13
Study of Antimicrobial Activity of ZnO Nanoparticles Using Leaves Extract of Ficus auriculata Based on Green Chemistry Principles
Gurpreet Kour, Ankit S. Bartwal, and Satish C. Sati
H.N.B. Garhwal University (A Central University), Department of Chemistry, Srinagar, Garhwal, Uttarakhand, 246174, India
13.1 Introduction
Microscopic particles with size usually ranged between 1 and 100 nm are known as nanoparticles, and they have been studied in a different branch of science called nano sciences and nanotechnology. Nanotechnology is fast emerging branch of science and technology, by which all the streams of society get benefitted day by day. Green synthesized nanoparticles are based on the principle of green chemistry, i.e. product should have less harsh effect with maximum efficacy, nontoxic, and ecofriendly [1–3], which shows effective chemical, physical, biological, and medicinal features such as size, distribution, and morphology. Completely novel and distinctive properties are exhibited by nanoparticles in comparison of its macroscaled counterparts which are efficient and useful in many fields of catalysis, environments, health, photonics, Surface‐enhanced Raman scattering (SERS) detection, and many more. Green synthesis of nanoparticle is an eco‐friendly technology that includes the synthesis of nanoparticle by bioreduction reaction [4]. So, the demand for green nanotechnology is increasing sharply. Consequently, the synthesis of nanoparticles by the biological approach becomes vital [5–10].
For the present study, leaves of Ficus auriculata were selected that belong to Moraceae family, a huge tropical and evergreen tree. It commonly occurs in India (The Outer Himalaya ascending up to 2000 m, from Uttarakhand, Jammu and Kashmir, South India to West Bengal), Myanmar, Pakistan, Bangladesh [11], Thailand, and Malaysia to S. China and Taiwan [12]. Ficus species are wealthy source of polyphenolic compounds, flavonoids that are responsible for strong antioxidant properties that help in prevention and therapy of various oxidative stress‐related diseases such as neurodegenerative and hepatic diseases [13].
Qualitative phyto‐constituents investigation of leaves extract of F. auriculata confirmed the presence of different types of secondary metabolites such as carbohydrates, flavonoids, terpenoids, tannins, phytosterols, resins, saponins, glycosides, phenolics, and proteins. The existence of compounds like betulinic acid, scopoletin, β‐sitosterol‐3‐O‐β‐D‐glucopyranoside, myricetin, lupeol, stigmasterol, bergapten, and quercetin‐3‐O‐β‐D‐glucopyranoside in leaves of F. auriculata, which shows the complex nature of the extract of the plant material [14], [15].
By virtue of its medicinal value like antioxidant, phytotoxic, anti‐inflammatory, hepatoprotactive, anti‐diabetic, anti‐microbial activity, etc. and presence of different organic molecules as phyto‐constituents in the leaves extract of F. auriculata, It was selected for present green synthesis and studies of biological activities [16], [17]. Therefore, we took the leaves of F. auriculata to synthesize zinc oxide nanoparticles (ZnONPs) and studied their antimicrobial activity.
13.2 Materials and Methods
13.2.1 Chemicals
Zinc acetate, Sodium hydroxide, Deionized water, well washed glasswares with millipore water and dried out in oven were used to proceed the work. For present study leaves of F. auriculata (Timla) were collected from VCSG, Government Medical College Campus, Srikot, Srinagar, Garhwal, Uttarakhand (Figure 13.1) and identified by Taxonomist, Department of Botany HNB Garhwal University Srinagar Garhwal.
13.2.2 Methodology
Fresh plant material was collected, dried, and crushed. In a clean flask 100 ml of millipore water was taken and 10 g of crushed leaves were boiled in it for one hour. The solution was allowed to cool and passed through Whatman filter paper no. 1. The leaf extract was collected for further analysis [11].
In the biosynthesis of ZnONPs, the leaf extract was added to freshly prepared zinc acetate solution. Four milliliter of plant extract was mixed with 50 ml of zinc acetate (0.02 M) solution with constant stirring. Subsequently 2 M NaOH was added dropwise to this mixture to adjust the pH and magnetic stirrer was again used for two hours. The separated out pale white precipitates were washed with Millipore water. It was then dried in oven and kept in dry, air tight container for further analysis.

Figure 13.1 Ficus auriculata (Timla) leaves.
After preparation of ZnONPs, characterization was done by following techniques:
- X‐ray diffraction (XRD) analysis
- Scanning electron microscopy (SEM)
- Transmission electron microscopy (TEM)
- Fourier transform infrared (FT‐IR) Spectroscopy
XRD data of the prepared sample were taken at normal room temperature by X‐ray diffractometer. Diffraction was carried out with the help of Smartlab (Rigaku) and was run at 40 kV, 40 mA with Cu Kα radiation at 2θ angle ranging from 20° to 80°. FESEM model Sigma (Carl Zeiss) was used to characterize the surface morphology and grain size of the prepared samples at an extra high voltage, high vacuum, and room temperature.
In our study, TEM measurements were done using a high‐resolution transmission electron microscopy (HR‐TEM) model Technai G2 30S‐Twin operating at an accelerating voltage of 300 kV. The IR spectra of ZnO nanoparticles on KBr disc were observed on Perkin Elmer spectrophotometer in the region 4000–400 cm−1.
13.2.3 Antimicrobial Activity
To analyze the antibacterial activity of the ZnO nanoparticles against the human pathogenic bacteria, viz Bacillus cereus and Klebsiella pneumoniae, the disk diffusion method using a Mueller–Hinton agar culture medium (Hi‐media) was used which is already discussed in our previous paper [18]. In petri dishes with MH agar medium the standard cultures were inoculated 108 CFU/ml and then the inoculated standard culture were laid on with paper disks of 5 mm diameter, and ZnONPs dissolved in dimethylsulfoxide (DMSO). Incubation of petri plates was done at 37 °C for 24 hours and by measuring the zone of inhibition around the disk, antimicrobial activity was determined. The antibacterial effect of Timla was recorded by the measurement of inhibited zone around the disc [18].
13.3 Results and Discussion
13.3.1 Characterization of Synthesized Zinc‐Oxide Nanoparticles (ZnONPs)
The structure, size, phase, and morphology of synthesized ZnONPs were characterized by the various standard characterization techniques.
13.3.1.1 XRD Analysis
XRD graph depicted through XRD studies, which is shown in Figure 13.2. The Debye–Sherrer formula (D = Kλ/βcos θ) was used to calculate the average crystallite size where K represents the Sherrer constant, λ stands for X‐ray wavelength, β is the peak width at half‐maximum, and θ symbolize the Bragg's diffraction angle.

Figure 13.2 XRD of ZnONPs of Ficus auriculata leaves.
The orientation and crystalline nature of ZnONPs was revealed by XRD pattern. The peak position with 2θ values of 31.841°, 34.507°, 36.324°, 47.592°, 56.634°, 62.895°, 66.426°, 68.064°, and 69.22° are indexed as (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes, which is found to be in good concurrence with those of powder ZnO acquired from the International Centre of Diffraction Data card (JCPDS‐36‐1451) verifying the formation of a crystalline hexagonal wurtzite structure [19]. The ZnONPs was found to be having an average size of nearly 20 nm.
13.3.1.2 FT‐IR Analysis
The FT‐IR spectrum of synthesized ZnONPs showed (Figure 13.3) presence of bands at 3444, 1549, 1434, 1085, 472 cm−1. Broad band at 3444 cm−1 is due to the presence of –NH stretching bond of amino group or due to –OH stretching vibration [20], [21]. Peak at 1549 cm−1, 1434 cm−1 may result from C<span class="dbond"></span>C stretching in aromatic nuclei. FT‐IR data reveal that phenolic group has the tendency to bind with metal, encap the nanoparticles, and thus stabilize them.
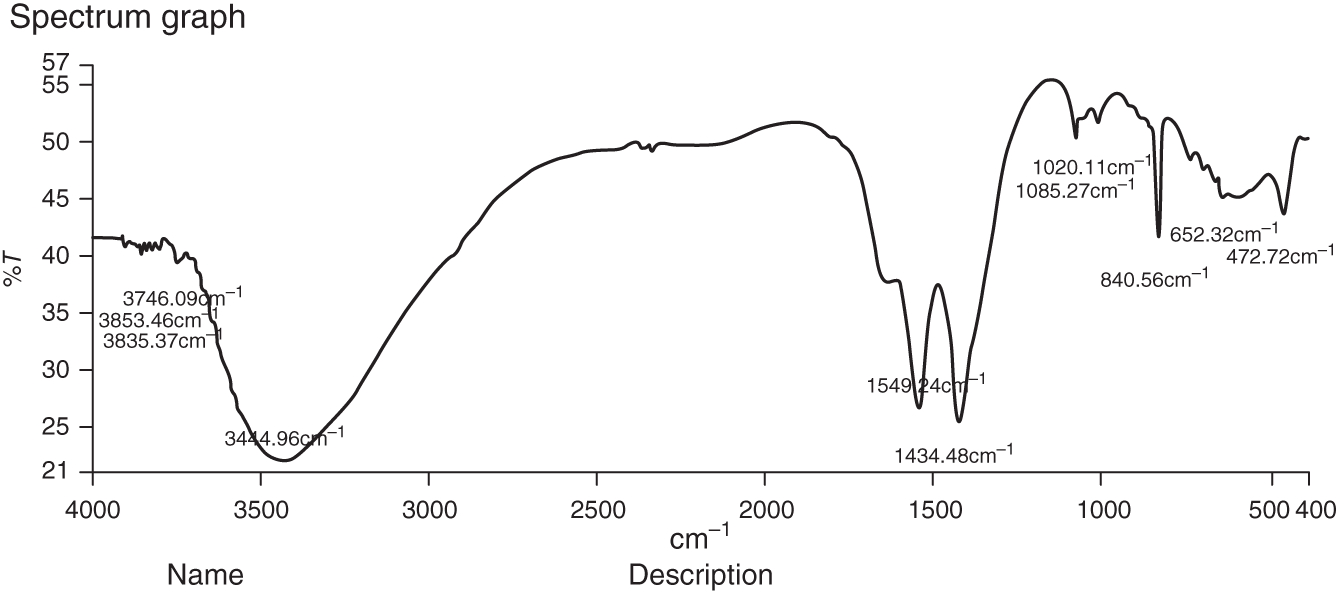
Figure 13.3 FT‐IR spectrum of ZnONPs of Ficus auriculata.
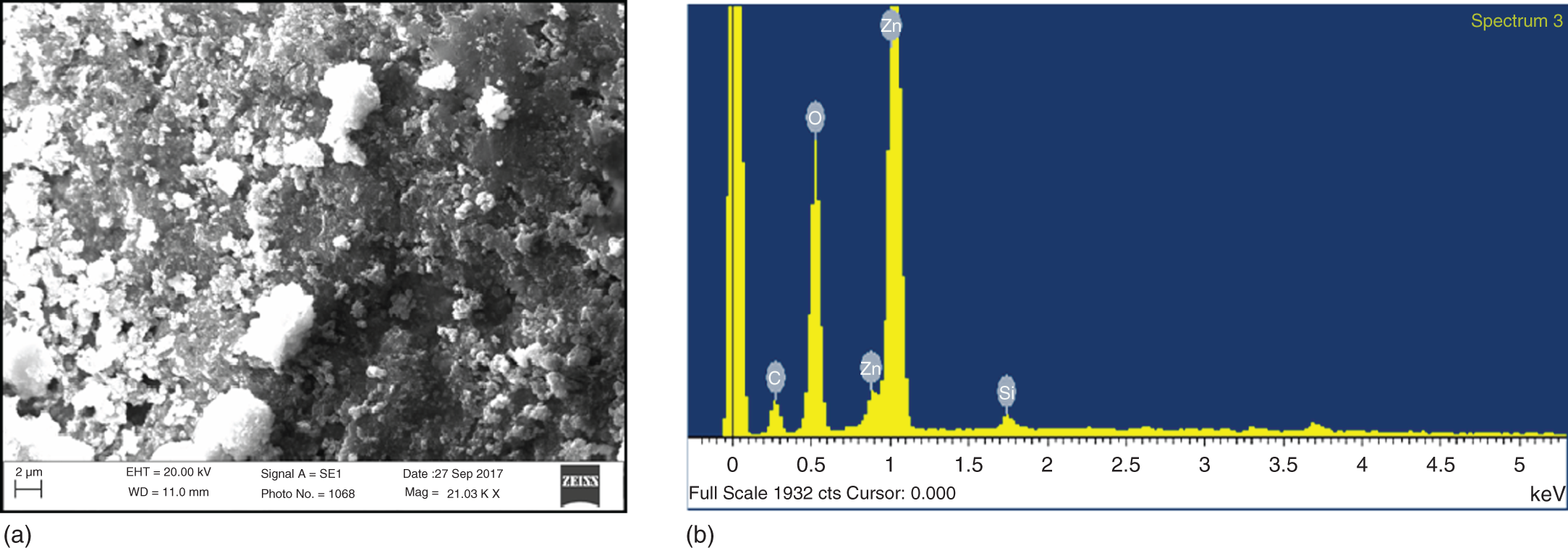
Figure 13.4 (a) SEM image and (b) EDX analysis of ZnONPs of Ficus auriculata.
Source: (b) Based on Bala et al. [22].
13.3.1.3 SEM Analysis
Further insight into the morphology of the ZnONPs was provided by SEM (see Figure 13.4a). The morphology of articles was nearly sphere like. The chemical composition of the ZnONPs was further analyzed by energy‐dispersive X‐ray spectroscopy (EDX).
The EDX analysis of ZnONPs is shown in the Figure 13.4b. It is an important tool for detecting the chemical composition of a specimen. EDX of the sample showed the occurrence of zinc and oxygen at stoichiometric ratio. EDX spectra also indicated the presence of carbon which might have originated from plant extract [22].
13.3.1.4 TEM Analysis
An idea about the size and shape of the nanoparticles can be achieved by TEM. The TEM picture clearly shows the distribution of spherical ZnONPs synthesized by F. auriculata extract (Figure 13.5). The ZnONPs were homogeneous and agglomerated with a particle size of 37 nm with some deviations [23].

Figure 13.5 TEM image of ZnONPs of Ficus auriculata.
13.3.2 Antibacterial Activity
The synthesized ZnONPs from plant extracts showed remarkable antibacterial activity. The biocapped ZnONPs obtained from F. auriculata leaves showed very good antimicrobial activity (Table 13.1) against B. cereus (21 mm) and K. pneumoniae (10 mm). It was found that green synthesized ZnONPs showed clear zone of inhibition. Here the positive control was streptomycin 25 µg/ml.
Table 13.1 Antibacterial activity of ZnONPs against B. cereus and K. pneumoniae.
| S. No. | Organisms | Zone of inhibition (mm) | Positive control (streptomycin‐25 µg/ml) |
|---|---|---|---|
| 1. | Bacillus cereus | 21 | 22.6 |
| 2. | Klebsiella pneumoniae | 10 | 18 |
13.4 Conclusion
Biosynthesis of ZnONPs by greener approach is valuable over the various chemical methods of nanoparticles synthesis. In this study, ZnONPs are prepared by using leaves extract of F. auriculata (Timla) without involving any synthetic reagents. Here the synthesis of ZnONPs was done by an ecofriendly, nonhazardous, cost effective and suitable green method. Different characterization methods such as XRD, FT‐IR, SEM, and TEM were used to characterize the nanoparticles. The nanoparticles were found to be crystalline, polydispersed, spherical in shape, and morphology with size of 37 nm. These nanoparticles also showed excellent antimicrobial activity against the selected pathogenic bacterial strains. This green cost‐effective method may be used as substitute of the chemical methods for the synthesis of zinc oxide NPs.
Acknowledgments
Authors are thankful to Department of USIC, HNB Garhwal University, Department of Microbiology, HNB Garhwal University, and VCSG Medical College, Srikot, Srinagar, Garhwal.
References
- 1 Arunachalam, K.D., Annamalai, S.K., and Hari, S. (2013). Int. J. Nanomed. 8: 1307.
- 2 Poliakoff, M., Fitzpatrick, J.M., Farren, T.R., and Anastas, P.T. (2002). Science 297 (5582): 807–810.
- 3 Raveendran, P., Fu, J., and Wallen, S.L. (2006). Green Chem. 8, 34 (1): –38.
- 4 Bartwal, A.S., Sati, S.C., and Ringwal, S. (2020). Appl. Innovative Res. 2: 39–43.
- 5 Li, L.S., Wald, J., Manna, L., and Alivisatos, A.P. (2002). Nano Lett. 2 (6): 557–560.
- 6 Zhang, H., Hussain, I., Brust, M., and Cooper, A.I. (2004). J. Adv. Mater. 16, 27 (1): –30.
- 7 Albrecht, M.A., Evans, C.W., and Raston, C.L. (2006). Green Chem. 8 (5): 417–432.
- 8 Kearns, G.J., Foster, E.W., and Hutchison, J.E. (2006). Anal. Chem. 78 (1): 298–303.
- 9 Veerasamy, R., Xin, T.Z., Gunasagaran, S. et al. (2011). J. Saudi Chem. Soc. 15: 113–120.
- 10 Parashar, V., Parashar, R., Sharma, B., and Pandey, A.C. (2009). Dig. J. Nanomater. Biostruct. 4: 45–50.
- 11 Sati, S.C., Kour, G., Bartwal, A.S., and Sati, M.D. . Biochemistry.https://doi.org/10.1021/acs.biochem.0c00388
- 12 Chaudhary, L.B., Sudhakar, J.V., Kumar, A. et al. (2012). Taiwania 57: 193.
- 13 Kour, G., Sati, S.C., and Sati, M. (2017). Int. J. Pharma Bio. Sci. 8 (2): 276–282.
- 14 John, J., Aravindakumar, C.T., and Thomas, S. (2018). SAJ Biotechnol. 4 (103): 19–21.
- 15 Stankovic, M.S. (2011). Kragujevac J. Sci. 1, 33: 63–72.
- 16 El‐Fishawy, A., Zayed, R., and Afifi, S. (2011). J. Nat. Prod. 4: 184–195.
- 17 Bertoletti, L.L., Skoronski, E., Schittler, L., and Kempka, A.P. (2018). Agri. Conspec. Sci. 9, 83 (4): 321–328.
- 18 Kour, G., Sati, S.C., and Mir, M.A. (2018, 2018). Asian J. Pharm. Pharmacol. 4: 192.
- 19 Yedurkar, S., Maurya, C., and Mahanwar, P. (2016). Open J. Synth. Theory Appl. 5: 1–14.
- 20 Susanto, H., Feng, Y., and Ulbricht, M. (2009). J. Food Eng. 139: 150.
- 21 Song, J.Y., Jang, H.K., and Kim, B.S. (2009). Process Biochem. 44: 1133.
- 22 Bala, N., Saha, S., Chakraborty, M. et al. (2015). RSC Adv. 5: 4993–5003.
- 23 Singh, R.P., Shukla, V.K., Yadav, R.S. et al. (2011). Adv. Mat. Lett. 2 (4): 313–317.
