Chapter 4
Enzyme Immobilization on Nanoparticles: Recent Applications
Cheng-Kang Lee and Ai-Nhan Au-Duong
4.1 Introduction
Enzymes have been extensively employed in many applications of our daily life such as dairy products, beverage processing or baking in food industries, personal care products, detergent industries, textile industries, paper and pulp making industries, biofuels industries, and diagnostic biosensors [1, 2]. Enzymes are also becoming favorable catalysts in green chemistry because of their high substrate specificity and much milder reaction conditions than those carried out by using traditional metal-based catalysts. With the advance of recombinant DNA technology and high-throughput screening technology, the cost for finding and employing a suitable enzyme for a specific reaction has also been significantly reduced. Due to their proteinaceous nature, however, the main drawback for employing enzymes in any reaction is their lack of stability. Most enzymes frequently lose their activity during the long-term reaction and/or storage mainly due to the denaturation of their tertiary structure and/or active site poisoning. As a water-soluble catalyst, the recovery of enzymes from the reaction medium for product purification and repetitive usage is also a big challenge for their industrial applications [1, 3]. Immobilization of enzymes to a solid support not only can retain the enzyme activity but also can make the recovery of enzyme from the reaction medium much easier. Once immobilized, stability of the enzyme is usually significantly enhanced due to the fact that the conformation of flexible enzyme tertiary structure is fixed by attaching to the rigid surface. Enzyme immobilization has become very popular research topic since 1960s, and various enzyme immobilization methods, such as adsorption, covalent bonding, entrapment, and cross-linking, have been developed based on different materials employed [4, 5]. Many good review papers [3, 6, 7] and books [1, 8] providing detailed enzyme immobilization techniques and their specific applications have been reported. Over the last few years, with the advances of nanotechnology, various nanomaterials have been developed for applications in many different areas. The nanoparticles not only have very high specific surface area but also due to their size have quite unique optical, electrical, electronic, thermal, chemical and mechanical, and catalytic (ability to facilitate electron transfer) properties [1]. Enzymes immobilized or conjugated onto nanoparticles for the preparation of enzyme nanoparticles (EnNPs) offer the advantages of high enzyme loading, improved enzyme stability, and ease of separation from products when employed for various chemical reactions [4, 9]. This present article consists mainly of two parts: (i) general EnNP preparation methods and some newly reported EnNP literature are briefly reviewed, and (ii) recently developed applications of EnNPs in the field of biomedicine, biosensors, and biofuel production are discussed.
4.2 Preparation of Enzyme-Immobilized Nanoparticles
The first immobilized enzyme, reported by Nelson and Griffin, was prepared in 1916 on an artificial aluminum hydroxide carrier by simple adsorption [4, 10]. Up to 1960, techniques for enzyme immobilization were developed and included covalent attachment and entrapment on different carriers [4, 5]. Then, in the late 1960s, in order to enhance the enzyme stability and the amount of enzyme loaded, the supports for enzyme immobilization took advantage of the porous polysaccharide beads such as cross-linked cellulose, dextran, or agarose [5]. There is, however, no perfect method or carrier material for enzyme immobilization that is application specific. In general, the method for enzyme immobilization can be classified according to the type of enzyme attachment, namely, physical adsorption, entrapment, encapsulation, covalent attachments, cross-linking, and bioaffinity interactions (Figure 4.1). A combination of steps for immobilization of enzyme by adsorption followed by cross-linking or covalent bonding was sometimes also employed.
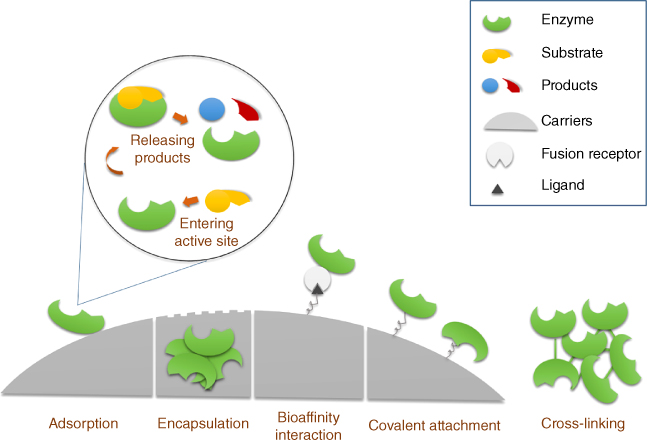
Figure 4.1 Illustration of general enzyme immobilization methods and reaction of immobilized enzyme.
4.2.1 Physical Adsorption
Physical adsorption relies mainly on van der Waals forces, hydrogen bonding, hydrophobic interaction and electrostatic interactions between enzyme molecules, and the surface of insoluble carriers. It can prevent enzymes from deactivation often caused by chemical modification on the enzyme structure as in the covalent attachment and cross-linking methods. In contrast to the conventional “added-on” immobilization, lysozyme [11, 12] and glucose oxidase (GOx) [13] have also been used to induce the formation of Au nanoparticles from its salt solution, and as a consequence, the enzymes were conjugated with the nanoparticles to form EnNPs. Besides, the in situ growth of self-assembly enzyme–inorganic hybrid nanocrystal also creates EnNPs with high surface area to achieve an improved enzymatic activity and stability [14, 15]. These EnNPs prepared by physical adsorption mainly found use in biomedical applications. This simplest immobilization method also has its disadvantage in that enzyme molecules may leak from EnNPs under some unfavorable conditions during their use and storage.
4.2.2 Encapsulation/Entrapment
The encapsulation or entrapment method for enzyme immobilization is basically to keep the enzyme trapped inside inert and porous materials. This is usually achieved by inducing the enzyme to interact with polymeric or inorganic salt solutions to form insoluble particles. The enzymes entrapped inside the solid particles are well protected from the external harsh reaction environments, and, as a consequence, the stability of the enzyme is maintained. A disadvantage, however, is probably a lower apparent activity of the enzyme due to the mass transfer resistance generated from the encapsulating matrix [16, 17]. Several natural polymer materials like cellulose, alginate, collagen, chitosan, and starch, mesoporous silica nanoparticles, and some conductive polymers such as polyaniline and Nafion have been used for encapsulating and entrapping enzymes [18–22]. Nevertheless, there are not many reports on enzymes encapsulated or entrapped in nanoparticles probably due to the difficulty of recovery of nanoparticles during practical applications. Biopolymeric chitosan nanoparticles have been used as carriers for immobilized enzymes by entrapment and covalent attachment [18]. Trypsin, encapsulated in chitosan as EnNPs, has been used as a zymogen-like enzyme in the gastrointestinal tract of fish [19]. However, the EnNPs prepared by encapsulation or entrapment may have limitations in reacting with large substrate molecules due to their significant mass transfer resistance across the encapsulating matrix.
4.2.3 Covalent Attachments
Covalently linking an enzyme to an insoluble carrier via the activated functional groups on the surface of the carrier generates a robust immobilized enzyme without the problems of enzyme leakage. Since the tremendous development of nanoparticles that have unique optical, electrical, chemical, and catalytic (ability to facilitate electron transfer) properties associated with the very high specific surface area, various enzymes have been immobilized onto nanoparticles via covalent attachment [1]. Only a few examples, based on the four most often used functional groups on an enzyme structure, have been covalently immobilized onto the surface of nanoparticles (Table 4.1). Such immobilized enzymes also have to deal with harmful reaction environments. Besides, the enzyme activity, the loss of enzyme molecular dynamics, and the enzyme conformation may change upon immobilization due to the limited accessibility of substrate to the active site of enzyme, which is not properly oriented [30].
Table 4.1 Examples illustrated for general covalent attachments of enzyme immobilization
| Type of covalent | Reaction groups | Catalyst | References | |
| attachment | Enzyme | Nanoparticles | ||
| Amino with carboxyl reaction | Amino | Carboxyl | NHS and carbodiimide | [23, 24] |
| Carboxyl | Amino | |||
| Aldehyde with amino reaction | Amino | Aldehyde | With/without reducing agents | [25, 26] |
| Amino with epoxide reaction | Amino | Epoxide functionalized | Neutral pH | [27] |
| Thiol linker | Thiol | Unsaturated carbonyls (e.g., maleimide) | Physiological pH (6.5–7.5) | [28, 29] |
4.2.4 Cross-Linking
Cross-linked enzymes (CLEs) as an immobilization method were demonstrated in the 1960s by Quiocho and Richards for cross-linking carboxypeptidase [31] and by Habeeb for cross-linking trypsin [32]. The enzyme molecules were cross-linked to each other by cross-linking agent (glutaraldehyde was most commonly used reagent) or sometimes by adding an inert protein like bovine serum albumin as proteic feeder in the cases of low-concentration and/or susceptible enzymes [10, 33]. Recently, cross-linked enzyme aggregates (CLEAs) have also been developed for enzyme immobilization by cross-linking the enzyme precipitate formed in the presence of added salts, or water-miscible organic solvents or nonionic polymers [34–37]. The enzymes physically aggregated by non-covalent bonding without perturbation of their tertiary structure and so were still able to maintain enzyme activity at a higher level. The CLEA method has been employed to form enzyme nanoparticles of penicillin acylase [33, 38], horseradish peroxidase (HRP) [39], trypsin [40], cholesterol oxidase (ChOx) [36], uricase [37], and lipase [33, 41] with advantages of forming stable and reusable biocatalyst of lower production cost. Although cross-linking is a relatively simple process with minimal enzyme leakage, the inevitable activity loss resulted from enzyme structural conformation change due to the cross-link bonding is usually encountered [42–44].
4.2.5 Bioaffinity Interactions and Other Methods
Various protein–protein and protein–small molecule specifically binding interactions such as histidine-tagged protein with chelated transition metals [45] and avidin or streptavidin to biotin-functionalized enzymes [46, 47] have been investigated and developed for enzyme immobilization. Maltose-binding protein has also been fused to enzymes for affinity adsorption and immobilization on magnetic nanoparticles [48]. The bioaffinity interaction immobilization provides the advantage that enzyme can be fixed in a specific orientation so that the active site of the enzyme will not be blocked by any of the insoluble carriers. In addition to the mentioned immobilization methods, a possible combination of these methods has also been developed to inherit each of the specific features for the preparation of EnNPs [46, 49]. For example, GOx covalently immobilized on silica nanoparticles was physically entrapped within photopolymerized hydrogels of poly(ethylene glycol) (PEG) to provide higher water content and larger mesh size to enhance mass transfer during quantitative glucose determination in a biosensor [49].
4.3 Application of Enzyme Nanoparticles
4.3.1 EnNP for Biomedical Application
Enzymes possess a great potential in the treatment of cardiovascular [50], oncological [51], viral and hereditary [52], intestinal [53], and other illnesses [54–57]. However, the daily clinical use of enzymes in practice is less common because of their short lifetime in storage or in the human body and the risk of causing toxic and systemic immune reactions [54]. These limitations can be overcome by targeted delivery of therapeutic enzymes to the desired site of interest. The use of nanoparticles as enzyme carriers for the targeted delivery provides the possibility to optimize the performance of nanodrugs through controlling the ratio between the attached enzyme and the nanocarriers with consideration of immunogenicity of the enzyme [58]. Although a number of therapeutic EnNPs have shown great promise in vitro and in animal studies, none of such constructs have reached early stages of clinical testing with human patients [58].
4.3.1.1 EnNP for Thrombolytic Therapy
Enzymes like tissue-type plasminogen activator (tPA), streptokinase, or urokinase-type plasminogen activator (uPA) have been used after acute myocardial infarction or cerebral microthrombosis due to their ability of inhibiting blood clot formation [58, 59]. Nanoparticles such as magnetic nanoparticles, liposomes, polymeric nanoparticles, or red blood cells have been employed for carrying these thrombolytic enzymes to the blood clot sites in order to eliminate the risk of hemorrhagic side effects caused by nontargeting and nonspecific activation of these native thrombolytic enzymes [55]. Magnetic nanoparticles with their attractiveness in magnetism were a popular nanocarrier in targeted therapies under magnetic guidance for localizing and concentrating the thrombolytic enzymes near the blood clot sites [56, 60–62]. Particularly, Torchilin et al. [60] have demonstrated that magnetic carrier could successfully deliver streptokinase to the vicinity of a dog carotid artery thrombosis under the guidance of an external magnetic field. The thrombolytic activity could be significantly increased by using mesoporous magnetic nanoparticles in which 30-fold enhancement of urokinase loading capacity was achieved in comparison with that of non-mesoporous magnetic nanoparticles [59]. tPA was also immobilized into echogenic liposomes to achieve ultrasound facilitated thrombolysis [63]. tPA and antifibrin antibodies have also been covalently attached to 40 nm polystyrene latex nanoparticles for direct delivery of tPA to the clot site through fibrin-specific antibody to lower the risk of systemic toxicity [64].
4.3.1.2 EnNP for Inflammation and Oxidative Stress Therapy
Reactive oxygen species (ROS) including a large variety of free oxygen radicals are unstable yet able to induce oxidative stress on cell structures [65]. ROS are produced both enzymatically and nonenzymatically and cause damage in cells including epithelial cells, macrophages, neutrophils, eosinophils, monocytes, and lymphocytes by electron transfer reactions in response to a variety of stimuli [65–67]. Intracellular ROS production happens in innate immune cells at sites of inflammation associated with chronic inflammatory diseases or in areas with high level influence of oxygen and external agents like cigarette smoke in the lung [66]. In addition, enzymatic sources, for example, NADPH oxidases located on the cell membrane of activated neutrophils and macrophages, generate the most aggressive reactive oxygen superoxide [66, 67]. Cells can be initially protected against inflammation and oxidative stress through catalytic reaction carried out by superoxide dismutase (SOD) [68] and catalase [46, 69]. To protect catalase from proteolytic degradation, substrate-permeable polymeric nanoparticles of PEG and polylactic–polyglycolic acid (PEG–PLGA) copolymer and oleate coated magnetite nanoparticles were employed as protease-impermeable nanocarriers. Targeting the polymeric nanoparticles with encapsulated catalase or peroxidase to endothelial cell (EC) was employed to protect against vascular oxidative stress in cell culture and mouse lung studies [69]. For example, endothelial targeting of polymeric nanoparticles loaded with catalase or SOD could achieve 33% of injected dose in the pulmonary vasculature after 30 min of intravenous injection and strong protection from acute inflammatory effects of mouse lung injury/inflammation caused by endotoxin [46]. Moreover, SOD has been investigated as an antiapoptotic drug, a free radical scavenger, and an anti-inflammatory agent for the central nervous system (CNS) using targeted delivery of SOD loaded nanoparticles to penetrate the blood–brain barrier. SOD-functionalized polybutylcyanoacrylate (PBCA) nanoparticles achieved remarkable penetrating capacity of the blood–brain barrier and targeted the CNS without significant difference in enzymatic activity or receptor-binding ability [70–73]. Recently, a new strategy for effective intracellular delivery of SOD has also been developed based on cell-penetrating peptide fusion of the SOD embedded in mesoporous silica nanoparticles [74].
4.3.1.3 EnNP for Antibacterial Treatment
Several recent research has paid much attention to use enzyme-conjugated nanoparticles for antimicrobial applications in order to lower the rate of antibiotic resistance of human pathogens such as M. tuberculosis, E. faecium, S. aureus, and P. aeruginosa [75–77]. Lysozyme has been conjugated onto polystyrene nanoparticles and used to enhance the delivery to the infected macrophages and liver by reticuloendothelial system uptake. Lysozyme together with antibody has been immobilized onto polystyrene nanoparticles for targeting to Gram-positive Listeria monocytogenes and shows superior activity over free lysozyme or lysozyme nanoparticles without any antibody [75]. With the assistance of lysozyme, the antimicrobial activity of coated silver nanoparticles has been proved to be a more effective against various silver ion resistance bacterial strains [77].
4.3.2 EnNP for Biosensor Applications
A biosensor is used for the detection of an analyte with the combination of a biological sensing component connected to a physicochemical detector that converts an observed response into a measurable signal that is directly proportional to the concentration of an analyte [1, 78]. Enzymes are the most often used biological component in a biosensor to generate a signal by reacting with the analyte. According to the signal transducing format, an enzyme-based sensor can be divided into electrochemical, optical, piezoelectric, and thermal/calorimetric biosensors. Nanoparticles have also been used as carriers for enzyme immobilization on the electrodes of biosensors as listed in Table 4.2. The nanoparticles not only can provide high specific area for the enzyme to be attached and therefore achieve high loading capacity but also can stabilize enzyme activity by fixing its structural conformation [1, 78]. Besides, nanoparticles such as gold, silver, and metal-like nanoparticles can significantly improve the electrical conductivity of enzyme layer immobilized on the electrode that leads to an enhanced detection sensitivity [82, 83]. Gold nanoparticles can also act as a catalyst to increase electrochemical reactions, typically catalysis of oxidation and reduction of H2O2 in the construction of glucose biosensor [12, 13, 80]. Recently, a number of nanostructured electrochemical biosensors based on the combination of magnetic nanoparticles and other materials such as carbon nanotubes, electroconductive polymers, and chitosan have been developed as electrocatalytic magnetoswitchable biosensors [84–89]. In general, employing enzyme-immobilized nanoparticles in biosensors can amplify their analytical performance evaluated by the dynamic parameters of the biosensor such as decreased response time and increased sensitivity [78, 80, 83, 84, 90]. Numerous biosensor devices associated with various enzymes such as GOx, HRP, urease, ChOx, penicillin acylase, and nanoparticles have been widely and successfully applied for clinical, biomedical, environmental, industrial, and pharmaceutical analysis [78, 83, 90].
Table 4.2 Some immobilized enzyme nanoparticles in biosensor applications
| Type of biosensors | Nanoparticles | Properties used | Typical application |
| Optical [79] | Glucose oxidase (GOx)-immobilized silver nanoparticles | – Change in localized surface plasmon resonance with glucose – Simplifying and inducing optical sensitivity |
Potential test kits for determining glucose concentrations |
| Electrochemical [36] | Cholesterol oxidase (ChOx) aggregate | – Change in electrical properties with cholesterol – Increasing stable, active and shelf life for immobilized enzyme | Amperometric biosensor for measuring of cholesterol concentrations (e.g., in human serum) |
| Electrochemical and optical [80] | GOx–gold nanoparticles | – Transduction in electrochemical impedance spectroscopy and surface plasmon resonance – Enhancing sensitive response and good reproducibility | Optical biosensor and electrochemical biosensors in detection of glucose concentrations |
| Colorimetric [81] | β-Galactosidase-immobilized gold nanoparticles | – Colorimetric development by released enzyme – Fast and sensitive quantification | Visual test strip format in detection of microbial contamination (e.g., model analyte Escherichia coli) |
4.3.3 EnNP for Biofuel Production
Recently, the study of biofuels has been of great interest because it is considered as one of the potential renewable energies for eliminating global dependence on fossil fuels. A rapid growth in biofuel production involving enzyme utilization has also been observed due to the high conversion and fast reaction kinetics that could be achieved [23, 91–95]. Enzyme-catalyzed production is more advantageous than a chemical method due to its high selectivity and mild reaction conditions. The instability of enzyme activity can be prevented by immobilization of enzymes onto nanoparticles. Such immobilization also increases the reusability of enzymes and flexibility of reactor design. Cellulases and lipases are two primary candidates for large-scale biofuel and biodiesel production. Cellulases immobilized on silica [96, 97] and magnetic nanoparticles [94] have been successfully used to hydrolyze cellulose for obtaining fermentable sugars for biofuel production. Typically, cellulases immobilized on magnetic nanoparticles were demonstrated to be superior in thermostability (up to 80 °C) and have longer storage half-life and efficiency of recovery for reuse [94]. It has been reported that three kinds of cellulases could be co-immobilized on gold-doped magnetic silicon nanoparticles to facilitate the single-step hydrolysis of complex cellulose substrates for biofuel fermentation [93]. Magnetic CLEAs of crude lipase, prepared by cross-linking aggregates formed from magnetic nanoparticles and a lipase mixture, could achieve very high conversion of biodiesel (80–85%) when olive oil, microalgal oil, and several non-edible vegetable oils or even waste cooking oil were employed as substrates [95]. To cope with lipase deactivation problem during biodiesel production, gold nanoparticles were used by Lv et al. [98] for lipase immobilization in a reusable reactor. The gold nanoparticles not only provide high surface area for immobilization but also work as intermediate ligands for non-covalently attached enzymes. This facilitates the regeneration of deactivated bioreactors by removing the deactivated enzyme using 2-mercaptoethanol and subsequent immobilizing the fresh one. Feasibility and potential application of this enzymatic reactor to produce biodiesel from kitchen oil through the transesterification of triacylglycerides have been demonstrated [98]. Carbon nanotube, graphene oxide nanosheets, and magnetic multi-walled carbon nanotubes as carriers for lipase or cellulase immobilization have also been reported for biofuel production with improved stability [99–101].
4.4 Conclusion and Perspectives
Over recent years, the advances of recombinant DNA and high-throughput screening technologies have made it easier to obtain an enzyme with desired properties. The rapid development in nanotechnology also makes the preparation of various nanoparticles more affordable. In comparison with other carriers, immobilization of enzymes onto nanoparticles (EnNPs) can bring the benefits of high enzyme loading, improved enzyme stability, and easy recovery of enzyme activity. EnNPs especially hold a great promise in biomedical applications because EnNPs not only possess therapeutic and diagnostic enzyme activity but also inherit some intrinsic properties of nanoparticles, which benefit biosensing, diagnostics, cell killing, healing, and many other areas in the biomedical field. However, beyond the very impressive results reported, several unexpected disadvantages of EnNPs such as aggregation, precipitation, and bioincompatibility are still challenging in health, environmental, and economic concerns. The development of EnNPs is still in its early state but is expected to be further investigated in the area of nanobiotechnology.
References
- 1. Pundir, C.S. (2015) in Enzyme Nanoparticles, vol. 69 (ed. C.S. Pundir), William Andrew Publishing, Boston.
- 2. Li, S. et al. (2012) Technology prospecting on enzymes: application, marketing and engineering. Comput. Struct. Biotechnol. J., 2 (3), 1–11.
- 3. Brady, D. and Jordaan, J. (2009) Advances in enzyme immobilisation. Biotechnol. Lett., 31 (11), 1639–1650.
- 4. Cao, L., Langen, L.v., and Sheldon, R.A. (2003) Immobilised enzymes: carrier-bound or carrier-free? Curr. Opin. Biotechnol., 14 (4), 387–394.
- 5. Brena, B. and Batista-Viera, F. (2006) in Immobilization of Enzymes, in Immobilization of Enzymes and Cells (ed. J. Guisan), Humana Press, pp. 15–30.
- 6. Willner, I., Basnar, B., and Willner, B. (2007) Nanoparticle-enzyme hybrid systems for nanobiotechnology. FEBS J, 274 (2), 302–309.
- 7. Ansari, S.A. and Husain, Q. (2012) Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol. Adv., 30 (3), 512–523.
- 8. Minteer, S.D. (ed.) (2011) Enzyme Stabilization and Immobilization, Methods in Molecular Biology, vol. 679, Humana Press.
- 9. Polizzi, K.M. et al. (2007) Stability of biocatalysts. Curr. Opin. Chem. Biol., 11 (2), 220–225.
- 10. Zhang, Y., Ge, J., and Liu, Z. (2015) Enhanced activity of immobilized or chemically modified enzymes. ACS Catal., 5 (8), 4503–4513.
- 11. Cai, H. and Yao, P. (2013) In situ preparation of gold nanoparticle-loaded lysozyme-dextran nanogels and applications for cell imaging and drug delivery. Nanoscale, 5 (7), 2892–2900.
- 12. Yang, T. et al. (2007) Synthesis, characterization, and self-assembly of protein lysozyme monolayer-stabilized gold nanoparticles. Langmuir, 23 (21), 10533–10538.
- 13. Sharma, B., Mandani, S., and Sarma, T.K. (2014) Enzymes as bionanoreactors: glucose oxidase for the synthesis of catalytic Au nanoparticles and Au nanoparticle-polyaniline nanocomposites. J. Mater. Chem. B, 2 (26), 4072–4079.
- 14. Ge, J., Lei, J., and Zare, R.N. (2012) Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol., 7 (7), 428–432.
- 15. Yin, Y. et al. (2015) An enzyme-inorganic hybrid nanoflower based immobilized enzyme reactor with enhanced enzymatic activity. J. Mater. Chem. B, 3 (11), 2295–2300.
- 16. Won, K. et al. (2005) Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem., 40 (6), 2149–2154.
- 17. Sharma, M., Sharma, V., and Majumdar, D.K. (2014) Entrapment of amylase in agar beads for biocatalysis of macromolecular substrate. Int. Sch. Res. Notices, 2014, 8.
- 18. Tang, Z.X., Qian, J.Q., and Shi, L.E. (2007) Preparation of chitosan nanoparticles as carrier for immobilized enzyme. Appl. Biochem. Biotechnol., 136 (1), 77–96.
- 19. Kumari, R. et al. (2013) Chitosan nanoencapsulated exogenous trypsin biomimics zymogen-like enzyme in fish gastrointestinal tract. PLoS One, 8 (9), e74743.
- 20. Wang, Y. and Caruso, F. (2005) Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater., 17 (5), 953–961.
- 21. Nemzer, L.R., Schwartz, A., and Epstein, A.J. (2010) Enzyme entrapment in reprecipitated polyaniline nano- and microparticles. Macromolecules, 43 (9), 4324–4330.
- 22. Zhu, Z. et al. (2014) A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun., 5, 3026.
- 23. Yu, C.-Y. et al. (2013) Optimized production of biodiesel from waste cooking oil by lipase immobilized on magnetic nanoparticles. Int. J. Mol. Sci., 14 (12), 24074–24086.
- 24. Li, D. et al. (2007) Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability. Biochem. Biophys. Res. Commun., 355 (2), 488–493.
- 25. Wang, S. et al. (2013) Magnetic nanoparticles coated with immobilized alkaline phosphatase for enzymolysis and enzyme inhibition assays. J. Mater. Chem. B, 1 (12), 1749–1754.
- 26. Hou, J. et al. (2015) Preparation of titania based biocatalytic nanoparticles and membranes for CO2 conversion. J. Mater. Chem. A, 3 (7), 3332–3342.
- 27. Preety and Hooda, V. (2014) Immobilization and kinetics of catalase on calcium carbonate nanoparticles attached epoxy support. Appl. Biochem. Biotechnol., 172 (1), 115–130.
- 28. Zhang, S. et al. (2005) Immobilization of glucose oxidase on gold nanoparticles modified Au electrode for the construction of biosensor. Sens.Actuators B: Chemical, 109 (2), 367–374.
- 29. Holland, J.T. et al. (2011) Engineering of glucose oxidase for direct electron transfer via site-specific gold nanoparticle conjugation. J. Am. Chem. Soc., 133 (48), 19262–19265.
- 30. Secundo, F. (2013) Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev., 42 (15), 6250–6261.
- 31. Quiocho, F.A. and Richards, F.M. (1964) Intermolecular cross linking of a protein in the crystalline state: carboxypeptidase-A. Proc. Natl. Acad. Sci. U.S.A., 52 (3), 833–839.
- 32. Habeeb, A.F.S.A. (1967) Preparation of enzymically active, water-insoluble derivatives of trypsin. Arch. Biochem. Biophys., 119, 264–268.
- 33. Shah, S., Sharma, A., and Gupta, M.N. (2006) Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem., 351 (2), 207–213.
- 34. Sheldon, R.A. (2007) Cross-linked enzyme aggregates (CLEA®s): stable and recyclable biocatalysts. Biochem. Soci. Transac., 35 (6), 1583–1587.
- 35. Liu, G. et al. (2005) Enzyme nanoparticles-based electronic biosensor. Chem. Commun., 27, 3481–3483.
- 36. Chawla, S. et al. (2013) Preparation of cholesterol oxidase nanoparticles and their application in amperometric determination of cholesterol. J. Nanopart. Res., 15 (9), 1–9.
- 37. Chauhan, N., Kumar, A., and Pundir, C.S. (2014) Construction of an uricase nanoparticles modified Au electrode for amperometric determination of uric acid. Appl. Biochem. Biotechnol., 174 (4), 1683–1694.
- 38. Cao, L., van Rantwijk, F., and Sheldon, R.A. (2000) Cross-linked enzyme aggregates: a simple and effective method for the immobilization of penicillin acylase. Org. Lett., 2 (10), 1361–1364.
- 39. Šulek, F. et al. (2011) Immobilization of horseradish peroxidase as crosslinked enzyme aggregates (CLEAs). Process Biochem., 46 (3), 765–769.
- 40. Chen, J. et al. (2006) Synthesis of cross-linked enzyme aggregates (CLEAs) in CO2-expanded micellar solutions. Colloids Surf. B: Biointer., 48 (1), 72–76.
- 41. Guauque Torres, M., Foresti, M., and Ferreira, M. (2013) Cross-linked enzyme aggregates (CLEAs) of selected lipases: a procedure for the proper calculation of their recovered activity. AMB Express, 3 (1), 1–11.
- 42. Eissa, A.S. et al. (2006) Enzymatic cross-linking of β-lactoglobulin: conformational properties using FTIR spectroscopy. Biomacromolecules, 7 (6), 1707–1713.
- 43. Majumder, A.B. et al. (2008) Designing cross-linked lipase aggregates for optimum performance as biocatalysts. Biocatal. Biotransform., 26 (3), 235–242.
- 44. Sheldon, R.A. (2011) Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol., 92 (3), 467–477.
- 45. Sommaruga, S. et al. (2014) Immobilization of carboxypeptidase from sulfolobus solfataricuson magnetic nanoparticles improves enzyme stability and functionality in organic media. BMC Biotechnol., 14 (1), 1–9.
- 46. Hood, E.D. et al. (2014) Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials, 35 (11), 3708–3715.
- 47. Yu, C.-C. et al. (2012) Site-specific immobilization of enzymes on magnetic nanoparticles and their use in organic synthesis. Bioconjugate Chem., 23 (4), 714–724.
- 48. Zhou, L. et al. (2012) Magnetic nanoparticles for the affinity adsorption of maltose binding protein (MBP) fusion enzymes. J. Mater. Chem., 22 (14), 6813–6818.
- 49. Jang, E. et al. (2010) Fabrication of poly(ethylene glycol)-based hydrogels entrapping enzyme-immobilized silica nanoparticles. Polym. Adv. Technol., 21 (7), 476–482.
- 50. Ghouri, N., Preiss, D., and Sattar, N. (2010) Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology, 52 (3), 1156–1161.
- 51. Wald, M. (2008) Exogenous proteases confer a significant chemopreventive effect in experimental tumor models. Integr. Cancer Ther., 7 (4), 295–310.
- 52. Buchholz, F. and Hauber, J. (2013) Engineered DNA modifying enzymes: components of a future strategy to cure HIV/AIDS. Antiviral Res., 97 (2), 211–217.
- 53. Thummel, K.E. (2007) Gut instincts: CYP3A4 and intestinal drug metabolism. J. Clin. Invest., 117 (11), 3173–3176.
- 54. Torchilin, V.P. (1988) Immobilised enzymes as drugs. Adv. Drug Deliv. Rev., 1 (3), 270.
- 55. Vellard, M. (2003) The enzyme as drug: application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol., 14 (4), 444–450.
- 56. Driscoll, C.F. et al. (1984) Magnetic targeting of microspheres in blood flow. Microvasc. Res., 27 (3), 353–369.
- 57. De Strooper, B., Vassar, R., and Golde, T. (2010) The secretases: enzymes with therapeutic potential in alzheimer disease. Nat. Rev. Neurol., 6 (2), 99–107.
- 58. Vertegel, A., Reukov, V., and Maximov, V. (2011) Enzyme–nanoparticle conjugates for biomedical applications, in Enzyme Stabilization and Immobilization (ed. S.D. Minteer), Humana Press, pp. 165–182.
- 59. Wang, M. et al. (2012) Targeted thrombolysis by using of magnetic mesoporous silica nanoparticles. J. Biomed. Nanotechnol., 8 (4), 624–632.
- 60. Torchilin, V.P., Papisov, M.I., and Smirnov, V.N. (1985) Magnetic Sephadex as a carrier for enzyme immobilization and drug targeting. J. Biomed. Mater. Res., 19 (4), 461–466.
- 61. Ma, Y.-H. et al. (2007) Intra-arterial application of magnetic nanoparticles for targeted thrombolytic therapy: a rat embolic model. J. Magn. Magn. Mater., 311 (1), 342–346.
- 62. Capitanescu, C. et al. (2016) Molecular processes in the streptokinase thrombolytic therapy. J. Enzym. Inhib. Med. Chem., 31(6), 1411–1414.
- 63. Tiukinhoy-Laing, S.D. et al. (2007) Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb. Res., 119 (6), 777–784.
- 64. Yurko, Y. et al. (2009) Design of biomedical nanodevices for dissolution of blood clots. Mater. Sci. Eng. C, 29 (3), 737–741.
- 65. Sharma, P. et al. (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot., 2012, 26.
- 66. Bargagli, E. et al. (2009) Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir. Med., 103 (9), 1245–1256.
- 67. Kliment, C.R. and Oury, T.D. (2010) Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radical. Biol. Med., 49 (5), 707–717.
- 68. Lee, S. et al. (2007) Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug. Chem., 18 (1), 4–7.
- 69. Dziubla, T.D. et al. (2008) Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials, 29 (2), 215–227.
- 70. Begley, D.J. (2004) Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol. Ther., 104 (1), 29–45.
- 71. Alam, M.I. et al. (2010) Strategy for effective brain drug delivery. Eur. J. Pharm. Sci., 40 (5), 385–403.
- 72. Schroeder, U. et al. (1998) Nanoparticle technology for delivery of drugs across the blood-brain barrier. J. Pharm. Sci., 87 (11), 1305–1307.
- 73. Reukov, V., Maximov, V., and Vertegel, A. (2011) Proteins conjugated to poly(butyl cyanoacrylate) nanoparticles as potential neuroprotective agents. Biotechnol. Bioeng., 108 (2), 243–252.
- 74. Chen, Y.-P. et al. (2013) A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J. Am. Chem. Soc., 135 (4), 1516–1523.
- 75. Yang, H. et al. (2007) Enhancing antimicrobial activity of lysozyme against Listeria monocytogenes using immunonanoparticles. J. Food Prot., 70 (8), 1844–1849.
- 76. Satishkumar, R. and Vertegel, A. (2008) Charge-directed targeting of antimicrobial protein-nanoparticle conjugates. Biotechnol. Bioeng., 100 (3), 403–412.
- 77. Ashraf, S. et al. (2014) Lysozyme-coated silver nanoparticles for differentiating bacterial strains on the basis of antibacterial activity. Nanoscale Res. Lett., 9 (1), 565.
- 78. Wilson, G.S. and Hu, Y. (2000) Enzyme-based biosensors for in vivo measurements. Chem. Rev., 100 (7), 2693–2704.
- 79. Endo, T. et al. (2008) Stimuli-responsive hydrogel-silver nanoparticles composite for development of localized surface plasmon resonance-based optical biosensor. Anal. Chim. Acta, 611 (2), 205–211.
- 80. Bourigua, S. et al. (2013) A new design of electrochemical and optical biosensors based on biocatalytic growth of Au nanoparticles – example of glucose detection. Electroanalysis, 25 (3), 644–651.
- 81. Miranda, O.R. et al. (2011) Colorimetric bacteria sensing using a supramolecular enzyme–nanoparticle biosensor. J. Am. Chem. Soc., 133 (25), 9650–9653.
- 82. Karunakaran, C., Rajkumar, R., and Bhargava, K. (2015) Chapter 1 - Introduction to biosensors, in Biosensors and Bioelectronics, Elsevier, pp. 1–68.
- 83. Sistani, P. et al. (2014) A penicillin biosensor by using silver nanoparticles. Int. J. Electrochem. Sci., 9 (11), 6201–6212.
- 84. Liao, M.-H., Guo, J.-C., and Chen, W.-C. (2006) A disposable amperometric ethanol biosensor based on screen-printed carbon electrodes mediated with ferricyanide-magnetic nanoparticle mixture. J. Magn. Magn. Mater., 304 (1), e421–e423.
- 85. Baby, T.T. and Ramaprabhu, S. (2010) SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta, 80 (5), 2016–2022.
- 86. Pal, S. and Alocilja, E.C. (2009) Electrically active polyaniline coated magnetic (EAPM) nanoparticle as novel transducer in biosensor for detection of Bacillus anthracis spores in food samples. Biosens. Bioelectron., 24 (5), 1437–1444.
- 87. Wang, S. et al. (2008) Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles–chitosan nanocomposite. Biosens. Bioelectron., 23 (12), 1781–1787.
- 88. Díez, P. et al. (2012) Supramolecular immobilization of redox enzymes on cyclodextrin-coated magnetic nanoparticles for biosensing applications. J. Colloid Interface Sci., 386 (1), 181–188.
- 89. Stanciu, L. et al. (2009) Magnetic particle-based hybrid platforms for bioanalytical sensors. Sensors, 9 (4), 2976–2999.
- 90. Putzbach, W. and Ronkainen, N.J. (2013) Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: a review. Sensors, 13 (4), 4811–4840.
- 91. Mangas-Sánchez, J. and Adlercreutz, P. (2015) Highly efficient enzymatic biodiesel production promoted by particle-induced emulsification. Biotechnol. Biofuels, 8 (1), 1–8.
- 92. Babaki, M. et al. (2016) Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: effect of water, t-butanol and blue silica gel contents. Renew. Energ., 91, 196–206.
- 93. Cho, E.J. et al. (2012) Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun., 48 (6), 886–888.
- 94. Abraham, R.E. et al. (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels, 7 (1), 1–12.
- 95. Cruz-Izquierdo, Á. et al. (2015) Magnetic cross-linked enzyme aggregates (mCLEAs) of Candida antarctica lipase: an efficient and stable biocatalyst for biodiesel synthesis. PLoS One, 9 (12), e115202.
- 96. Lupoi, J.S. and Smith, E.A. (2011) Evaluation of nanoparticle-immobilized cellulase for improved ethanol yield in simultaneous saccharification and fermentation reactions. Biotechnol. Bioeng., 108 (12), 2835–2843.
- 97. Chang, R.H.-Y., Jang, J., and Wu, K.C.W. (2011) Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem., 13 (10), 2844–2850.
- 98. Lv, Y. et al. (2014) Preparation of reusable bioreactors using reversible immobilization of enzyme on monolithic porous polymer support with attached gold nanoparticles. Biotechnol. Bioeng., 111 (1), 50–58.
- 99. Raghavendra, T. et al. (2013) Robust nanobioconjugates of Candida antarctica lipase B – multiwalled carbon nanotubes: characterization and application for multiple usages in non-aqueous biocatalysis. Bioresour. Technol., 140, 103–110.
- 100. Mubarak, N.M. et al. (2014) Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B: Enzym., 107, 124–131.
- 101. Pavlidis, I.V. et al. (2010) Functionalized multi-wall carbon nanotubes for lipase immobilization. Adv. Eng. Mater., 12 (5), B179–B183.
