240 A. UNITS AND SCIENTIFIC NOTATION
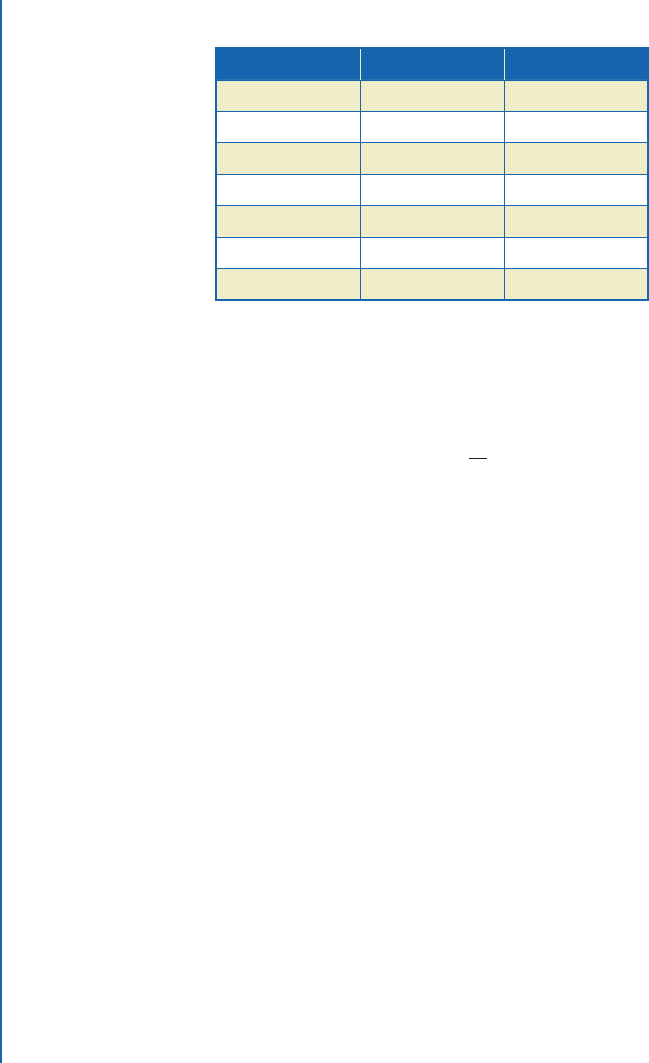
Table A.1: Common SI units
Dimension Unit Abbreviation
Length Meter m
Time Second s
Mass Kilogram kg
Temperature Kelvin K
Force Newton N
Energy Joule J
Power Watt W
to divide m=s by seconds? We can do that just fine, and we get m=s=s D m=s
2
(called “meters
per second squared”). Many of the units in Table A.1 are actually derived combinations of other
units. For example, the newton is actually a combination of kilograms, meters, and seconds:
1 N D 1 kg
m
s
2
: (A.1)
ese base units can be modified by any one of a number of official prefixes, which then
multiplies the unit by some power of 10. ese prefixes and their abbreviations are listed in
Table A.2, although some are more commonly used than others. For example, “milli” means
“ˆ1=1000.” And so a millimeter (abbreviated mm) is one thousandth of a meter.
A.2 SCIENTIFIC NOTATION
We have used scientific notation for the values in Table A.2. Physical quantities in nature can
vary by many powers of 10. And so for example the light given off by the Sun, it’s power, P, is
many times greater than the light given off by a 60 W light bulb:
P
Sun
D 667000000000000000000000000P
lightbulb
: (A.2)
After the 667, there are 24 zeros there. What if I had mistyped (or you miscounted) and
you found 23 zeros instead? Well that number would be ten times too small. And so clearly, when
dealing with numbers like this, we need a better way. And so we use what is called scientific
notation. Written this way, the above equation becomes:
P
Sun
D 6:67 ˆ 10
26
P
lightbulb
: (A.3)
e ˆ 10
26
part means, ˆ100000000000000000000000000. But in practical terms this
also means, “take the decimal point in 6.67, and move it 26 places to the right, filling in with
zeros as needed.”

A.2. SCIENTIFIC NOTATION 241
Table A.2: Prefixes for SI units
Prefi x Abbreviation Meaning
Femto f
× 10
–15
Pico p
× 10
–12
Nano n
× 10
–9
Micro
μ
× 10
–6
Milli m
× 10
–3
Centi c
× 10
–2
Deci d
× 10
–1
Hecto h
× 10
2
Kilo k
× 10
3
Mega M
× 10
6
Giga G
× 10
9
Tera T
× 10
12
Raising something to a negative power means the same thing as dividing 1 by that same
thing, but raised to the same positive power. For example:
27
´3
D
1
27
3
: (A.4)
And so we can also use negative numbers in scientific notation; it means simply divide by the
power of 10 instead of multiplying by it. And as with positive powers, we can also express this
as a decimal equivalent:
3:27 ˆ 10
´5
D 3:27 ˆ
1
10
5
D
3:27
10
5
D 0:0000327: (A.5)
Here we can see that 3:27 ˆ 10
´5
means, “take the decimal place in 3.27 and move it 5 places
to the left, filling in with zeros as needed.”
is has a couple of advantages. For one thing, we can see at a glance the most important
part numerically: how many powers of ten. Second, when we write it this way, we don’t need the
zeros for place holders. And so if I put them there, it means I believe that they are significant.
And so, 6:67 ˆ 10
26
and 6:670 ˆ 10
26
are not really the same number, although they will
both appear the same on a calculator. 6:67 ˆ 10
26
could possibly be 6:673 ˆ 10
26
or even 6:668 ˆ
10
26
. If I do not include any more decimal places, then I am making a statement that, based
on my uncertainty in the measurement of that quantity, I have no idea what the value of the
next decimal place would be. If, on the other hand, I write 6:670 ˆ 10
26
then I am saying that I
believe (even if with some uncertainty) that it really is 6:670 ˆ 10
26
and not, say, 6:673 ˆ 10
26
.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
