Conductors and Superconductors
AG Clegg, MSC, PhD, University of Sunderland (Sections 5.1 and 5.2)
NG Dovaston, BSC, PhD, MPRI., University of Sunderland (Section 5.1.3)
5.1 Conducting materials
A major part of the world’s production of copper is used in the unalloyed form, mainly in the electrical industries. Copper has the highest electrical and thermal conductivity of the common industrial metals with good mechanical properties, resistance to corrosion, easy jointing, ready availability and high scrap value.
5.1.1.1 Conductivity
Pure copper has a volume resistivity at 20° C of 1.697 × 10−8 Ω-m, lower than any known material except silver. In 1913 the International Electrochemical Commission established the International Annealed Copper Standard (IACS) by which the conductivity of all other grades and purities of copper and its alloys should be measured. The standard chosen was an annealed copper wire of length 1 m and cross-sectional area 1 mm2, having a resistance of 0.17421 Ω. The corresponding volume resistivity at 20°C was assigned at 0.17421 × 10−8 Ω-m representing 100% IACS. The percentage IACS for any other material can then be calculated as
Since the standard was adopted in 1913, higher purity copper is now commonly produced, explaining why electrical conductivities of up to 101.5% are frequently quoted.
The relative conductivity at 20°C of other metals compared to that of copper (= 100) is silver 104, aluminium 60, nickel 25, iron 17, platinum 16, tin 13 and lead 8.
All impurities tend to lower the conductivity of copper but the worst effects are produced when solid solutions are formed. Precipitation by heat treatment can sometimes be used to minimise these effects. The worst impurities include phosphorus, arsenic, antimony and nickel which are commonly found in some grades of copper. Silver, cadmium and zinc produce only a marginal decrease in conductivity whilst improving mechanical properties considerably. Figure 5.1 shows the effect of a range of impurities on the electrical resistance of copper. The requirements for copper wires for various electrical purposes are given in BS 4109 and BSEN 12166. More detailed information about standards is given at the end of the chapter.
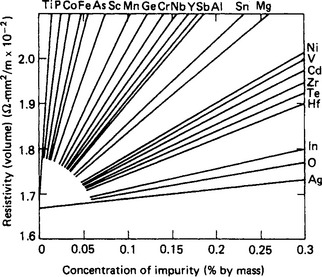
Figure 5.1 Effect of added elements on the electrical resistivity of copper. (Courtesy of the Copper Development Association)
The resistivity of copper, like that of all pure metals, increases with increasing temperature. The temperature coefficient of resistivity, αT, for pure copper is 3.95 × 10−3/°C. For accurate work the temperature coefficient must take account of dimensional changes due to thermal expansion. However omission of this correction for copper leads to errors of less than 0.5%.
5.1.1.2 Mechanical properties and electrical conductor applications of copper and its alloys
Copper, as cast, has a tensile strength of 150–170 MN/m2. Subsequent rolling, drawing or other hot and cold working can raise the tensile strengths to 230 for the annealed material and to a maximum of 450 MN/m2 for hard-drawn wire. Over this range of strengths, tensile moduli increase from 110 to 130 GN/m2, Vickers hardness values increase from 50 to 110–130 and ductilities decrease from 45–60% to 5–20%. Cold drawn materials start to recrystallise and lose their strength at temperatures in the range 110–200°C. The increase in strength due to cold working is associated with a loss of conductivity. For hard-drawn material of tensile strength T in the range 300–400 MN/m2, there is a loss of conductivity of 0.007T% relative to IACS copper.
For low-strength, low temperature applications, fire refined tough pitch (TP) copper is used. Small amounts of oxygen (0.04–0.05%), present as finely dispersed copper oxide, produce some strengthening and act to concentrate harmful impurities such as bismuth, preventing them from forming brittle intergranular films. Because oxygen has negligible solubility in the copper matrix (less than 0.002%) the conductivity is only slightly reduced (less than 1 % IACS). Tough-pitch copper is unsuitable for intricate castings or applications where gas welding or brazing is involved. Reaction of oxides with the gas flame leads to hydrogen embrittlement. For these applications, the slightly more expensive oxygen free high conductivity copper (OFHC) containing less than 0.001% oxygen, is used. These two materials are most widely used for wire and strip conductors, for windings of a.c. and d.c. motors and generators and for transformers. Heavier gauge bar, strip and channel section material is used for bus-bars. Large quantities of high-conductivity wire and strip are used in telephone and power cables and as the outer sheathing of mineral insulated copper clad cable for fire and abrasion resistant applications.
Alloying of copper with cadmium, chromium, silver, beryllium and zirconium is used to improve mechanical properties and resistance to wear, especially at high temperatures. Such improvements are always at the expense of some increase in resistivity. Hardening of contacts by dispersions of refractory oxides improves resistance to ablation, fusion and wear.
Copper alloys containing 0.7–1.0% cadmium have greater strengths under both static (up to 750 MN/m2) and alternating stresses and greater resistance to wear, making them useful for contacts and telephone wires, although there is little improvement in strength retention at elevated temperatures. Conductivity is between 80 and 97% of that of IACS material, depending on the degree of cold work.
Alloys containing 0.77% chromium can be heat treated to retain their enhanced hardness and tensile strength (up to 480 MN/m2) even after exposure to more than 1000 h at 340°C when the tensile strength of OFHC or tough—pitch copper would be reduced to 170–200 MN/m2. Both tensile strength and conductivity depend on the heat treatment, high-temperature (solution) treated materials having typical values of 230 MN/m2 and 45% IACS while for annealed (precipitation hardened) materials the values are 450 MN/m2 and 80%. These materials are used in electrical engineering for welding electrodes and for light current-carrying springs. Copper-beryllium and copper-zirconium alloys have similar properties and applications with superior notch-fracture resistance.
For applications where the highest conductivity is essential and enhanced creep strength at high temperature is required (e.g. for the rotor conductors in large turbogenerators and for components which have to be tinned, soldered or baked during fabrication), copper alloys containing up to 0.15% silver are used. These have the same conductivity as IACS copper and retain their mechanical properties to 300°C.
Alloys containing tellurium (0.3–0.7%), sometimes with small amounts of nickel and phosphorus, have machining properties approaching those of free-cutting brass and retain their tensile strength (275 MN/m2) to 315°C with improved oxidation resistance. Tellurium additions alone produce only small reductions in conductivity as the solubility of tellurium in copper is only about 0.003% at 600°C. Copper-sulphur (0.4%) and copper lead (0.8%) alloys are also finding application because of their easy machining and electroplating properties.
For castings, electrolytically refined copper is sometimes used as the raw material, but more commonly, deoxidised tough-pitch copper is employed. The usual deoxidant for copper is phosphorus which produces large increases in resistivity (as little as 0.04% will reduce the electrical conductivity to about 75% of that for pure copper) and hence more expensive deoxidants such as silicon, lithium, magnesium, beryllium, calcium or boron are required. Cadmium-copper and chromium-copper alloys are also used for castings.
5.1.1.3 Other applications of copper and its alloys
Springs The material selected depends on whether the spring itself is required to carry current. For low conductivity spring materials, phosphor bronze (3.75–6.75% Sn, 0.1% P) and nickel silver (10–30% Ni, 10–35% Zn) are widely used and have conductivities in the ranges 12–27% and 6–8% IACS, respectively. Beryllium-copper alloys are used in more critical applications; a 2% beryllium alloy can be cold worked and heat treated to give tensile strengths of 1000–1500 MN/m2, while retaining a conductivity of 25–35% IACS. With other compositions and heat treatments even higher conductivities can be obtained.
Resistance and magnetic materials Copper alloys containing manganese (9–12%), aluminium (0–5%) and nickel (0–10%) are widely used as resistance materials because they have high resistivities (38-48 × 10−8 and 44-175 × 10−8 Ωm, respectively), low or zero temperature coefficients of resistance (-0.03 1+to.4 × 10−3/°C over the range 0–100°C) and low thermal e.m.f.s relative to copper (see page 2/9 section 2.2.2.6). Copper is also a constituent of many magnetic materials, both hard and soft.
Contacts Palladium-copper and silver-copper (7.5–50% copper) alloys are suitable for light current applications. For heavy-duty contacts, sintered copper-tungsten materials are tough and durable; similar materials are used for electric discharge machining electrodes.
Electroplating alloys Copper alloys containing nickel (7–23%) and zinc (10–35%), known as German or nickel silvers, are widely used for electroplating as they form durable corrosion-resistant coatings with reflectivities equivalent to that of standard silver.
Heat-transfer materials Copper alloys are extensively used in nuclear and fossil fuel steam plant for electric power generation for heat exchangers such as feedheaters and condensers, although there is an increasing tendency towards the substitution of mild steel for the former and titanium for the latter. The principal condenser alloys are Admiralty brass (70% Cu, 30% Zn with small arsenic and zinc additions to prevent dezincification) and aluminium brass (76% Cu, 2% Al, 30–10% Ni with 1–2% Fe, 1–2% Mn). These are used where improved corrosion resistance is required. In more erosive conditions caused by suspended solids in the cooling water, cupronickel alloys (70–90% Cu, 30–10% Ni, with 1–2% Fe, 1–2% Mn) are used at higher initial capital cost and with some penalty in heat transfer. For feedheater applications cupronickel alloys are most widely used in older plant.
Memory-effect alloys Some copper-zinc-aluminium alloys (8–14% Al, 0–14% Zn, 0.3% Ni) have the useful property of existing in two distinct shapes above and below a critical transformation temperature (within the range − 70°C to+130°C) due to a structural change. These alloys are finding applications in temperature-sensitive actuating devices, replacing bimetallic strip or thermistor controlled relay devices.
Superconducting alloys Dendritic copper-niobium alloys (20–30% Nb) may be plated or diffused with tin and reacted in situ to form fine superconducting Nb3Sn filaments intimately incorporated in a copper matrix. Such materials have high critical current densities in the 8–14 K temperature range and an improved tolerance to strain.
5.1.2 Aluminium
Aluminium and its alloys are widely used in the electrical industry because of their good electrical and thermal conductivity, generally excellent mechanical properties and corrosion resistance, ease of fabrication, low density and non-magnetic properties. World production of aluminium has steadily increased and has overtaken that of copper which it has replaced in many electrical applications. Weight for weight aluminium is a cheaper and better conductor than copper.
5.1.2.1 Resistivity
Pure aluminium has a resistivity of 2.64 × 10−8 Ωm at 20°C with a mean temperature coefficient over the range 0–100°C of 4.2 × 10−3/°C. Thus it has about 66% of the conductivity of pure copper or 66% of that of the International Annealed Copper Standard (IACS) at 20°C. The density of aluminium is 2.7 compared with 8.9 for copper and hence, weight for weight, the conductivity of aluminium is 2.1 times that of copper and exceeds that of all known materials except the alkali metals.
5.1.2.2 Mechanical properties and electrical conductor applications of aluminium and its alloys
The mechanical properties of aluminium depend upon the purity as well as the degree of cold work. Ordinary aluminium of commercial purity contains 99.2% aluminium whilst superpurity grades contain 99.99%. For special purposes aluminium may be zone refined to give a purity of 99.9995%. Tensile strengths and hardness are at a minimum in the high purity 99.99% annealed grades giving values of 59 MN/m2 and 15 Brinell hardness, respectively. Commercial purity, 99.2% aluminium has tensile strength of about 91 MN/m2 and Brinell hardness of 22. Cold working will increase these values by a factor of 2. Low strength and hardnesses mean that in most applications aluminium is used in the alloyed form.
Overhead-line conductors All-aluminium alloy conductors (AAAC), made from alloys containing 0.3–1.0% silicon, 0.4–0.7% manganese and small amounts of iron and manganese, are being used increasingly for high-voltage (≥ 400 kV) overhead lines. These alloys can be precipitation hardened to give tensile strengths in the range 310–415 MN/m2, while retaining conductivities of 52–60% of that of IACS copper.
All-aluminium (AAC) and steel-cored aluminium conductors (ACSR) for overhead lines are constructed to IEC 60207,9 (BS 215) with a maximum permitted resistivity for the aluminium of 2.83 × 10−8 Ωm at 20°C and a constant-mass temperature coefficient of resistivity of 4.03 × 10−3/°C. All-aluminium alloy conductors (AAAC) are constructed to IEC 60208 (BS 3242) which specifies a maximum resistivity of 3.28 × 10−8 Ωm at 20°C and a constant-mass temperature coefficient of resistivity of 3.6 × 10−3/°C.
Reinforced cables Where cables are steel reinforced the requirements for the steel core wires are given in IEC 60888 (BS 4565) and for aluminium in BSEN 1172 and BSEN 1652–4. In order to prevent galvanic corrosion, all steel wires and fittings must be galvanised—suitable specifications are given in IEC 60888 (BS 4565) and ISO 1459–61 (BS 729). Complete greasing of the central steel core wires and of the inner aluminium wires is necessary to minimise corrosion in marine or industrially polluted environments.
Because of the widely different mechanical properties of aluminium and steel, special jointing and anchoring systems have been designed. These generally take the form of compression fittings applied using hydraulic or mechanical crimping tools. Aluminium conductor alloy reinforced (ACAR) cables overcome many of the shortcomings of steel-reinforced cables in terms of corrosion and jointing, they offer a good combination of conductivity, strength and weight.
Bus-bars Bus-bars are commonly constructed from aluminium and aluminium alloys of similar compositions to those employed in overhead-cable manufacture. Specification in terms of strength is lower, permitting lower resistivity requirements of less than 3.133 × 10−8 Ωm to be achieved. Alloy materials have lower temperature coefficients of resistivity than pure aluminium so that performance in terms of current-carrying capacity improves relative to pure aluminium as the temperature increases. Aluminium bus-bars of the same current-carrying capacity as copper bus-bars have the added advantage of greater heat dissipation and only half the weight.
Jointing of aluminium or aluminium alloy bus-bar material requires more care than copper. Aluminium brazing, soldering or inert-gas welding is employed. Alternatively, mechanical jointing may be employed. Where steel or copper fittings are involved, precautions similar to those used with steel-reinforced cables should be used to avoid galvanic corrosion. Sealing with greases, tapes, mastics or paints containing corrosion inhibitors will reduce the need for maintenance. Anodised or aluminium clad alloys are often employed in severe environments.
5.1.2.3 Other applications of aluminium and its alloys
Switchgear, generators, motors and transformers Aluminium casting alloys containing 0–12% silicon with small additions of iron, copper, magnesium and nickel are widely used for switchgear, transformers, rotors for small induction motors and generator castings where lightness is essential. Tensile strengths range up to 345 MN/m2 and conductivities to 50% of that of IACS copper.
Aluminium strip and wire are also used for transformer windings and for field coils in starter motors for automotive applications.
Heat sinks and heat exchangers Aluminium and its alloys with copper, silicon, magnesium and other metals are widely used as heat-sink and heat-exchanger materials. The thermal conductivity of pure aluminium is 2.38 W/m/K, which is 56% of that of pure copper. Again on an equal weight basis, the thermal conductivity of aluminium is almost twice that of copper.
Capacitors High-purity aluminium is widely used as a capacitor-plate material. High-capacity, low-volume capacitors can be fabricated by anodising aluminium electrolytically; the oxide layer produced forming the dielectric. Alternatively, plastic film materials may be aluminised on both sides forming the electrodes of film capacitors for a range of electronic applications.
5.1.2.4 Standards
Each country has in the past had its own standards for materials. Over the past twenty years or so there has been a movement towards international standards, which for electrical materials are prepared by IEC (International Electrotechnical Commission). More general standards are prepared by ISO (International Standards Organisation). When an international standard is produced the member countries copy this standard and issue it under their own covers. This now applies to BSI, ASTM, DIN, and the French standards organisation. Where appropriate, standards are also issued as applying to all the European Union Countries.
Leading standards for electrical materials are shown as follows in Table 5.1.
Table 5.1
International and British Standards for Copper and Aluminium
| Copper | ||
| BS 1432,3 & 4: 1970–1991 | IEC 60356 | Specifications for copper for electrical purposes. |
| BS 4109: 1991 | BSEN 12166 (replaces BS 2873) ASTM B1 — B3 | Specifications for copper for electrical purposes. Wire for general electrical purposes and for insulated cables and flexible cords. (Includes wire tables with diameter, resistance (ω/km), and mass (kg/km).) |
| BS 7884: 1997 | Specifications for copper and copper-cadmium stranded conductors for overhead electric traction and power transmission systems. | |
| ASTM B258 | Standard nominal diameters and cross-sectional areas of AWG sizes of solid round wires used as electrical conductors. | |
| Aluminium | ||
| BS 215 parts 1 & 2: 1970–1985 | IEC 60207 | Aluminium stranded conductors. (BS part 1 includes physical properties and wire tables including diameter, resistance (ω/km) and breaking load.) |
| BS 2627: 1985 | ASTM B396 & B398 | Wrought aluminium for electrical purposes—wire. |
| BS 3242: 1970 | IEC 60208 | Aluminium alloy stranded conductors for overhead power transmission. |
| BS 3988: 1970 | IEC 60121 | Specification for aluminium for electrical purposes—solid conductors for insulated cables. |
| BS 6360: 1991 | IEC 60228 and 60228A | Specifications for conductors in insulated cables and cords. |
| BS 4565: 1990 | IEC 60888 | Specifications for galvanised steel for aluminium conductors, steel reinforced. |
| BSEN 1301, 2 & 3 | Aluminium and aluminium alloys. Drawn wire. Inspection, mechanical properties and tolerances. | |
| BSEN 60889 | IEC 60889 | Hard drawn aluminium for overhead line conductors. |
| BS 729: 1994 | ISO1459-61 | Specification for hot dip galvanised coatings, on iron and steel articles. |
| BSEN 61232 | IEC 61232 | Aluminium-clad steel wires for electrical purposes. |
Further information may be obtained about copper and its alloys from The Copper Development Association, Verulam Industrial Estate, 224 London Road, St Albans, Herts, AL1 1AQ, UK. Phone 0172 773 1200, or The Copper Development Association, 260 Madison Avenue, New York, NY 10016, USA. Phone 212 251 7200
Further information may be obtained about aluminium and its alloys from The Aluminium Federation Ltd, Broadway House, Calthorpe Road, Birmingham, B15 1TN, UK. Phone 0121 456 1103, or Aluminium Association, 900 19th Street, NW, Washington, DC 20006, USA, phone 202 862 5178.
5.1.3 Carbon
Carbon occurs in nature in two crystalline forms, graphite and diamond, the former being thermodynamically more stable at ambient temperature and pressures. Synthetic carbons prepared from coke or other precursors may be predominantly graphitic or amorphous depending on the conditions of manufacture. Diamonds may be prepared from graphite using high-pressure techniques but the resulting products are usually suitable only for industrial applications; new techniques have been reported for the production of gem-quality stones by vapour deposition onto small specimens.
The engineering applications of carbon exploit the following properties:
(1) it is thermally stable and in the absence of an oxidising atmosphere retains most of its mechanical strength to a temperature of 3500°C at which, under atmospheric pressure, it sublimes;
(2) its oxides are gases and leave no surface film;
(3) it is dimensionally stable, does not swell in water and can be machined to close tolerance;
(4) it has a low expansion coefficient and low density (each about one-quarter that of steel);
(5) it is a good conductor of heat, with a high specific heat and a great resistance to thermal shock;
(6) it is not wetted by molten metals;
(8) it is self-lubricating under normal atmospheric conditions; and
(9) it is electrically conductive and has high contact resistance with metals.
Electrical resistivity is lowest in the graphitic materials, which in the form of graphitised carbon black finds wide use in the manufacture of electrical carbons. Resistivity is dependent upon origin, composition and manufacture; typical values are in the region of 10−5 Ωm.
5.1.3.1 Carbon brushes
Current collection in moving contacts forms one of the most important electrical applications of graphitic carbon. Brushes must carry heavy current without excessive overheating or wearing of the parts contacted. Low friction, high contact resistance and infusibility are amongst the most desirable characteristics. Table 5.2 gives data on some widely used brush grades.
Table 5.2
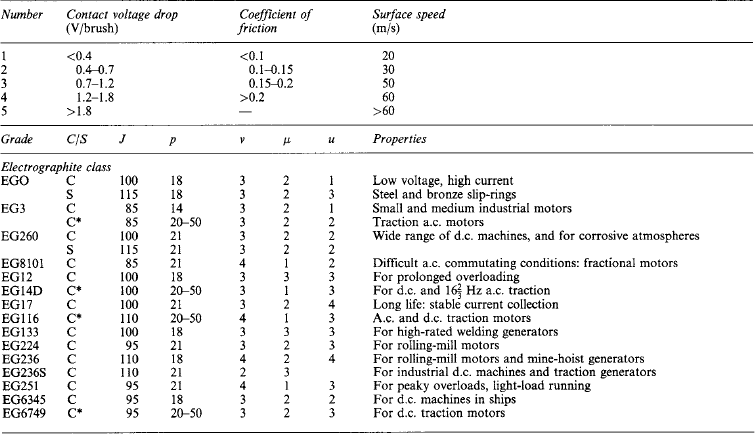
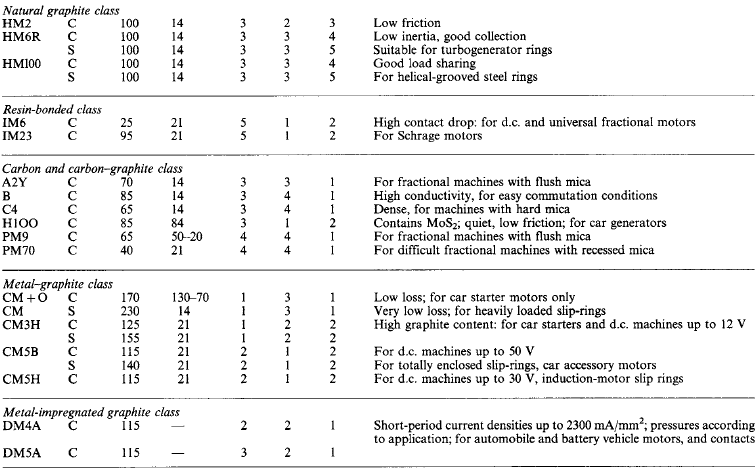
The characteristics v, μ and u are indicated by numbers:
GradeMorganite Grade coding
C/SFor commutator/slip-ring application
C*For traction commutator
JNormal current density, mA/mm 2
pNormal pressure, mN/mm2
vContact volt drop per brush
μCoefficient of friction
uNormal maximum operating speed
Commutators Contact resistance is of major importance in commutation. High contact resistance reduces losses due to high circulating currents, minimising problems due to over-heating and sparking. Commutator machines use non-metallic 100% carbon brushes, except in low-voltage applications where metal—graphite grades are necessary to reduce voltage drop and hence losses. Current density and surface speed must be considered in selection of suitable grades. Low-friction materials and good design serve to reduce chatter, ensuring good contact between brush and commutator bars. Friction characteristics may be affected by a number of factors, including the chemical and mechanical properties of the commutator metal as well as humidity, temperature, contaminants and abrasives. The ash content of the brush material is important in determining friction and abrasion characteristics and in some types of commutator may serve to wear down insulation between commutator segments. The performance of a brush on a commutator machine is influenced by its position on the commutator, i.e. the circumferential and axial stagger.
Slip-rings When commutation phenomena are absent, brush grades with low contact drops, particularly those in the metal—graphite class, can be employed. Again, current density and surface speed must be taken into account; at the highest surface speeds it is necessary to select a grade from the natural graphite class. For applications in instrumentation, such as pick-ups for thermocouples and strain gauges, silver—graphite (SM) brushes on pure silver rings are needed to give minimal and constant contact drop.
Wear The rate of wear of brushes is not directly related to the hardness of the brush material but more to the grade, the current density and quality of mechanical features (surface roughness, eccentricity, stability of brush holders and brush arms and angle of brush relative to the pick-up surface).
Spring pressure The pressure specified for a particular brush grade is determined from laboratory and field tests to give the optimum performance and life, and should be carefully adhered to. Pressures are normally of the order of 14–21 mN/mm2, but in conditions of considerable vibration (as in traction and in aircraft) pressures may be as high as 28–50 mN/mm2.
Brush materials Brushes using lampblack base are widely used for medium- and high-voltage machines, d.c. motors and generators and universal motors. These materials possess high contact resistance which is necessary for good commutation. Graphitised petroleum coke is used for non-metallic slip-ring brushes where high contact resistance is not required. Metal powders, copper or silver, blended with graphite may be used for slip-ring or low-voltage commutating machinery. The bond in most non-metal brush grades is carbon from pyrolysis of the coal-tar pitch binder. Some graphite grades are bonded with synthetic resins. Final properties are controlled by use of impregnating agents combined with heat treatments which are designed to control contact resistance, filming action and friction characteristics.
Brush design For standards of brush design, reference should be made to BS 4999 and to IEC Publication 136, on which the British Standard is based.
5.1.3.2 Linear current collection
This is the reverse of the machine condition in that the conductor is stationary and the collector (equivalent to the brush) is moving. Current collection of this type is found on rail and trolley traction systems, cranes and line process plants. In many applications the collector and conductor are open to aggressive environments including adverse weather conditions. Arcing which results from such situations roughens the conductor and accelerates wear in the collector. Carbon as a contact material provides the real benefits of a very low rate of conductor wear, long collector life and good contact stability. The carbon element, which needs no applied lubrication, is very hard and strong. Carbon shoes are in competition with brass or bronze wheels and copper shoes. Radio interference is minimised by use of carbon shoes; the grades most generally used are Link CY and Link MY, the latter being metal impregnated.
5.1.3.3 Carbon contacts
Electrical contacts include components for switches, circuit-breakers, contactors, relays and sliding contacts. The properties of carbon and graphite of importance in such applications include: self-lubricating and filming characteristics; non-welding; unwetted by molten metals; and high thermal stability. These properties make carbon the ideal contact material, particularly where arcing may occur under severe contact-bounce conditions. Carbon requires higher voltages to maintain arcing conditions than do many metals and the effects of arcing on carbon are less significant as carbon sublimes rather than melts at temperatures in excess of 3500°C. The material does not tarnish, giving constant contact resistance in the absence of any surface films.
Selection of carbon contact grade for such applications is in accordance with the required contact resistance, the metal-impregnated and metal—graphite classes giving the lowest and the carbon class the highest values.
5.1.3.4 Resistance brazing and welding
Using the relatively high resistance characteristics of carbon, it is possible to obtain a heating effect that can be used for the joining of metals. The major application is in resistance brazing where one component in the system is melted, or a low-melting alloy solder is introduced to complete the bond.
The absence of melting of carbon at high temperatures prevents sticking or welding of the carbon to the work pieces and prevents distortion of the tips of the tool even at white heat under pressure. Design of the carbon tips, in terms of shape and area of contact, is used to control the amount of heat generated in the joint. The lower strength of carbon, compared to the alternative water-cooled metal electrode, limits its use in resistance-welding and spot-welding applications; plain carbon or electrographites are used, the latter generally giving better life.
5.1.3.5 Arc welding
Electrodes of carbon are very suitable for arc welding. The weld is achieved by either a fusion process (i.e. fusion of butting edges) or by feeding a weld rod into the arc formed between the workpiece and the electrode. Carbon arcs find limited use in metal cutting, typical applications being in the cutting of risers from castings or the grooving of metal plates. Carbon arcs are widely used for brazing of thin mild-steel sheet.
5.1.3.6 Granules
Carbon granules for microphone applications are made in sizes of 60–700 μm, the largest being suitable for maximum response but developing more background noise. The smallest granules give better quality but a lower response. For normal telephone work, granules of the smaller range of sizes are employed to give good response and acceptable noise. Carbon is superior to metal powders in these applications due to high specific and contact resistance and the absence of any tendency to oxidise or tarnish. The result is a stable and reproducible performance essential to audio applications.
Telephone granular carbon is a crushed product made from petroleum coke; particle size and shape are important in determining performance characteristics. Special non-ageing grades are available.
5.1.3.7 Fibres
Fibres are the latest form in which carbon is manufactured; they possess a near-perfect orientation of the graphitic structure parallel to the fibre axis. Fibres have high strengths and elastic moduli which may be controlled by the maximum temperature attained during heat treatment. Low density confers high specific strength and stiffness of particular value in aerospace applications. Selective use of carbon fibres in composites is employed to maximise properties in desired directions. Carbon fibres are graphitic structures produced by carefully controlled pyrolysis of polyacrylonitrile or cellulose fibres under tension. More recently, petroleum or coal-tar pitch have been used.
5.2 Superconductors
Superconducting materials lose all electrical resistance below a temperature called the critical temperature (Tc) which is different for different materials. The phenomenon was first observed in mercury in 1911. Engineering interest in superconductors became really significant in the early 1960s when materials capable of carrying high current densities (up to 109 A/m2) in high magnetic fields (several tesla) were discovered. These opened the way for high-field electromagnets. The interest was reinforced by advances (partly spurred by superconductor development) in the technology of large-scale helium refrigeration (hundreds of watts cooling at 4K) which could produce cold gaseous or liquid helium for cooling purposes. The discovery of the Josephson effect (1962) led to small, low-field, superconducting electronic devices (see Section 5.2.5). More recently the interest has multiplied with the discovery of the high temperature superconductors in 1986 and there are now power transformers and power cables at the prototype stage.
Two groups of materials have superconducting properties. The ‘classical’ or low-Tc superconductors (LTS) are metals or alloys all of which show superconducting properties below 23 K. Virtually all industrial applications use materials from this category, in particular, NbTi or Nb3Sn. The second group are metal oxide compounds based on copper oxide (CuO2) subunits. These are known as ceramic or high temperature superconductors (HTS) and the compound with the highest known critical temperature (HgBaCaCuO; Tc 133 K (-140°C)) belongs to this group. The coolant for LTS material is liquid helium which is liquid below 4.2 K and for HTS material the coolant is liquid nitrogen which is liquid below 77.3 K (-195.8°C).
Once cooled below the critical temperature, superconductors can carry resistanceless current up to a maximum determined by the ambient magnetic field and the temperature. If the limiting combination of current, magnetic field and temperature is exceeded, the material reverts to an electrically ‘normal’ condition. The temperature, magnetic field and current density are independent variables, the critical values of which characterise the material. The capability to carry large lossless currents in magnetic fields of several tesla is a primary requirement of any superconductor if it is to be used in electrical plant.
5.2.1 Low temperature superconductors
For engineering purposes there are two varieties of superconductor—surface superconductors and bulk superconductors—determined as much by the way they are used as by their intrinsic properties. Surface superconductors carry superconducting current only in a surface layer and only in low magnetic fields. The magnetic field at the surface is equal to the current per unit width of the surface. In general, the resistance of superconductors to a.c. is not zero, though small; that of surface superconductors can be virtually zero. The small power losses that do then occur arise mainly from factors such as surface irregularities.
Bulk superconductors can carry currents throughout their section. Losses under changing current are relatively high and bulk superconductors are difficult to use at power or high frequencies. The loss is effectively a hysteresis loss. Both the high-Tc superconductors and ultrafilamentary superconductors (see Section 5.2.2) may ameliorate this problem. Some bulk superconductors, often referred to as ‘hard’ superconductors, can carry large current densities in strong magnetic fields (see Table 5.3). Materials with good superconducting properties tend to have high normal resistivities.
Table 5.3
Tcsome lowsuperconducting materials and their properties
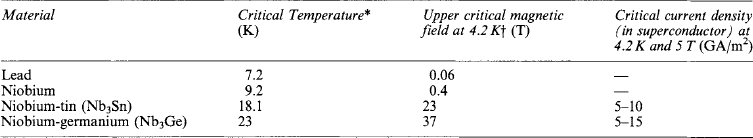
*All the superconductors listed are type II except lead (type I). Critical current densities are very dependent on the way in which the material is prepared.
†4.2 K is the boiling point of liquid helium.
Physicists divide superconductors into types I and II. Type I materials are perfectly diamagnetic below the critical magnetic field, they carry surface currents only and exclude all magnetic flux from their bulk. The depth of current and field penetration is determined by quantum mechanical considerations and is typically 10−7 m say 1000 atoms. Above the critical field type I materials lose all diamagnetic properties. Type II materials also show diamagnetism but lose the property gradually as the field is raised above a first critical field. The magnetic field penetrates the bulk in quantised flux bundles or fluxons (2 × 10−15 Wb/bundle) which can be detected with sensitive techniques. Electrical resistance returns above the critical field.
The quantum theory of superconductivity envisages a low-temperature condensed state of electrons in which they interact, through the atomic lattice, to form temporary pairs. Each member of the pair distorts the lattice in such a way as to attract the other. Because of their energy state, these pairs are not readily scattered and flow unimpeded. This mechanism is well established for metallic superconductors but the exact coupling mechanism which generates the pairs in high-Tc materials is uncertain. Without detailed prior knowledge of the electronic and atomic structure of a material, the theory is not sufficiently quantitative to predict whether or not the material will exhibit superconducting properties, let alone what the properties will be. Analogy with known superconductors has proved the best guide to new ones.
Typical low Tc materials used for engineering purposes are shown in Table 5.3. Niobium is the surface superconductor most commonly envisaged for use in a.c. equipment such as power cables. Lead is cheaper but not usually preferred unless for special reasons a low-field capacity is satisfactory. Bulk superconductors can be used in low fields as surface superconductors, e.g. niobium-tin (Nb3Sn). Of the bulk superconductors, niobium-titanium is a ductile alloy, which needs to be cold-worked to produce good current carrying capacity. Niobium-tin is a compound and very brittle; it is usually made by diffusion of tin into niobium, sometimes from a bronze, sometimes in ribbon form to reduce mechanical stresses during handling. The diffusion is often produced by heat treatment of the magnet coil after it has been wound.
5.2.2 Stabilisation of magnet conductors
Bulk superconductors suffer from electrothermal instability, especially when used near their critical conditions. A small increase in temperature lowers the critical current density, which can lead to a further increase in temperature, ultimately driving the material into its normal resistive state; heat generation then becomes very rapid and the whole coil becomes ‘normal’. Stabilisation can be achieved in several ways. All methods use a composite conductor in which ordinary conductors, frequently of copper, are in intimate contact with the superconductor. The copper has a much lower electrical resistance than the superconductor in its normal state. The most robust stabilisation system is the so-called cryostatic method. The design provides plenty of copper in parallel with the superconductor and good cooling of the composite. It envisages some agent causing the temperature to rise and current to transfer into the low-resistance copper over a short length of conductor. The thermal conditions are designed to be such that the composite conductor will cool so that once more the superconductor element regains its superconducting capacity. This method requires coolant access to most of the winding.
A more compact scheme for coils aims to limit localised temperature rises. Magnetically induced electrical losses in the superconductor during current changes are reduced by using a composite of fine strands of superconductor co-processed in a matrix, often of copper. The diameter of the superconducting filaments can be as small as 100 nm and each composite wire may contain upwards of half a million strands. Such ultra-fine filamentary conductors have very low a.c. losses and may potentially be used at power frequencies. Although normally the superconducting filaments have diameters in excess of 1 μm, strands can be twisted and the matrix incorporates components with high resistance, such as cupronickel, to control circulating currents and losses. The need for additional metal means that current density in terms of the total conductor cross-section is up to 10 or more times lower than that in the superconductor itself. This is especially so for Nb3Sn and Nb3Ge. Perhaps most importantly, the winding can be encapsulated in resin to prevent frictional heating as the winding takes up mechanical loads; preventing cracking of the resin is an important part of the technology.
The thermal instability is a consequence of the very small specific heat of most materials at liquid helium temperatures and, consequently, a localised energy input can easily lead to a significant increase in temperature. However, at liquid nitrogen temperatures the specific heat is some orders of magnitude greater and thereby the problem is considerably eased.
5.2.3 Applications of superconducting magnets
To produce magnetic fields greater than about 2T using conventional copper windings can require powers of the order of megawatts. The outstanding success of superconductors has been in providing strong fields for medical imaging, laboratory experiments and for high energy nuclear physics equipment without a massive power burden. The power to drive the helium refrigerators or liquefiers is a factor of 100 or even 1000 less. This advantage can extend to lower fields, especially when the field is required over a large volume. Magnets generating fields in excess of 15 T are readily obtainable with sufficient stability and homogeneity for use in magnetic resonance experiments.
5.2.3.1 Magnetic resonance imaging
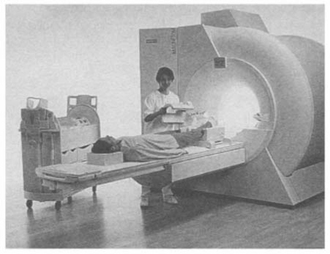
The main commercial market for low-Tc superconductors is as magnets for medical imaging systems based on the magnetic resonance of hydrogen and other nuclei. More than 1500 magnetic resonance imaging (MRI) units are installed world-wide. A superconducting solenoid with a large room temperature is the heart of the system and represents about 25% of the total cost. Although these MRI units have to be topped up with liquid helium, with the latest sophisticated designs, this is usually required only annually. They have been further improved by the use of high temperature superconductor tape for the leads which reduces the heat leaking to the liquid helium by 90%. These MRI instruments provide a non-invasive diagnostic technique without the dose restrictions of conventional X-ray tomography. MRI can provide information concerning the structure of tissue and the flow of fluids in the body by the measurement of various relaxation times which, combined with its anatomical imaging ability allows detailed diagnostic characterisation. Although whole-body scanners using superconducting solenoids generating fields of more than 2T are in use, a typical system has a room temperature bore diameter of 1.2 m with a central field of less than 1.5 T, and a homogeneity of about 10 ppm over half the bore. An example of a MRI system illustrating the superconducting magnet is shown in Figure 5.2.
5.2.2.2 High energy physics
Many superconducting magnets are used for particle accelerators, for beam handling and for analysing particle reactions in bubble chambers. Beam-bending dipoles are made in large numbers and when the US superconducting supercollider (SSC) is built it will represent a market of a size similar to that of the MRI industry. The electron-proton colliding beam facility at Deutches Electronen-Synchrotron (DESY) in Hamburg, Germany has for example 416 dipoles each 8.8 m long with a central field of 4.7 T. Radiofrequency cavities employing surface superconductors are also used in some accelerators. The good performance of all these devices and the associated refrigeration plant has shown that equipment using superconductors can run economically and reliably. If magnetically confined thermonuclear fusion is to be a future power source, the windings needed will have to be superconducting. Such magnets have already been built for experimental investigation.
5.2.3.3 Magnetic separators
Superconducting magnets can be used for extracting weakly magnetic materials. This is a very good application for low temperature superconductivity magnets which are readily available. A fine magnetic particle will move in a magnetic field gradient and will be attracted onto the magnet providing the gradient. This technique has been used throughout the world for removing iron oxide from china clay to give a good white colour and to prevent low temperature localised melting during firing of the clay.
The strong magnetic fields and field gradients are also used for separating quite weakly magnetic materials such as ferro-manganese and ferro-titanium oxides. It is also used for seperating haemoglobin from blood.
5.2.3.4 Traction and machines
A pilot development for trains uses superconducting coils mounted underneath the carriages. The field from the coils provides lift from a conducting surface placed between the rails when the train is moving fast enough. The principle offers a possible efficient alternative to the wheels used on very high speed trains.
Homopolar d.c. motors using superconducting exciter coils have been built and work satisfactorily. A generator and motor operated back-to-back can form a quiet efficient ‘gearbox’ for ship propulsion.
An a.c generator with a superconducting exciting winding on the rotor is more efficient than one with a conventional copper winding: about 0.5–1% more output is achievable for a given mechanical input. Prototype superconducting rotors have been built and tested up to 400 MVA using NbTi technology. Continued development indicates that these machines will eventually become available.
5.2.3.5 A.c. power switches and current limiters
At the moment a superconductor becomes ‘normal’ it very quickly regains its resistance. The transition phenomena can be used as the basis of a simple power switch, but such applications have been limited by the poor a.c. performance of conventional superconductors. High-Tc superconductors should improve the position.
The conventional transductor intended for fault current limitation in electric power systems has a d.c. bias winding to saturate the core of an inductor, and so reduce the a.c. impedance of a coil on the same core. The core can come out of saturation on one half-cycle if the a.c. current attempts to rise excessively; the impedance of the transductor then increases. Superconductors offer an attractive method of providing the d.c. bias.
5.2.3.6 Other power applications
If hydrodynamic generators are to be used to produce electricity efficiently, their windings will have to be superconducting.
For energy storage, gigantic inductors holding perhaps 500 MWh are being discussed. Such a store could retain electricity generated cheaply at night for use during the day. A small superconducting energy store for controlling stability in a power system is being used on an experimental basis.
Because superconductors that carry a.c. do not function satisfactorily in strong fields, they give no benefit to power transformer design. However, ultra-fine filamentary NbTi has a sufficiently improved a.c. performance that consideration of such a design is possible. A small 70 kV-A transformer using such wires has been constructed.
5.2.4 Power transmission
High power superconducting cables, either a.c or d.c. appear to be more economical than conventional ones for power transfers in excess of a few gigawatts; such power transfers in single circuits are not at present required. A.c. cables would use superconductors in a surface mode to avoid excessive hysteresis losses.
5.2.5 Electronic devices
When two pieces of superconductor are connected together by a ‘weak’ link, many intriguing phenomena occur which form the basis for a variety of electronic devices. Niobium and lead are commonly used and the link, rather poorly conducting or even insulating, may consist of a point contact, a fine constriction or a thin (2–5 nm) layer of oxide. The resulting junction can carry supercurrents (typically up to a few hundred microamps) by tunnelling of electron pairs through the barrier without any voltage drop. This is the d.c. Josephson effect. If the current exceeds some critical value, which depends on the magnetic flux in the barrier, the junction switches to a resistive state with a voltage drop of typically a few millivolts. When there is a voltage across the junction, the supercurrent component oscillates at a very high frequency determined by the voltage (483.6 GHz/mV). This is the a.c. Josephson effect: it can be used as a precise voltage standard.
5.2.5.1 SQUIDs
An extremely sensitive measuring device may be made from a pair of Josephson junctions connected in parallel by a superconductor; this is known as a SQUID (superconducting quantum interference device). The total critical current then depends periodically on the flux (the period being the flux quantum) within the loop formed by the two junctions. Field changes as low as 10−14 T can be detected. Careful screening, together with differential techniques, are needed to eliminate unwanted responses. Small voltages in low-resistance circuits (e.g. 10−10 V in 1 Ω) and small currents may be measured by using the SQUID to determine the associated magnetic field. Sometimes just one junction is used in a superconducting loop with the flux being sensed by its effects on an inductively coupled radiofrequency bias circuit. With sufficiently high bias frequencies, measurements into the microwave region are possible.
Instruments incorporating SQUIDs are finding application particularly in geophysics (studies of rock magnetism, exploration based on anomalies, determination of deep earth conductivity) and in biomagnetism (e.g. investigation of electrical activity in the heart, brain and muscles).
5.2.5.2 Computer elements
Many groups are exploring the possibility of using SQUIDs and related devices in computers. The bistable characteristics, small size, ultra-short switching times (as low as 10 ps) and very low power consumption make SQUIDs attractive components for large high-speed computers which must be compact to reduce signal-transit times. The need for liquid helium cooling is not seen as a serious disadvantage.
As a logic gate, currents in control lines overlying the SQUID induce transitions between the zero voltage and resistive states by altering the flux linking the device. For memory cells, superconducting loops may be used with the binary values 1 and 0 being represented by the presence or absence of a persistent circulating current. One Josephson junction in series with the loop and one inductively linked to it are required to ‘write’ and ‘read’, respectively. Arrays of junctions may be deposited on a substrate using many of the processes developed for fabricating silicon microcircuits. Using such technology with niobium superconductor random-access-memory (RAM) devices of 1-kbit capacity and access times of less than 0.5 ns can be made.
5.2.5.3 Electronic devices
Devices other than digital circuits have been fabricated. Infrared and millimetre wavelength receivers based on superconducting-insulator-superconducting (SIS) quasi-particle heterodyne mixers and on bolometric detection are readily available. Real-time signal processing with bandwidths approaching 10 GHz are required in many front-end radar and communication applications. Superconducting analogue signal-processing components with bandwidths exceeding 2 GHz have been achieved in striplines, microstrips and co-planar devices. They exploit the unique property of superconducting thin films, i.e. low radiofrequency surface resistance. This property allows the fabrication of delay lines or high-Q resonators in compact planar structures. The component is manufactured in a similar manner to conventional microwave stripline, etc., except for the substitution of a superconducting film for copper.
5.2.5.4 Outlook
While many of the applications above are in everyday use it appears that eventually the low Tc superconductors will be replaced by high Tc superconductors. This may take some time particularly for MRI instruments which are part of a many million dollar industry. The coming use and application of high Tc superconductors is discussed below.
5.2.6 High temperature superconductors
Although certain metal oxides were known to superconduct at a few degrees kelvin for many years, they were only of academic interest until 1986 when an LaSrCuO compound was discovered which had a Tc around 30 K, a full 7 K higher than any previously known Tc. This discovery has generated world-wide activity and a number of other compounds have been discovered. The principal superconducting compounds to emerge are compounds of YbaCuO (YBCO), BiSrCaCuO (BISCO), TlBaCaCuO and HgBaCaCuO, all of which have critical temperatures significantly above the boiling point of liquid nitrogen (77.4 K). They each form homologous series, the superconductivity depends on the exact stoichiometry of the particular compound. Table 5.4 lists some of the more important compounds together with their properties. The use of liquid nitrogen as the coolant is a major breakthrough because it simplifies the cryogenic engineering, it gives a 50-fold reduction in the refrigeration power requirement, and, being about 1/30 of the cost of liquid helium, it gives a substantial reduction in the cryogenic costs. In addition, significantly better thermal properties at liquid nitrogen temperatures improve the superconductor’s stability (see section 5.2.2). Below 30 K certain classes of compounds (e.g. BiSrCaCuO) have very good current-transport properties and a remarkable ability to carry current in fields up to 25 T, out-performing the best conventional metallic superconductors.
Table 5.4
Properties of some high Tc superconductors
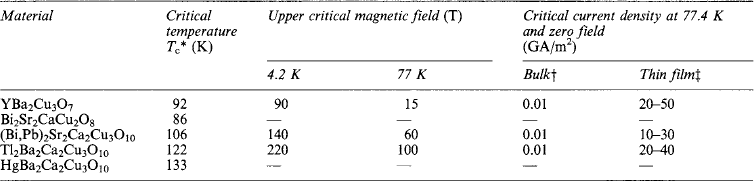
*Critical temperature depends on the exact stoichiometry.
†Sintered untextured material.
‡Thin film deposited on MgO or SrTiO3 substrates—as indication of potential.
5.2.6.1 Applications
Developments to replace the conventional conductors copper or aluminium by high temperature superconductors (HTS) are very active in many parts of the world including USA, Europe and Japan. There are prototypes of power transformers, underground power cables, large motors and generators and fault current limiters in active development and in use. The electricity supply of the city of Geneva in Switzerland is completely provided by power transformers wound with HTS conductors. It is expected that there will be definite power savings with the use of HTS and this will provide a considerable contribution to the reduction of environmental pollution. All of the applications for low temperature superconductors mentioned in sections 5.2.3.2 to 5.2.5.3 are candidates for replacement by HTS.
These HTS materials are better than LTS for 50/60 Hz a.c. current and can even be used for radiofrequency and microwave applications. Here the main requirement is a low radiofrequency surface resistance for antenna arrays and microwave resonators and the HTS tape has this property.
5.2.6.2 Tape
High temperature superconductors are very brittle and this has provided a considerable challenge for the production of suitable conductors. This is gradually being mastered and narrow composite tape of the HTS is currently being produced. There are a number of variations of the substrates but the following is typical. A nickel tape is used as the base and a buffer layer of palladium followed by ceria and yttriazirconia layers are applied to the tape. This is the basic substrate and the high temperature superconductor is then deposited onto the tape using pulsed laser deposition. The tape is 3 mm wide and cables up to 100 metres in length are currently under test at various locations. The production of this tape is essential to all the power applications and the production rates are increasing and the cost is decreasing.
5.2.6.3 Outlook
The outlook for HTS is very good with many prototypes of applications at the test stage in utilities and elsewhere. While there is need for cheaper tape, this will inevitably come as production increases. The highest Tc has remained at 133 K for the last few years however it has been found that the Tc can be increased by pressure. This may provide a lead for producing materials with higher values of Tc. The dream of a Tc higher than room temperature may remain unfulfilled but we must remember that the highest Tc remained at 23 K for over 20 years and we can always hope for a further break through.
My thanks are due to the staffs of The City of Sunderland College and the University of Sunderland for their assistance with this section.
Low temperature superconductors
Super-conducting Applications SQUIDs and machines. In: Foner S., Schwartz B.B., eds. NATO Advanced Study Institutes Series. Pittsburgh, USA: Plenum Press, 1977.
Rose-Innes, A.C., Rhoderick, E.H. Introduction to Superconductivity, 2nd edition. New York: Pergamon Press; 1997.
Wilson, M.N. Superconducting Magnets. Oxford: Oxford University Press; 1983.
High temperature superconductors
Bednorz, J.G., Muller, K.A. Perovskite-type oxides-the new approach to high Tc superconductivity. Reviews in Modern Physics. 1988;60:585.
Sheahen, T.P. Introduction to High Temperature Superconductivity. Oxford: Plenum Press; 1994.
Various Authors. Superconductivity in electric power a special report, IEEE Spectrum. July 1997:18 to 49.
, Handbook of Applied Superconductivity. 2 vols. Seeber B. New York: Institute of Physics, 1998.
8. Lawrence L. R., Cox C., Broman D. High Temperature Superconductivity The Products and their benefits 2000 edition, ORNL/Sub/450006921