CHAPTER 23
Heat Treatment
Learning Objectives
23.1 INTRODUCTION
Heat treatment is a process to control the mechanical properties of engineering materials by heating, cooling and alloying the metal as per requirement. It deals with change in properties by alloying different elements to the metal at various temperatures. The various mechanical properties such as hardness, toughness, ductility, machinability, and grain refinement are controlled by heat treatment process. In this chapter, we deal only with steel and its properties. Some of the basic heat treatment processes such as hardening, normalizing, annealing, tempering, with iron-carbon diagram and time temperature transformation diagram have been introduced.
23.2 IRON–CARBON PHASE DIAGRAM
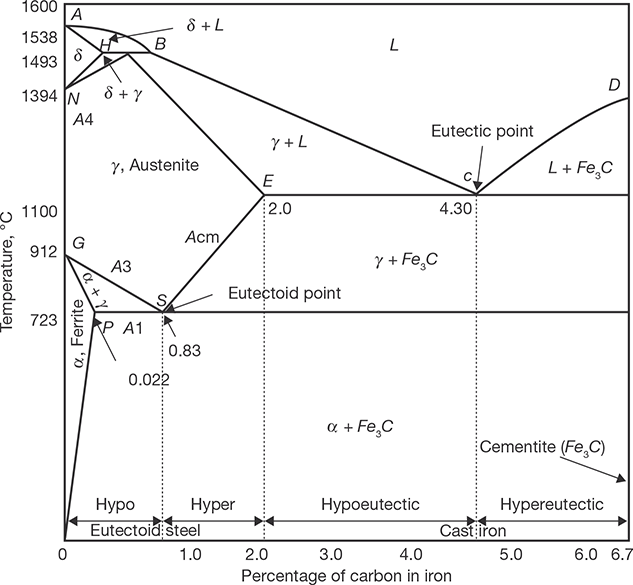
Iron-carbon (Fe-C) phase diagram shows the solubility of carbon in iron at different temperature and the corresponding structure of the steel. For describing the Fe-C phase diagram, the equilibrium between Fe and C is considered as metastable.
The larger phase field of γ-iron (austenite) compared with that of α-iron (ferrite) reflects the greater solubility of carbon in γ-iron, with a maximum value of just over 2% at 1147°C (E) as shown in Figure 23.1. This high solubility of carbon in γ-iron is of extreme importance in heat treatment when solution treatment in the γ-region followed by rapid quenching to room temperature allows a supersaturated solid solution of carbon in iron to be formed.
The α-iron phase field is severely restricted, with a maximum carbon solubility of 0.02% at 723°C (P), so over the carbon range encountered in steel from 0.05 to 1.5%, α-iron is normally associated with iron carbide in one form or another. Similarly, the δ-phase field is very restricted between 1390 and 1534°C and disappears completely when the carbon content reaches 0.5% (B).The great difference in carbon solubility between γ- and α-iron leads normally to the rejection of carbon as iron carbide at the boundaries of the γ phase field. The transformation of γ to α-iron occurs via a eutectoid reaction, which plays a dominant role in heat treatment. The eutectoid temperature is 723°C while the eutectoid composition is 0.80% C. On cooling alloys containing less than 0.80% C slowly, hypo-eutectoid ferrite is formed from austenite in the range 910–723°C with enrichment of the residual austenite in carbon, until at 723°C the remaining austenite, now containing 0.8% carbon transforms to pearlite (a lamellar mixture of ferrite and cementite). In austenite with 0.80 to 2.06% carbon, on cooling slowly in the temperature interval 1147°C to 723°C, cementite first forms progressively depleting the austenite in carbon, until at 723°C, the austenite contains 0.8% carbon and transforms to pearlite.
Steels with less than about 0.8% carbon are thus hypo-eutectoid alloys with ferrite and pearlite as the prime constituents, the relative volume fractions being determined by the lever rule which states that as the carbon content is increased, the volume percentage of pearlite increases until it is 100% at the eutectoid composition. Above 0.8% C, cementite becomes the hypereutectoid phase, and a similar variation in volume fraction of cementite and pearlite occurs on this side of the eutectoid composition.
There are several temperatures or critical points in the diagram, which are important, both from the basic and from the practical point of view.
Firstly, there is the A1, temperature at which the eutectoid reaction occurs, which is 723°C in the binary diagram.
Secondly, there is the A3, temperature when α-iron transforms to γ-iron. For pure iron, this occurs at 910°C, but the transformation temperature is progressively lowered along the line GS by the addition of carbon.
The third point is A4 at which γ-iron transforms to δ-iron, 1390°C in pure iron, but this is raised as carbon is added. The A2, the point is the Curie point when iron changes from the ferro- to the paramagnetic condition. This temperature is 769°C for pure iron, but no change in crystal structure is involved. The A1, A3 and A4 points are easily detected by thermal analysis during cooling or heating cycles.
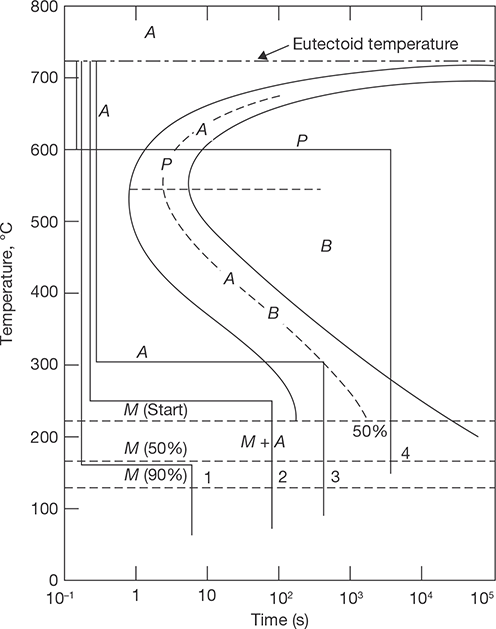
23.3 TTT (TIME–TEMPERATURE–TRANSFORMATION) DIAGRAM
The time-temperature-transformation curves correspond to the start and finish of transformations which extend into the range of temperatures where austenite transforms to pearlite. Above 550°C, austenite transforms completely to pearlite. Below 550°C, both pearlite and bainite are formed and below 450°C, only bainite is formed. The horizontal dotted line that runs between the two curves marks the beginning and end of isothermal transformations. The dashed line that runs parallel to the solid line curves represents the time to transform half the austenite to pearlite.
The transformations through various paths are described below as:
Path 1: The specimen is cooled rapidly to 160oC and left for 20 minutes.. The cooling rate is too rapid for pearlite to form at higher temperatures; therefore, the steel remains in the austenitic phase until the Ms temperature is passed, where martensite begins to form. Since 160°C is the temperature at which half of the austenite transforms to martensite, the direct quench converts 50% of the structure to martensite. Holding at 160°C forms only a small quantity of additional martensite, so the structure can be assumed to be half martensite and half retained austenite.
Path 2: The specimen is held at 250°C for 100 sec, which is not long enough to form bainite. Therefore, the second quench from 250°C to room temperature develops a martensitic structure.
Path 3: An isothermal hold at 300°C for 500 sec produces a half-bainite and half-austenite structure. Cooling quickly would result in a final structure of martensite and bainite.
Path 4: Austenite converts completely to fine pearlite after eight seconds at 600°C. This phase is stable and will not be changed on holding for 100,000 seconds at 873 K. The final structure when cooled, is fine pearlite.
23.4 NORMALIZING
Normalizing is a process of heating about 30 to 50°C above higher critical point for the time duration of 15 minutes and cooling in still air.
The purposes of the process normalizing are: (a) to reduce the grain size of steel, (b) to remove the internal stress caused by working, and (c) to improve some of the mechanical properties. The products obtained are ferrite and pearlite for hypoeutectoid steel and pearlite and cementite for hypereutectoid steel. The normalized structure of these two steel consists of sorbite and ferrite. The properties of normalized steel are higher yield point, ultimate tensile strength, impact strength and lower ductility. It is advantageous for low and medium carbon steel. For alloy steel, it is possible with time duration of 2 h cooling in the furnace.
23.5 ANNEALING
The purposes of annealing are: (a) to soften the metal for easy machining, (b) to remove internal stress caused by working, (c) to increase ductility, to refine grain size, and (d) to modify electrical and magnetic properties. Normalized steel is less ductile and has more yield point and tensile strength than the annealed steel. There are two types of annealing—process annealing and full annealing.
Process Annealing: This is a process of heating the metal below or very close to lower critical temperature, i.e., 650°C for steel and slow cooling to form new grain structure. The purposes of the process are: (a) to increase the ductility of cold worked metal and (b) to remove internal stress. This is frequently used in wire drawing to increase the plasticity of the metal.
Full Annealing: The purposes of full annealing are: (a) to soften the steel, (b) to refine grain structure above the upper critical limit by 20 to 30°C for 0.9% C-steel and by the same amount below the critical point for high carbon steel. Carbon-steel is cooled 100° to 200°C per hour. It is essential that the steel should not hold less than 4 to 8 min for heating. To prevent the steel for carburization and oxidization workpiece is closed in a metal box and put into the furnace. Austenite changes to pearlite and mixture of pearlite and ferrite.
23.6 SPHEROIDIZING
Spherodizing is used to improve the machinability of steel. The workpiece is heated to 730–770°C, slightly above the lower critical temperature, and cooled 25-30°C per hour.
23.7 HARDENING
The purposes of hardening are: (a) to harden the steel to resist wear, (b) to enable it to cut other metal. The metal is heated 30–50°C above the upper critical temperature for hypoeutectoid steel and above the same amount above the lower critical temperature for hypereutectoid steel. It is left for soaking for considered time. Quenching of high carbon steel heated to 1100–1300°C is done in a current of air. Quenching 150–200°C per sec in solution 3–10% caustic soda and 5–15% salt is more rapid than the quenching effect in water at 20oC and 32–42°C for oil quenching.
23.8 TEMPERING
Tempering is a process of reheating of hardened steel below critical range and cooled at the decreased rate (approximately 4 to 5 minutes for each mm of the section). There is the partial transformation of martensite to secondary constituent troosite and sorbite. The purposes of tempering are: (a) to reduce some amount of hardness produced during hardening and increase the ductility and (b) to remove strain produced during heating.
Low-temperature Tempering: Steel is heated to 150–250°C and cooled down. This is used to remove internal stress, reduce hardness, and increase ductility without changing the steel structure.
Medium-temperature Tempering: Steel is heated to 350–450°C and cooled down. Martensite is changed is changed to secondary troosite. It results in a reduction in strength and hardness, and increase in ductility. It is used for the part which is to be used in impact loadings such as chisel, hammer, spring, and spring plates.
High-temperature Tempering: Steel is heated to 500–600°C and cooled down. Martensite is changed to sorbite. Internal stress is relieved completely. This is used for the part subjected to high impact and stress such as gear wheels, shafts, and connecting rod, etc.
23.8.1 Austempering
This is the method that can be used to overcome the restrictions of conventional quench and tempering. The quench is interrupted at a higher temperature than for Martempering to allow the metal at the center of the part to reach the same temperature as the surface. By maintaining that temperature, both the center and the surface are allowed to transform to Bainite and are then cooled to room temperature.
The austempering heat treatment consists of three steps—austenitization in the temperature range of 840–950°C for a time sufficient to produce fully austenitic matrix, rapid cooling of the entire part to an austempering temperature in the range of 230–450°C without any transformations, and isothermal treatment at the austempering temperature, at which during the transformation only bainitic ferrite forms in a favorable case. Advantages of austempering are less distortion and cracking than Martempering, no need for final tempering, improvement of toughness, and Improved ductility. Limitation of austempering is that the austempering can be applied to parts where the transformation to pearlite can be avoided. This means that the section must be cooled fast enough to avoid the formation of pearlite. Thin sections can be cooled faster than the bulky sections.
23.8.2 Martempering
Martempering or marquenching permits the transformation of Austenite to Martensite to take place at the same time throughout the structure of the metal part. By using interrupted quench, the cooling is stopped at a point above the martensite transformation region to allow sufficient time for the center to cool to the same temperature as the surface. The cooling is continued through the martensite region, followed by the usual tempering.
Martempering of steel (and of cast iron) consists of quenching from the austenitizing temperature into a hot fluid medium (hot oil, molten salt, molten metal, or a fluidized particle bed) at a temperature usually above the martensite range (Ms point), holding in the quenching medium until the temperature throughout the steel is substantially uniform, and cooling (usually in air) at a moderate rate to prevent large differences in temperature between the outside and the center of the section
The advantage of martempering lies in the reduced thermal gradient between surface and center as the part is quenched to the isothermal temperature and then is air cooled to room temperature. Residual stresses developed during martempering are lower than those developed during conventional quenching. Martempering also reduces or eliminates susceptibility to cracking. Another advantage of martempering in a molten salt is the control of surface carburizing or decarburizing.
23.9 CARBURIZING
Carburizing is a heat treatment process in which iron or steel absorbs carbon liberated when the metal is heated in the presence of a carbon rich atmosphere, such as charcoal or carbon monoxide, with the intent of making the metal harder. Depending on the amount of time and temperature, the affected area can vary in carbon content. Longer carburizing times and higher temperatures lead to greater carbon diffusion into the part as well as increased depth of carbon diffusion. When the iron or steel is quenched, the higher carbon content on the outer surface becomes hard via the transformation from austenite to martensite, while the core remains soft and tough as a ferritic and/or pearlite microstructure. It is applied to low-carbon workpieces; workpieces are in contact with a high-carbon gas, liquid or solid; it produces a hard workpiece surface; workpiece cores largely retain their toughness and ductility, and it produces case hardness depths of up to 6.4 mm.
Gas Carburizing: It is a heat treatment process, which improves the case depth hardness of a component by diffusing carbon into the surface layer to improve wear and fatigue resistance. The workpieces are pre-heated and then held for a period of time at an elevated temperature in the austenitic region of the specific alloy, typically between 820 and 940°C. During the thermal cycle the components are subject to an enriched carbon atmosphere such that nascent species of carbon can diffuse into the surface layers of the component. The rate of diffusion is dependent on the alloy and carbon potential of the atmosphere. Care must be taken to ensure that only sufficient carbon is available in the atmosphere at any one time to satisfy the take-up rate of the alloy to accept the carbon atoms.
Pack Carburizing: It is a heat treatment process in which carbon monoxide derived from a solid compound decomposes at the metal surface into nascent carbon and carbon dioxide. The nascent carbon is absorbed into the metal, and the carbon dioxide immediately reacts with carbonaceous material present in the solid carburizing compound to produce fresh carbon monoxide. The formation of carbon monoxide is enhanced by energizers or catalysts, such as barium carbonate, calcium carbonate, potassium carbonate, and sodium carbonate that are present in the carburizing compound. These energizers facilitate the reduction of carbon dioxide with carbon to form carbon monoxide. Thus, in a closed system, the amount of energizer does not change. Carburizing continues as long as enough carbon is present to react with the excess carbon dioxide. Pack carburizing is no longer a major commercial process.
23.10 CYANIDING
Steel parts may be surface-hardened by heating in contact with a cyanide salt, followed by quenching. Only a thin case is obtained by this method. Cyaniding is, however, a rapid and economical method of case hardening, and may be used in some instances for relatively unimportant parts. The work to be hardened is immersed in a bath of molten sodium or potassium cyanide from 30 to 60 minutes. The cyanide bath should be mainlined at a temperature to 760 to 899°C. Immediately, after removal from the bath, the parts are quenched in water. The case obtained in this manner is due principally to the formation of carbides and nitrides on the surface of the steel. The use of a closed pot and ventilating hood are required for cyaniding, as cyanide vapors are extremely poisonous.
23.11 NITRIDING
This method is advantageous due to the fact that a harder case is obtained than by carburizing. Many engine parts such as cylinder barrels and gears may be treated in this way. Nitriding is generally applied to certain special steel alloys, one of the essential constituents of which is aluminum. The process involves the exposing of the parts to ammonia gas or other nitrogenous materials for 20 to 100 h at 500–650°C. The container in which the work and Ammonia gas are brought into contact must be airtight and capable of maintaining good circulation and even temperature throughout. The depth of case obtained by nitriding is about 0.2 to 0.4 mm if heated for 50 h. The nitriding process does not affect the physical state of the core if the preceding tempering temperature was 500°C or over.
23.12 INDUCTION HARDENING
This process involves heating applied rapidly and locally to the steel component followed by quenching. High-frequency electric fields quickly heat the surface of the component via induction coils, which is then quenched using water. This results in a localized hardened layer at the surface. Different shaped inductor coils are available and can be made to suit. Induction Hardening offers a cost effective low distortion surface hardening treatment to steels, particularly large components where an increase in surface hardness is required whilst maintaining core properties.
RECAP ZONE
Points to Remember
- Heat treatment is a process to control the mechanical properties of engineering materials by heating, cooling and alloying the metal as per requirement.
- Iron-carbon (Fe-C) phase diagram shows the solubility of carbon in iron at different temperature and the corresponding structure of the steel.
- Firstly, there is the A1, temperature at which the eutectoid reaction occurs, which is 723°C in the binary diagram.
- Secondly, there is the A3, temperature when α-iron transforms to γ-iron. For pure iron this occurs at 910°C, but the transformation temperature is progressively lowered along the line GS by the addition of carbon.
- The third point is A4 at which γ-iron transforms to δ-iron, 1390°C in pure iron, hut this is raised as carbon is added.
- The time-temperature-transformation curves correspond to the start and finish of transformations which extend into the range of temperatures where austenite transforms to pearlite.
- Above 550°C, austenite transforms completely to pearlite. Below 550°C, both pearlite and bainite are formed and below 450°C, only bainite is formed.
- Normalizing is a process of heating about 30 to 50°C above higher critical point for the time duration of 15 min and cooling in still air.
- The purposes of the process normalizing are: (a) to reduce grain size of steel, (b) to remove internal stress caused by working, and (c) to improve some of the mechanical properties.
- The purposes of annealing are: (a) to soften the metal for easy machining, (b) to remove the internal stress caused by working, (c) to increase ductility, to refine the grain size, and (d) to modify the electrical and magnetic properties.
- Normalized steel is less ductile and has more yield point and tensile strength than the annealed steel.
- Tempering is a process of reheating of hardened steel below critical range and cooled at the decreased rate (approximately 4 to 5 min for each mm of the section). There is the partial transformation of martensite to secondary constituent troosite and sorbite.
- The purposes of tempering are: (a) to reduce some amount of hardness produced during hardening and increase the ductility and (b) to remove strain produced during heating.
- Carburizing is a heat treatment process in which iron or steel absorbs carbon liberated when the metal is heated in the presence of a carbon rich atmosphere, such as charcoal or carbon monoxide, with the intent of making the metal harder.
- Steel parts may be surface-hardened by heating in contact with a cyanide salt, followed by quenching.
- This process involves heating applied rapidly and locally to the steel component followed by quenching. High-frequency electric fields quickly heat the surface of the component via induction coils, which is then quenched using water.
REVIEW ZONE
Multiple-choice Questions
- The heat treatment process in which steel is heated above upper critical temperature and then cooled in air is known as:
- Annealing
- Normalizing
- Austempering
- Martempering
- The heat treatment process in which steel is heated above upper critical temperature and then cooled in furnace is known as:
- Annealing
- Normalizing
- Austempering
- Martempering
- In nitriding steel components, the following atmosphere is generally used in the furnace:
- Inert
- Nascent nitrogen
- Liquid nitrogen
- Ammonia
- Austempering is the heat treatment process used to obtain greater:
- Hardness
- Toughness
- Softness
- Brittleness
- Low carbon steel can be hardened by:
- Hardening
- Heating and quenching in oil
- Heating and quenching in water
- Carburizing and cyaniding
- The hardening strains are reduced and the toughness of the part increased by the following process after hardening:
- Annealing
- Tempering
- Carburizing
- Anodizing
- A small selected portion of the job can be hardened by:
- Flame and induction hardening
- Pack hardening
- Cyaniding
- Case hardening
- Which of the following is a case hardening process:
- Spherodising
- Tempering
- Cyaniding
- Parkerising
- Martensite is a supersaturated solution of carbon in:
- Iron
- Steel
- α-iron
- δ-iron
- Martensite is a structure obtained by:
- Quenching austenite
- Quenching austenite and heating into the range of 200 to 375°C
- Quenching austenite and heating into the range of 375 to 660°C
- Quenching austenite and heating into the range of 600 to 700°C
Fill in the Blanks
- 11. Troosite is the structure obtained by quenching austenite and heating at ___________°C.
- 12. Line A1 on iron-carbon diagram indicates completion of austenite transition to ___________.
- 13. Line Acm on iron-carbon diagram indicates limit of carbon solubility in ___________.
- 14. Line A3 on iron-carbon diagram indicates the beginning of transition fron austenite to ___________.
- 15. Eutectoid composition of carbon steel at room temperature is known as ___________.
Theory Questions
- What is heat treatment? Discuss its importance in metallurgy.
- Write the importance of FE-C diagram? Draw the diagram and explain the solubility of carbon in iron at the different temperature.
- Draw the TTT diagram and explain the isothermal transformation process.
- Differentiate between normalizing and annealing.
- Write notes on the process and full annealing.
- Differentiate between austempering and martempering.
- Differentiate between hardening and tempering.
- Write notes on: (i) case hardening, (ii) pack carburizing, (iii) nitriding, (iv) cyaniding, and (v) induction hardening.
- * What is tempering? What are its objectives?
- * Explain various case hardening processes of steel.