12.2. LIGHT: ELECTROMAGNETIC WAVES 167
-1
-0.5
0
0.5
1
A
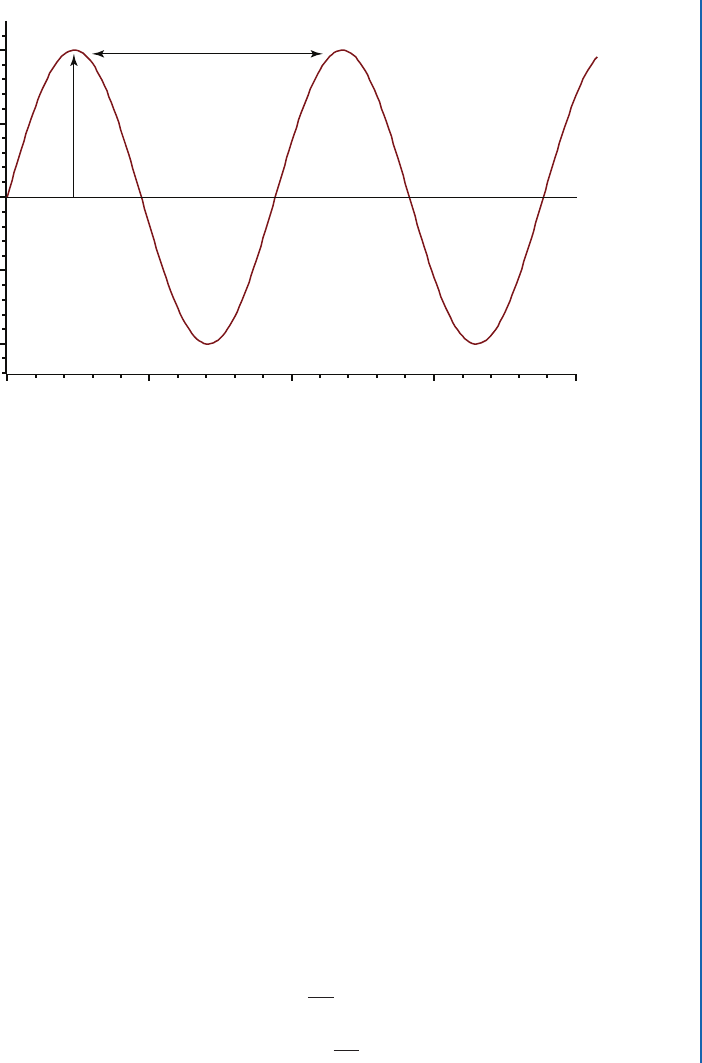
100 20 30 40
Height
Time (s)
Period (T)
Figure 12.2: Period (T) and amplitude (A) of a wave. Notice that the horizontal axis is time. e
period is the time over which the wave repeats itself at a particular point in space. e frequency
(f) of the wave is the reciprocal of the period.
speed. us, we have a relation between the frequency, f , the wavelength, , and the speed, v,
of a wave:
v D f : (12.2)
12.2 LIGHT: ELECTROMAGNETIC WAVES
e last two Maxwell equations—Faraday’s Law (11.16) and the Ampère–Maxwell Law
(11.17)—are in many ways the most intriguing. Let us consider in particular a simple but special
case—the vacuum of empty space itself. If there is nothing there at all, then certainly there is no
moving charge, and thus no charge density,
E
J. And so these two equations become simply:
r ˆ
E
E D ´
B
E
B
Bt
(12.3)
r ˆ
E
B D
0
0
B
E
E
Bt
: (12.4)
168 12. WAVES
ese two equations, taken together, say that a magnetic field,
E
B, that changes with time causes
there to be a swirling electric field,
E
E. But also, the opposite is true: an electric field,
E
E, that
changes with time causes there to be a swirling magnetic field,
E
B.
I can easily make a changing electric field; simply jiggle an electric charge back and forth.
Gauss’s law says that electric field diverges away from the charge. And so if the charge is moving
back and forth, the electric field at any given point in the empty space around it must also be
changing with time. But in that empty region of space, the changing
E
E will cause, according to
Equation (12.4), a magnetic field
E
B. And in this particular case the magnetic field induced will
also be changing with time.
You can see where this is going. e induced and changing magnetic field
E
B would then
produce in the space around it—by Equation (12.3)—a swirling electric field
E
E, which by Equa-
tion (12.4) would produce a changing magnetic field, and so on.
Maxwell was the first to realize the profound implications of Equations (12.3) and (12.4)
taken together, even for the empty vacuum of space. Once one produces a changing electric
or magnetic field (by, for example, jiggling a charged particle back and forth), the two fields
inevitably act upon each other to produce a changing pattern of electricity and magnetism that
moves rapidly through space—an electromagnetic wave.
In hindsight, it is only a matter of sophomore-level physics to demonstrate that Equa-
tions (12.3) and (12.4) can be combined mathematically to form the wave equation. In the pro-
cess, the speed of this wave can be calculated directly from the scale factors
0
and
0
—already
known in Maxwell’s time from simple laboratory experiments of electricity and magnetism. e
answer is this: the speed of the wave of electricity and magnetism is 2:998 ˆ 10
8
m s
´1
. I hope
that by now you recognize this speed as c, the speed of light.
And thus light is an electromagnetic wave—a rapidly changing pattern of electric and
magnetic field that can move through even the vacuum of space. An electromagnetic wave can
do this because the electric and magnetic fields are properties of space itself, and so there need
be nothing there at all. But if on the other hand there is something there—a charged particle for
example—the electromagnetic wave will do stuff to it as it passes by. For the fields exert forces
on charged particles.
And so, move a charged particle back and forth over here, and an electromagnetic wave
will radiate outward through space at the speed of light. e traveling electromagnetic wave
will then exert forces on a charged particle over there, and so cause stuff to happen. is is the
meaning of light.
12.2.1 THE ELECTROMAGNETIC SPECTRUM
We use the symbol c to represent the speed of light—the speed of an electromagnetic wave
traveling in a vacuum. And so for light, Equation (12.2) becomes:
c D f : (12.5)

12.2. LIGHT: ELECTROMAGNETIC WAVES 169
Because the speed of light, c, is a constant, we can see there is a simple relationship be-
tween wavelength and frequency for electromagnetic waves. us, any given wavelength corre-
sponds to a particular frequency, and vice versa. We can rearrange Equation (12.5) as follows:
f D
c
(12.6)
D
c
f
: (12.7)
ese are reciprocal relations; if frequency is larger, then wavelength is smaller, and vice versa. It
also means that, for light, we can choose either frequency or wavelength for our description. If
given one, the other can be easily calculated.
Different wavelengths (or frequencies) of electromagnetic waves interact with matter in
different ways. Since both the absorption and emission of light are examples of such interactions,
one would need different strategies to produce light of vastly different wavelengths. Likewise,
different methods are required to detect light of very different wavelengths.
e human eye is only sensitive to the very narrow range of wavelengths between about
0.4–0.7 millionths of a meter, and so it is this range of wavelengths that defines what we call
visible light.
e electromagnetic wave nature of light was unknown until the late 1800s. And it wasn’t
until the 20th century that most forms of electromagnetic waves were finally identified. Some
types had previously been detected, but it wasn’t recognized until much later that they were
just different wavelengths of electromagnetic waves. And so different ranges of wavelength of
electromagnetic waves have different names, in part for historical reasons.
Table 12.1 shows the ranges of possible wavelengths, along with their customary names.
Taken together, this is called the electromagnetic spectrum. Keep in mind that the ranges of wave-
lengths or frequencies are only approximate; the boundaries are fuzzy and overlap each other.
e names really come from the different ways in which we produce or detect them, and that
has changed over the years as technology has changed.
And so let us very briefly consider each of these basic parts in turn. I will start at the long-
wavelength bottom of the list; this may seem strange, but remember that long wavelength is the
same as low frequency.
• Radio waves are made by moving an electrical current back and forth in a wire, and radio
waves induce currents to oscillate back and forth in wires they pass through.
• Microwaves can be thought of as very high-frequency radio waves. ey can sometimes
be made in the same fashion, but other processes (besides electronic circuits) are also used
to produce them. Because of their shorter wavelength, they can be focused with special
mirrors and more easily guided along paths.
• Infrared light is usually produced in ways similar to visible light, but the wavelengths are
too long for the human eye to detect it.

170 12. WAVES
Table 12.1: e electromagnetic spectrum
Name Typical λ(m) Typical Size
f (Hz)
Gamma Ray
< 1 × 10
-11
Atomic nucleus
> 3 × 10
19
X-Ray 1 × 10
-11
– 3 × 10
-8
Atom 1 × 10
16
– 3 × 10
19
Ultraviolet 1 × 10
-8
– 4 × 10
-7
Virus 7.5 × 10
14
– 3 × 10
16
Visible Light 4 × 10
-7
– 7 × 10
-7
Bacteria 4.3 × 10
14
– 7.5 × 10
14
Infrared 7 × 10
-7
– 1 × 10
-3
Protozoa 3 × 10
11
– 4.3 × 10
14
Microwaves 1 × 10
-4
– 0.1 Person 3 × 10
9
– 3 × 10
12
Radio
> 0.1
Building
< 3 × 10
9
• Visible Light is the name for the narrow range of wavelengths (about 400–700 nm) to
which the human eye is sensitive. Different wavelengths of visible light produce different
color sensations; violet for short wavelengths and red for long wavelengths, with blue,
green, yellow, and orange in between.
• Ultraviolet light also is often produced in ways that are similar to visible light, but the
wavelengths are too short to be detected by our eyes.
• X-rays have wavelengths similar to the size of individual atoms, and so they usually interact
with matter in ways that involve individual atoms on a one-on-one basis.
• Gamma rays, with their enormous penetrating power, interact directly with the tiny nuclei
of atoms. And thus they are associated most strongly with nuclear reactions.
12.2.2 LIGHT AND ITS SPECTRUM
ere are many ways in which light can be created by matter. But any particular method for
producing light is essentially an interaction between light and matter, and the wavelength (or
frequency) of the light is of crucial importance.
For example, to make electromagnetic waves of a frequency of 10
6
Hz, simply use an elec-
tronic oscillator circuit to make a current go back and forth in a wire at that frequency. A fre-
quency of 10
6
Hz is in the radio part of the electromagnetic spectrum, and so this is essentially
the basis of a radio transmitter. But for visible light with frequencies several powers of ten higher,
this strategy simply will not work. Instead one can, for example, heat up a tungsten wire to a
high temperature, and the individual tungsten atoms will vibrate at high frequencies, and visible
(and infrared) light will be emitted.
Whenever light is created by matter, some wavelengths are made well while others are
made poorly or not at all. us, to really describe a particular source of light, we need to describe

12.2. LIGHT: ELECTROMAGNETIC WAVES 171
4,500 5,000 5,500 6,000
Double Star β Cygni (Albireo)
Wavelength (Angstrom)
Relative Brightness
β Cygni A
β Cygni B
1.4
1.2
1
0.8
0.4
0.2
Figure 12.3: e spectra of two stars making up the binary star system ˇ Cygni. Each colored line
represents the spectrum of one of the stars. e fine-scale squiggles result from noise (random
measurement uncertainty), but the overall trends in the spectra are accurate. e spectra span
the range from 4,200–6,000 Å (420–600 nm), thus covering the range from blue-violet on the
left to orange-red on the right (data from Beaver and Conger [2012]).
how much of each wavelength has been produced. Such a description is called a spectrum (plural,
spectra) of the light source, and the most useful way to represent one is with a graph.
Figure 12.3 shows the spectra of the two stars that make up the binary star system Albireo.
e horizontal axis of the graph is wavelength, with short wavelength on the left and long
wavelength on the right. e axis goes from about 420 ´600 nm, and this is visible light, within
the range of wavelengths sensitive to the human eye. e spectrum of one of the stars is marked
in red while the other in blue. Clearly, ˇ Cygni A emits more long-wavelength (red) light than
short-wavelength light (blue), while the opposite is true for ˇ Cygni B.
In the case of ˇ Cygni and other stars astrophysicists can determine many things about
their physical natures by analyzing the spectra of the light they emit. We will use this idea of
the spectrum of a light source many more times throughout this book; it is one of the most
important tools for understanding light.
12.2.3 THERMAL RADIATION
e term thermal radiation refers to electromagnetic waves emitted due to the normal motions
of atoms and molecules in some material. Whether it be liquid, solid, or gas, the atoms and
molecules that make it up are constantly in motion. Although the individual particles are moving
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
