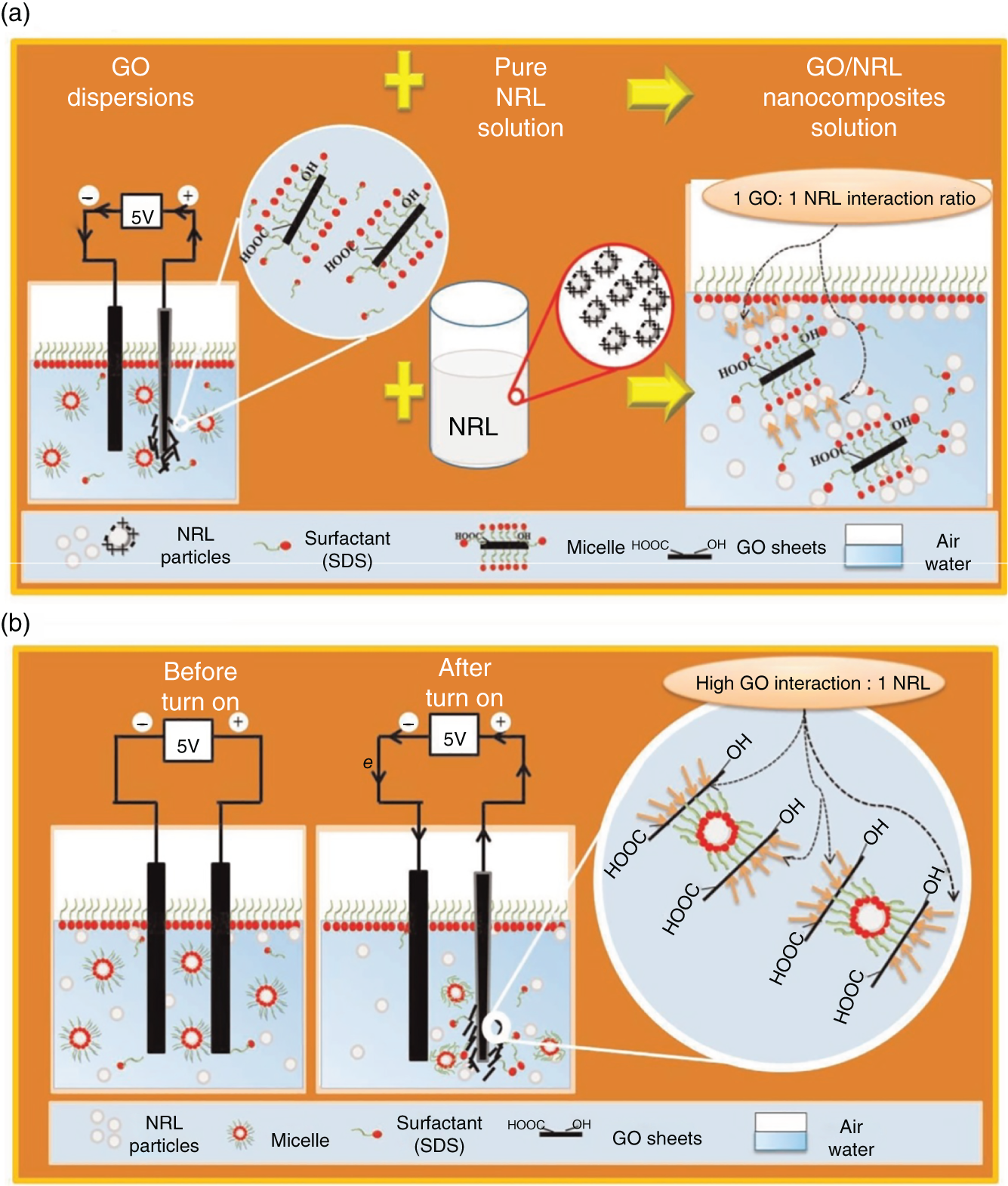
Figure 7.3 Schematic growth mechanism of GO/NRL nanocomposites.
Source: Suriani et al. [18]. Reproduced with permission from Elsevier.
GO/NRL nanocomposites were successfully synthesized under various applied voltages from 3 to 14 V by electrochemical exfoliation [19] (Table 7.2). The GO/NRL nanocomposites synthesized by applying 10 V showed a moderate crystallinity of 0.91. The synthesis of GO at 10 V showed more wrinkles indicating high exfoliation. However, when the voltage is increased from 5 to 14 V, the electrical conductivity of composites tends to decrease due to rapid and higher oxidation degree. The presence of higher amounts of oxygen functional groups affects the electron movement in GO and causes difficult formation of conductive pathways. Also, electrons confined in the agglomerated GO structures rarely tunnel from inner to outer parts of the composite and prevents the formation of effective conducting networks (Table 7.2 and Figure 7.4).
Table 7.2 Summary of GO/NRL nanocomposite produced using different applied voltage.
| Mechanical test | ||||||
|---|---|---|---|---|---|---|
| Sample description | Electrical conductivity, σ (S/cm) | Specific capacitance, Cs (F/g) | Max load (N) | Tensile strength, σb (MPa) | Young modulus, E (MPa) | Elongation at break, EB (%) |
| 3 V‐GO/NRL | 4.43 × 10–12 | 0.2 | 0.511 24 | 0.1973 | 2 490 | 115 |
| 5 V‐GO/NRL | 1.66 × 10–5 | 103 | 0.526 32 | 0.2009 | 3 110 | 89.4 |
| 7 V‐GO/NRL | 4.66 × 10–6 | 2.0 | 0.746 63 | 0.2083 | 5 360 | 72.8 |
| 10 V‐GO/NRL | 2.11 × 10–7 | 2.5 | 0.480 72 | 0.2380 | 8 950 | 66.6 |
| 12 V‐GO/NRL | 5.60 × 10–8 | 1.4 | 0.638 53 | 0.2460 | 13 200 | 59.5 |
| 14 V‐GO/NRL | 4.92 × 10–9 | 0.6 | 1.93 | 0.4176 | 20 200 | 53.6 |
Source: Nurhafizah et al. Reproduced with permission from Elsevier [19].
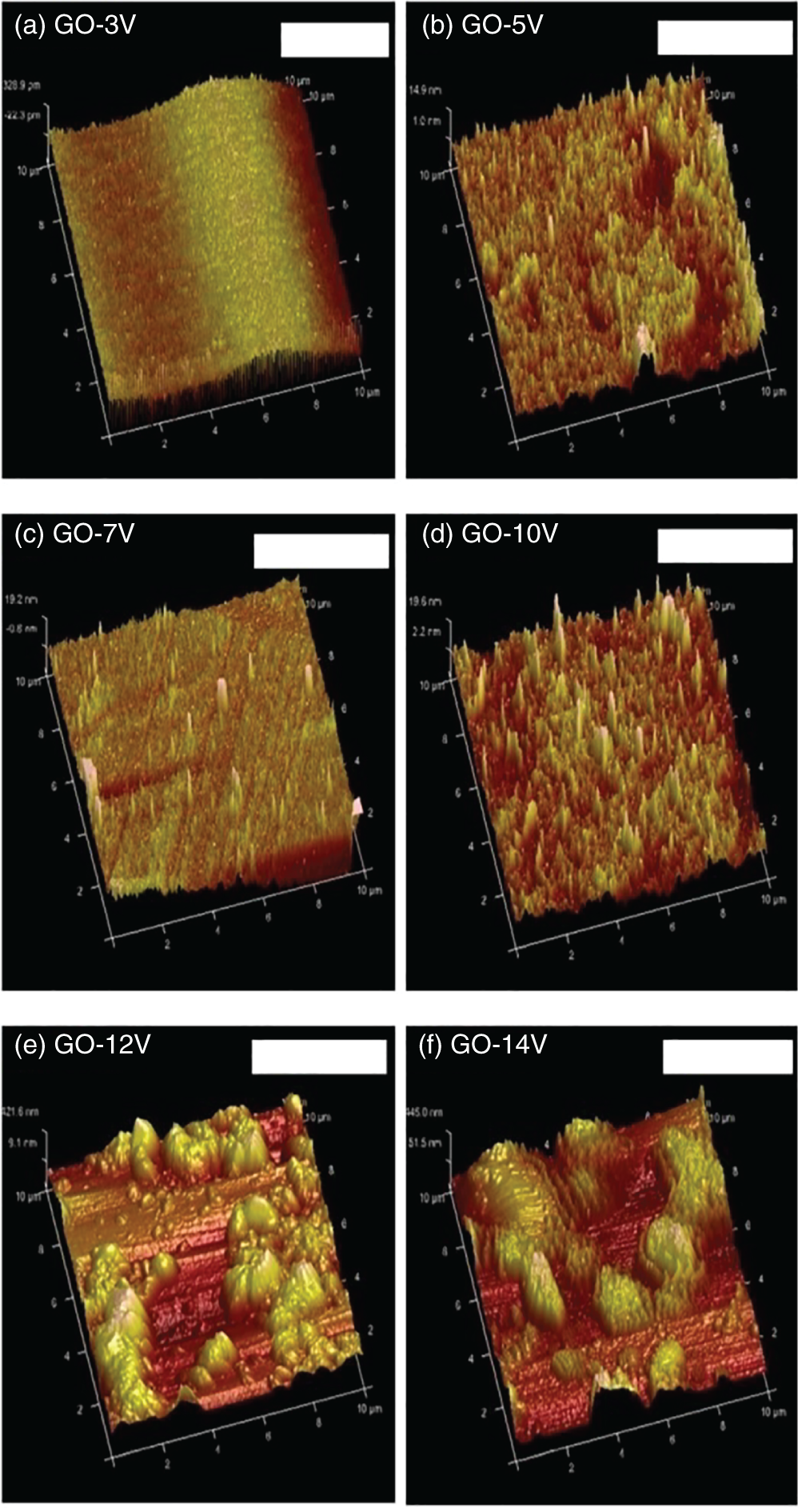
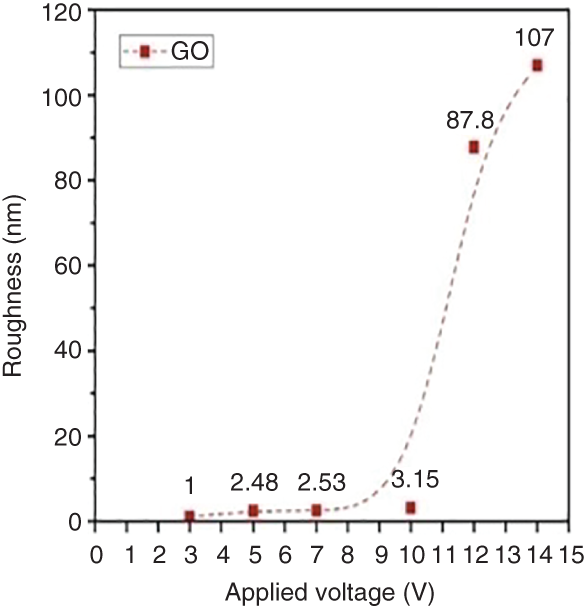
Figure 7.4 AFM images of GO synthesized at different applied voltages of 3, 5, 7, 10, 12, and 14 V.
Source: Nurhafizah et al. [19]. Reproduced with permission from Elsevier.
Berki et al. studied the effect of compounding technique (melt and latex method) on the properties of sulfur cured NR/GO nanocomposites [20]. In the case of latex compounded systems, the tensile and tear strengths were monotonously enhanced and the ductility reduced with increasing amount of GO reflecting the reinforcement effect of GO. This is attributed to better exfoliation of high aspect ratio GO in latex mixed samples compared to melt mixed composites. The tear strength and hardness of melt mixed composites are lower compared with composites produced via latex compounding. The cure characteristics of the GO/NR latex nanocomposites prepared through the latter technique were not affected, whereas it exhibited an unusual behavior in the case of nanocomposites fabricated through melt mixing, i.e. GO accelerated the curing and reduced its extent at higher GO loading. The authors were unable to provide a satisfactory explanation for this.
The surface morphology of NRL and NRL/graphene composite was explained using SEM [21]. The pure NRL showed a smooth surface with no agglomeration. As the concentration of graphene increases, the agglomeration starts to appear. The tensile strength and elongation at break of the latex‐graphene composite increased remarkably. The addition of graphene in NRL improved the tensile strength by 92%. The hybrid network structure could serve as sacrificial bonds and dissipates energy thereby increasing the toughness of rubber compounds.
Graphene as a nanofiller in a variety of NRL matrix, namely, in low ammonia NRL, RVNRL, and epoxy natural rubber latex 25 (ENRL 25) for the fabrication of graphene‐rubber nanocomposites as electrode materials were investigated [22]. The electrical conductivity and capacitive behavior of the nanocomposite samples were investigated under a four‐point probe and cyclic voltammetry measurements, respectively. The electrical conductivity of GO/RVNRL, GO/ENRL25, and GO/NRL nanocomposites was ∼8.6 × 10−4 S/cm, ∼3.1 × 10−4 S/cm, and ∼2.6 × 10−4 S/cm respectively. In addition, the GO/RVNRL polymer nanocomposites electrodes showed acceptable specific capacitance of 5 F/g (Figure 7.5).
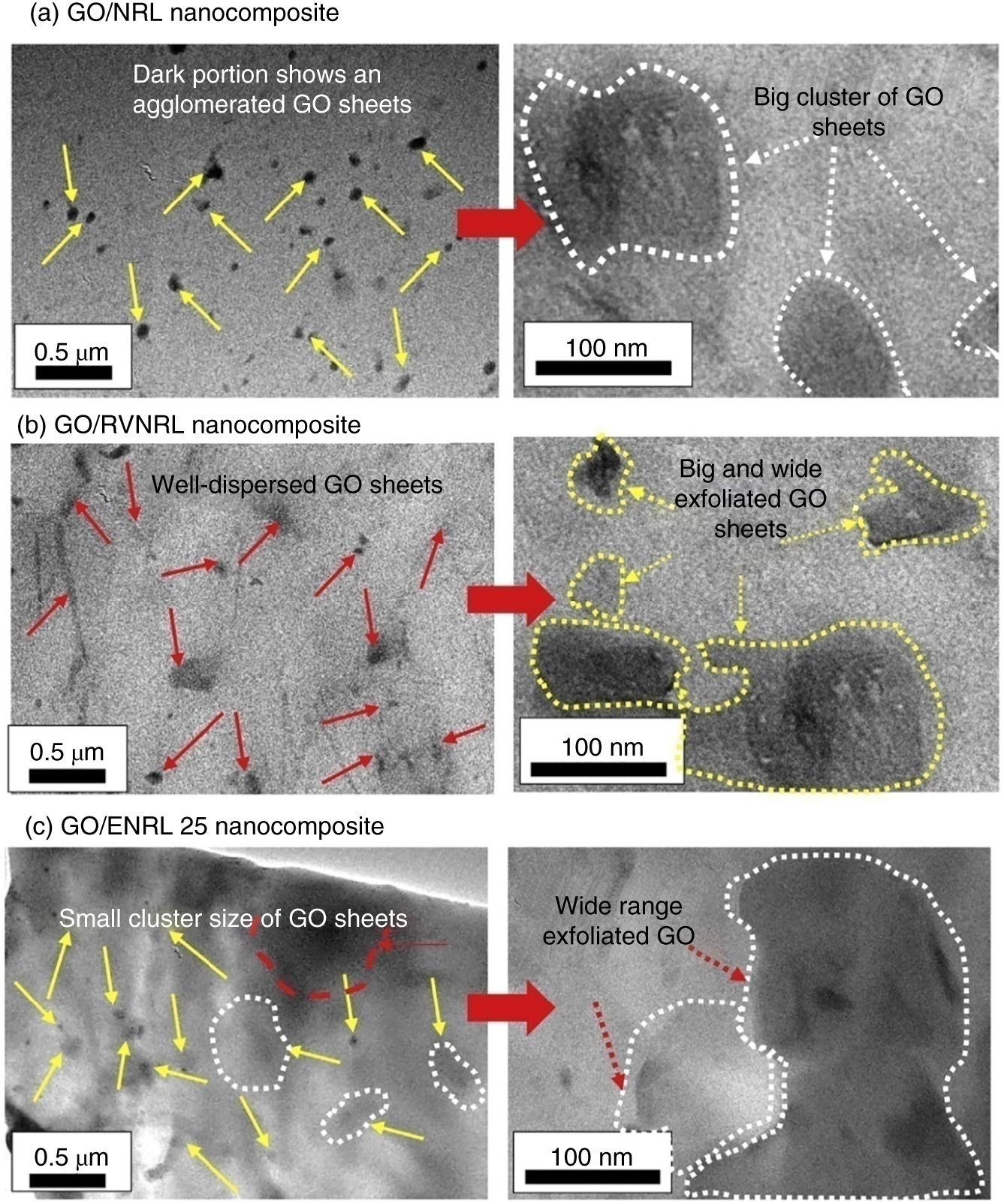
Figure 7.5 HRTEM images of the (a) GO/NRL, (b) GO/RVNRL, and (c) GO/ENRL 25 polymer nanocomposites.
Source: Nurhafizah et al. [22].
Lin et al. reported a facile, environmentally friendly approach to fabricate high‐radiation resistance poly(methyl methacrylate)/reduced graphene oxide (PMMA/RGO) composites through latex mixing of anionic PMMA latex particles and GO dispersion followed by coagulation and in situ hydrazine reduction [23]. The thermogravimetric analysis (TGA) results showed a delay in radiation‐induced oxidative degradation of PMMA/RGO nanocomposites compared to pure PMMA. The homogenous distribution of RGO can behave as free‐radical scavengers and was evident by the decrease in free radical concentration in PMMA/RGO composites with increased RGO loading during irradiation. Also, the Tg values of the composite increase with RGO loading.
Styrene‐acrylic latex polymer possesses excellent film formation ability, weatherability, heat, and corrosion resistance and hence they are widely used in coating and adhesive applications. Guan et al. fabricated latex nanocomposites by incorporating modified graphene oxide (mGO) into styrene‐acrylic latex using water as the solvent and studied the oxygen permeability of resulting nanocomposites films [24]. The fabricated latex nanocomposite exhibited 84% reduction in oxygen permeability (1.2 wt% mGO) compared to neat polymer.
Recently, Zhang et al. reported the fabrication of ultralight, water adhesive graphene/natural rubber latex hybrid aerogel (GA/NRL) by simple solution mixing route [25]. The utilization of the prepared GA/NRL composites on collecting water droplets and solar‐thermal harvest capacity was presented. The formation of hierarchical microstructures contributes to enhanced toughness, improved solar‐thermal conversion efficiency, and water‐adhesion capacity (Figures 7.6 and 7.7).
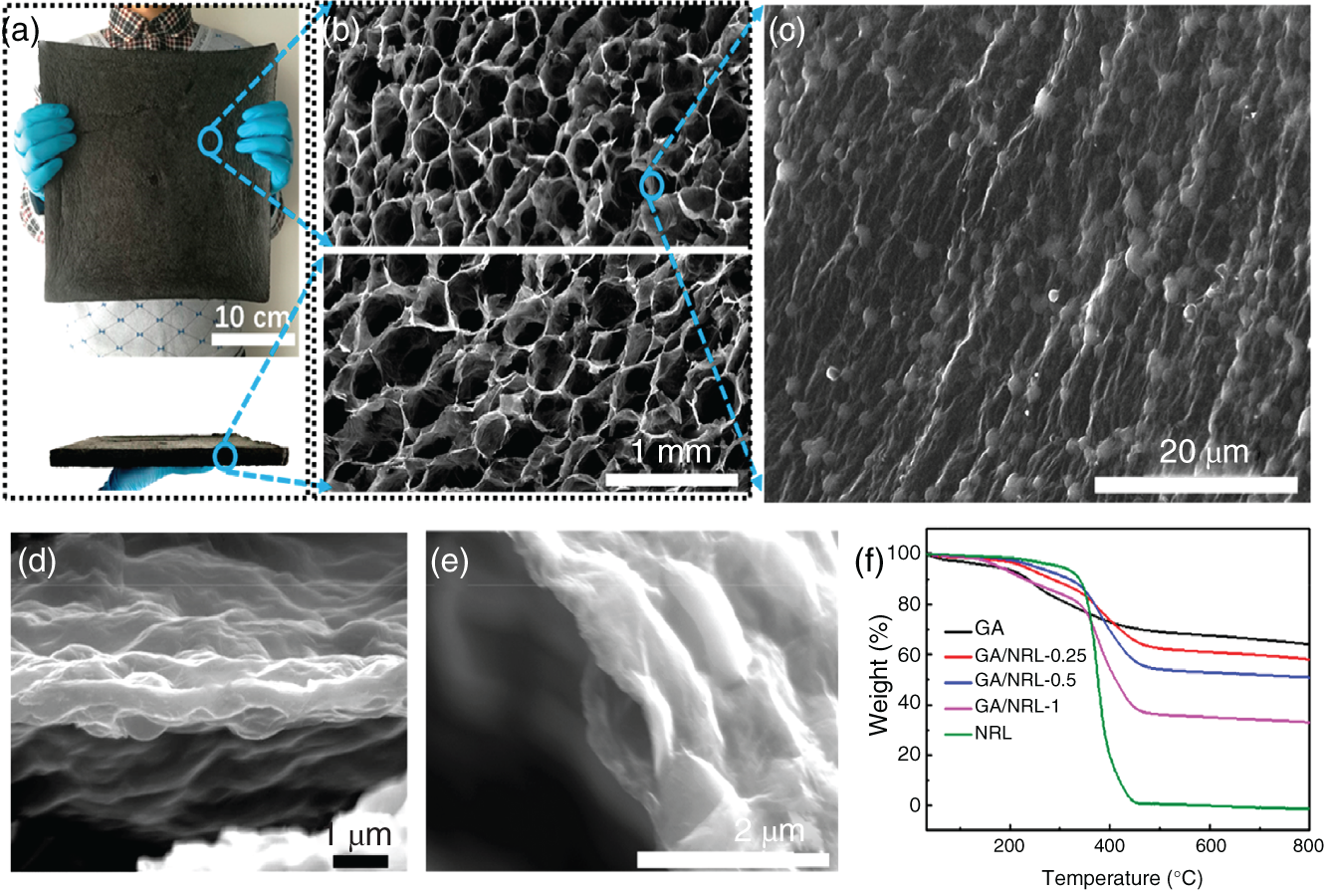
Figure 7.6 Morphology and thermal characterization of GA/NRL‐0.5 and GA/NRL aerogels. (a) Photographs of GA/NRL‐0.5 from different visual angles and (b) the corresponding SEM pictures. (c) Surface morphology of a single hybrid graphene wall to show the uniformly wrapped NRL particles akin to the rose petal surface and (d) the corresponding fracture location with sandwich like structure. (e) SEM image of the hybrid graphene wall with sandwich like structure. (f) TGA curves of GA, GA/NRL aerogels, and NRL under nitrogen atmosphere.
Source: Zhang et al. [25]. Reproduced with permission from American Chemical Society.
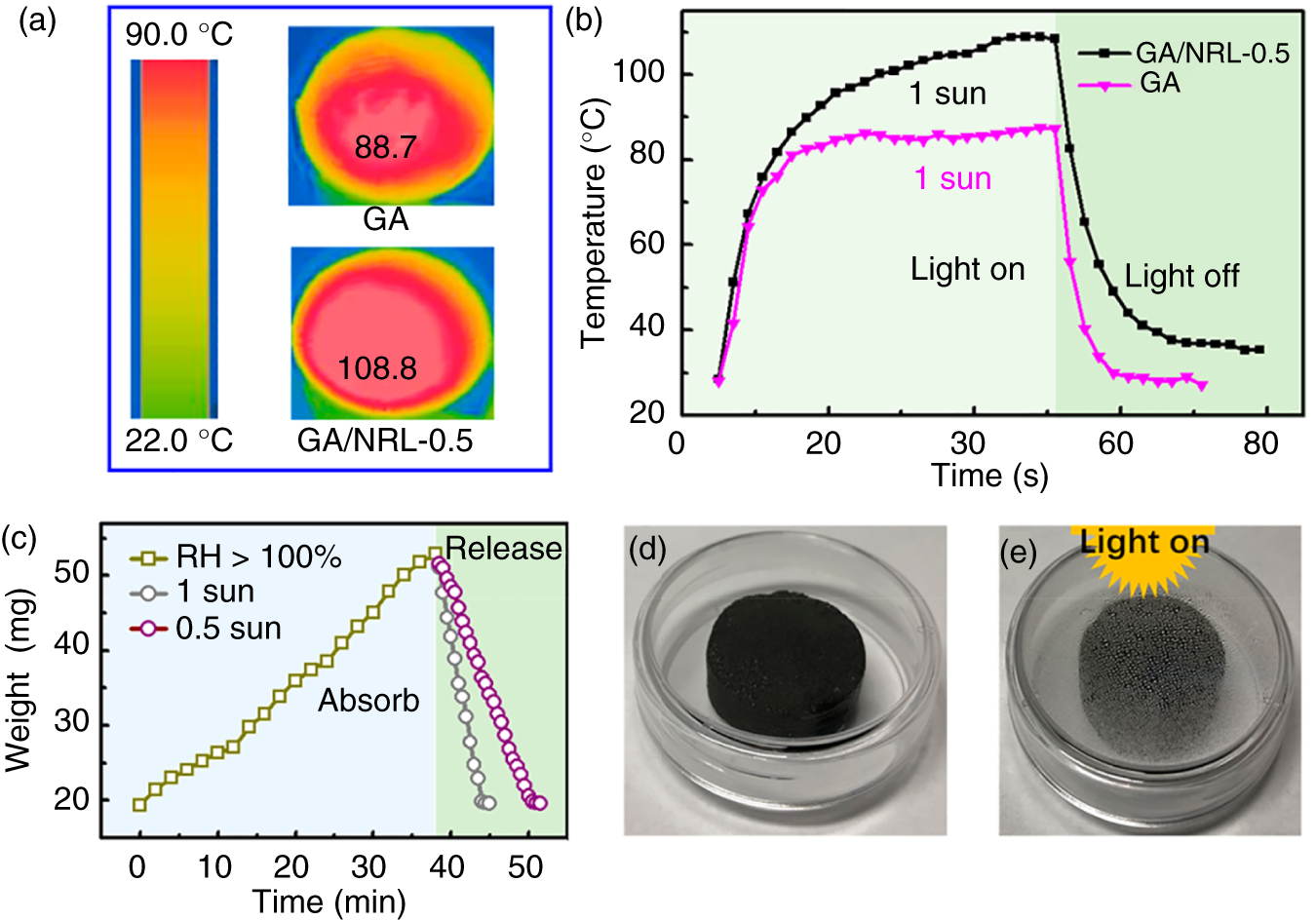
Figure 7.7 Solar−thermal properties of GA/NRL‐0.5 and its application in collecting water stored in GA/NRL. (a) Solar−thermal behavior of GA/NRL‐0.5 and GA under 1 sun. (b) Temperature changing curves of “light on” and “light off” under 1 sun. (c) Water harvest at RH > 100% and release behavior under different sun intensities. (d, e) Pictures of GA/NRL‐0.5 with stored water before (d) and after (e) exposure to 5 sun.
Source: Zhang et al. [25]. Reproduced with permission from American Chemical Society.
The process of making stable and homogenous dispersion of graphene in polyisoprene latex is quite challenging. Preparation of polyisoprene latex graphene nanocomposites having excellent film formation ability, good physical properties, and improved aging resistance is patented [26]. The inventors found a method of producing rGO without the use of a strong reducing agent. They also achieved preparation of homogenous composite dispersion of composite mixtures without the use of high shear mixing or ultrasonic mixing.
The central objective of incorporation of graphene on polymer latex is to improve the property of the materials as well as to impart specific functionalities to tailor them for various applications. When a conductive material such as graphene is added to insulating polymer matrix, the resultant composite becomes conductive due to the formation of interconnected graphene networks in the system. The fabrication of grapheme‐based latex nanocomposites received significant interest because of their potential applications in EMI shielding, functional coatings, energy storage devices, sensors, high‐performance composites, etc.
7.6 Conclusions
Polymer latex‐based nanocomposites received much attention among materials research community. The advantage with polymer lattices is that it can offer a wide range of choices of the production of materials with tuneable properties. Polymer latex (natural and synthetic) based products have huge market demand. For example, NRL, a biodegradable polymer is widely used in healthcare and biomedical industry for the preparation of gloves, catheters, etc. Similarly, synthetic polymer lattices is essential for paints and coatings industry. Hence, incorporation of graphene in polymer matrices (natural and synthetic) results in composites with enhanced physico‐chemical and mechanical properties. However, large scale production of defect‐free graphene and proper dispersion of these in polymer matrix are quite challenging. The dispersion‐related issue can be solved at large by proper functionalization of graphene or polymer latex. Graphene‐based latex nanocomposites demonstrate enhanced electrical conductivity at low filler loading, superior thermal conductivity, improved chemical and bacterial resistance, EMI shielding properties, etc. In the coming years, it is anticipated that the academia and industry community is going to invest in doing cutting‐edge research on the preparation and fabrication of latex‐graphene nanocomposites, which will result in development of high‐performance multifunctional materials for advanced technologies.
References
- 1 Makuuchi, K. (2003). An Introduction to Radiation Vulcanization of Natural Rubber Latex (ed. K. Makuuchi) Chapter 11, 144. Thailand: T.R.I Global Co., Ltd.
- 2 Blackley, D.C. (1966). Polymer Latices Science and Technology, vol. 2 (ed. D.C. Blackley). UK: Chapman & Hall.
- 3 Xu, Z., Liu, Y., Zhao, X. et al. (2016). Ultrastiff and strong graphene fibers via full‐scale synergetic defect engineering. Adv. Mater. 28 (30): 6449–6456.
- 4 Dasari, B.L., Nouri, J.M., Brabazon, D., and Naher, S. (2017). Graphene and derivatives – synthesis techniques, properties and their energy applications. Energy 140: 766–778.
- 5 Bhuyan, M.S.A., Uddin, M.N., Islam, M.M. et al. (2016). Synthesis of graphene. Int. Nano Lett. 6 (2): 65–83.
- 6 Lin, L., Peng, H., and Liu, Z. (2019). Synthesis challenges for graphene industry. Nat. Mater. 18 (6): 520–524.
- 7 Dehghanzad, B., Razavi Aghjeh, M.K., Rafeie, O. et al. (2016). Synthesis and characterization of graphene and functionalized graphene via chemical and thermal treatment methods. RSC Adv. 6 (5): 3578–3585.
- 8 Wang, X., Liu, X., Yuan, H. et al. (2018). Non‐covalently functionalized graphene strengthened poly(vinyl alcohol). Mater. Des. 139: 372–379.
- 9 Cheng, C., Jia, P., Xiao, L., and Geng, J. (2019). Tandem chemical modification/mechanical exfoliation of graphite: scalable synthesis of high‐quality, surface‐functionalized graphene. Carbon 145: 668–676.
- 10 Fang, F., Ran, S., Fang, Z. et al. (2019). Improved flame resistance and thermo‐mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self‐assembly in water. Composites, Part B 165 (December 2018): 406–416.
- 11 Paul, G., Shit, S., Hirani, H. et al. (2019). Tribological behavior of dodecylamine functionalized graphene nanosheets dispersed engine oil nanolubricants. Tribol. Int. 131 (June 2018): 605–619.
- 12 Muthoosamy, K. and Manickam, S. (2017). State of the art and recent advances in the ultrasound‐assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 39 (February): 478–493.
- 13 Zhao, D., Gao, X., Wu, C. et al. (2016). Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr(VI). Appl. Surf. Sci. 384: 1–9.
- 14 Zhou, S., Hao, G., Zhou, X. et al. (2016). One‐pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil‐water separation. Chem. Eng. J. 302 (May 2016): 155–162.
- 15 Wu, S.L., Shi, T.J., and Zhang, L.Y. (2016). Latex co‐coagulation approach to fabrication of polyurethane/graphene nanocomposites with improved electrical conductivity, thermal conductivity, and barrier property. J. Appl. Polym. Sci. 133 (11): 1–13.
- 16 Mohamed, A., Ardyani, T., Abu Bakar, S. et al. (2018). Rational design of aromatic surfactants for graphene/natural rubber latex nanocomposites with enhanced electrical conductivity. J. Colloid Interface Sci. 516 (January): 34–47.
- 17 Suriani, A.B., Nurhafizah, M.D., Mohamed, A. et al. (2016). Highly conductive electrodes of graphene oxide/natural rubber latex‐based electrodes by using a hyper‐branched surfactant. Mater. Des. 99: 174–181.
- 18 Suriani, A.B., Nurhafizah, M.D., Mohamed, A. et al. (2015). A facile one‐step method for graphene oxide/natural rubber latex nanocomposite production for supercapacitor applications. Mater. Lett. 161: 665–668.
- 19 Nurhafizah, M.D., Suriani, A.B., Mohamed, A., and Soga, T. (2020). Effect of voltage applied for graphene oxide/latex nanocomposites produced via electrochemical exfoliation and its application as conductive electrodes. Diam. Relat. Mater. 101: 107624.
- 20 Berki, P., László, K., Tung, N.T., and Karger‐Kocsis, J. (2017). Natural rubber/graphene oxide nanocomposites via melt and latex compounding: comparison at very low graphene oxide content. J. Reinf. Plast. Compos. 36 (11): 808–817.
- 21 Rahman, M.A., Tong, G.B., Kamaruddin, N.H. et al. (2019). Effect of graphene infusion on morphology and performance of natural rubber latex/graphene composites. J. Mater. Sci. Mater. Electron. 30: 12888–12894.
- 22 Nurhafizah, M.D., Aziz, A.A., Suriani, A.B. et al. (2020). Low‐temperature exfoliated graphene oxide incorporated with different types of natural rubber latex: electrical and morphological properties and its capacitance performance. Ceram. Int. 46 (5): 5610–5622.
- 23 Lin, Y., Liu, Y., Zhang, D. et al. (2017). Radiation resistance of poly(methyl methacrylate)/reduced graphene oxide nanocomposites fabricated through latex mixing and in situ reduction. Chem. Eng. J. 315: 516–526.
- 24 Guan, Y., Meyers, K.P., Mendon, S.K. et al. (2016). Ecofriendly fabrication of modified graphene oxide latex nanocomposites with high oxygen barrier performance. ACS Appl. Mater. Interfaces 8 (48): 33210–33220.
- 25 Zhang, X., Yang, G., Zong, L. et al. (2020). Tough, ultralight, and water‐adhesive graphene/natural rubber latex hybrid aerogel with Sandwichlike cell wall and biomimetic rose‐petal‐like surface. ACS Appl. Mater. Interfaces 12: 1378–1386.
- 26 Kim, C., Joshi, S., and Cauwenberghs, G. (2019). Patent Application Publication: US 2019/0253069 A1. Issued 2019.
