Chapter 32
Biosynthesis and Applications of Silver Nanoparticles
Bipinchandra K. Salunke and Beom Soo Kim
Concise Definition of Subject
Silver is a valuable metal and conversion of silver to nano form changes its physicochemical characteristics. The nano form of silver is an ideal and treasured compound for different fields of science and technology. As a result of diverse range of applications of silver nanoparticles (AgNPs), the research in the field of AgNP synthesis is getting increased attention. Physical, chemical, and biological methods can be used to produce AgNPs. However, biological methods for AgNP synthesis are becoming popular due to limitations associated with chemical and physical methods. Plant-mediated AgNP synthesis is more advantageous over microbial and other modes due to their green synthesis approach. Higher rate of nanoparticle synthesis with different shapes and sizes can be achieved due to flexibility in reaction parameters of plants. Biochemical diversity with pharmacological importance gives added advantage for plant-mediated AgNP synthesis approach. This chapter highlights the elementary features related to nanomaterials and methods of AgNP synthesis with specific emphasis on plant extract-mediated synthesis. Methods for characterization for AgNPs, parameters responsible for higher yield, mechanisms of plant-mediated synthesis of AgNPs, and applications including future promise and toxicity aspects of AgNPs are also deliberated.
32.1 Introduction
Nanoscience, a new and recently established multifaceted science, is devoted to study fundamental properties of nanomaterials [1, 2]. The prefix “nano” designates one billionth or 10−9 units. The clusters of atoms in the size range of 1–100 nm are broadly regarded as nanoparticles [3, 4]. As compared with their bulk materials, the nanometer-size metallic particles as a result of their high surface-to-volume ratio show unique and considerably changed physical, chemical, and biological properties [5]. Therefore, nanoparticles have attracted considerable scientific interest in recent years [6, 7]. The size- and shape-dependent unique properties are displayed by metallic nanoparticles that have been found useful for applications in medicine, healthcare, electronics, agriculture, and so on [4, 5, 8, 9]. The utilization of specific synthesis methods, reducing agents, and stabilizers is found to be valuable to synthesize nanoparticles with uniform size, shape, and size distribution [10–13]. The different types of properties or activities of nanoparticles vary with their size, structure, shape, size distribution, and chemical–physical environment. Therefore, there is increasing interest to obtain nanoparticles with uniform size, shape, and size distribution. For example, the uniform smaller-sized silver nanoparticles (AgNPs) exhibit better antimicrobial activity than the larger ones [14].
In general, two strategies are adopted for the synthesis of nanomaterials and fabrication of nanostructures, that is, top-down and bottom-up (Figure 32.1). Top-down strategy includes nanomaterial synthesis via photo-reduction, mechanical/ball milling, laser/thermal ablation, kinetic sputtering, thermal/chemical electro-explosion, lithography, and chemical etching. Bottom-up strategy utilizes chemical vapor deposition, atomic/molecular condensation, laser/spray/aerosol pyrolysis, sol/gel, supercritical fluid synthesis, template synthesis, spinning, chemical/electrochemical precipitation, and biological methods. In the top-down approach, the bulk materials are sliced or successively cut to get nanosized particles. Materials from the bottom, that is, atom by atom, molecule by molecule, or cluster by cluster, are built up in the bottom-up approach. Both approaches have advantages and disadvantages and are playing an important role in nanotechnology and modern industry. Top-down strategy is very good for bulk production of nanomaterials. Therefore, this approach will continue to play an essential role in the synthesis of nanomaterials. The concept of the bottom-up approach is based on long-standing principles. The growth of all the living things in nature occurs by employing this approach, which is practiced in industries for over a century, for example, producing salt and nitrate in chemical industry. The bottom-up approach has an important role in the fabrication and processing of nanomaterials.

Figure 32.1 Top-down and bottom-up strategies for the synthesis of nanomaterials and fabrication of nanostructures.
The metal nanoparticles can be synthesized by employing different physical, chemical, and biological methodologies. Biological mode is more advantageous than physical and chemical methods as a result of the simplicity of synthesis method with no requirement of high temperature, pressure, energy, and toxic chemicals besides eco-friendliness, cost-effectiveness, and scalability of the processes. Biological syntheses of nanoparticles have been reported using plants, bacteria, fungi, algae, yeast, actinomycetes, lichen, and so on [4, 15]. These biological agents or their active constituents have the ability to reduce metal precursors to their respective nanoparticles. The general scheme of biological synthesis of nanoparticles is exhibited in Figure 32.2. As the microbe-mediated processes require maintenance of aseptic conditions and pure cultures, industrial feasibility of the process becomes difficult. The additional cost of isolation of microorganisms, culture media, and specialized equipment and expert manpower can enhance the cost of the process. Due to the chances of biohazard, elaborate process of maintaining cell cultures and expertise is required to handle the cultures [16]. Among biological methods, plant-mediated synthesis method is more advantageous than the other modes, as the plant metabolites act as natural capping agents for the stabilization of AgNPs and the process of synthesis is eco-friendly, cost-effective, and suitable for diverse applications including medical applications [4].
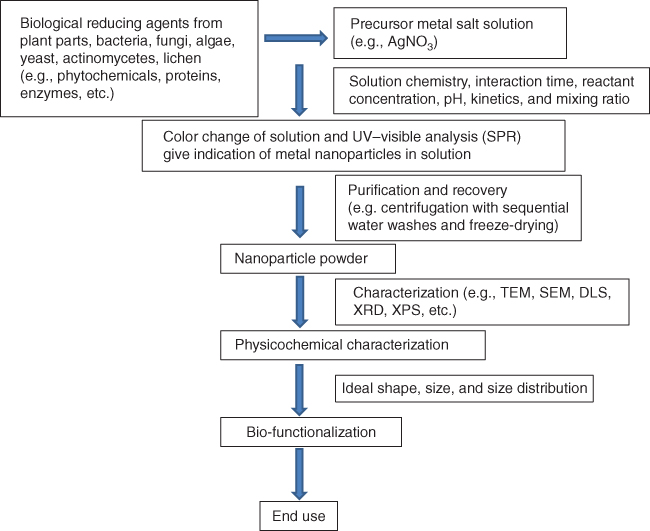
Figure 32.2 General scheme of biological synthesis of nanoparticles.
32.2 Silver Nanoparticles
In recent years, AgNPs as a result of their unique properties have become one of the attractive fascinating products among all noble metal nanoparticles in the field of nanotechnology. AgNPs display characteristics such as chemical stability, good conductivity, and catalytic activities [17]. They can be incorporated into composite fibers, cryogenic superconducting materials, cosmetic products, food industry, and electronic components [18, 19]. They are useful in biosensors [20], textiles [21], agriculture [22], and pest management [23]. They show antibacterial, antiviral, antifungal, and anti-inflammatory activities. They are useful in biomedical applications such as drug delivery [24], chemotherapeutics [25], wound dressings, topical creams, antiseptic sprays and fabrics, antimicrobials [26], and anti-tuberculosis agents [27]. They serve as antiseptic and display a broad biocidal effect against microorganisms through the disruption of their unicellular membrane thus disturbing their enzymatic activities. AgNPs can be synthesized via different approaches.
32.3 Plants in Nanoparticle Synthesis
As a result of rapidness, eco-friendliness, nonpathogenicity, and cost-effectiveness, a single-step process, the phyto-synthesis approach, is drawing increasing attention. A brief AgNP synthesis scheme is presented in Figure 32.2. Different phyto-constituents/active metabolites such as proteins, amino acids, enzymes, polysaccharides, alkaloids, tannins, phenolics, saponins, terpenoids, vitamins, and so on play a role in the reduction and stabilization of silver ions. Most of these metabolites are eco-friendly and have medicinal properties [4].
The general process of synthesis using plant involves the collection of plant materials and preparation of plant extracts. The plant materials are properly washed first with tap water. The plant materials are used either fresh or dried. Drying of plant materials is commonly carried out in the shade for 5–10 days. The materials are ground in a grinder to make fine powder. Five to 10 g of fresh plant materials or dried powder is usually added in 100 ml deionized distilled water and boiled for 5–10 min. The resulting broth is then filtered through Whatman filter paper. Plant broth (5–10%) is added in 1 mM AgNO3 solution, and synthesis of AgNPs through reduction of pure Ag(I) ions to Ag(0) is monitored by observing color change and UV–Vis spectra of the solution at regular intervals [4, 14, 28]. The synthesized AgNPs are purified by centrifugation at 15 000 rpm for 15–20 min and repeatedly washed two to three times. The settled AgNPs are recovered and freeze-dried to obtain AgNPs in powder form.
A range of different techniques such as UV–Vis spectroscopy, dynamic light scattering (DLS), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), X-ray diffractometry (XRD), atomic force microscopy (AFM), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) are used to characterize nanoparticles [4, 5, 29, 30]. These methods give ideas about the shape, size, fractal dimensions, pore size, surface area, and crystallinity besides dispersion, orientation, and intercalation of nanoparticles. UV–Vis spectroscopy provides indication about the sample formation by showing the surface plasmon resonance. DLS delivers clue of size distribution of particles. XRD delivers information about crystallinity of particles. TEM, SEM, and AFM analyses help to understand the morphology and size of particles. AFM analyses provide three-dimensional images that are useful to calculate particle height and volume.
32.4 Parameters Affecting Synthesis of AgNPs
32.4.1 Effect of pH
Shape, size, production rate, and stability of nanoparticles are found to vary with change of pH after use of plant and other sources for AgNP synthesis [31–36]. Alkaline condition favored AgNP synthesis, while acidic conditions did not produce AgNPs with the use of Azadirachta indica (neem) leaf extracts [37]. However, Gan and Li [38] reported that phyto-mediated synthesis can produce a large number of stable nanoparticles over wide pH range.
32.4.2 Reaction Time, Precursor to Plant Extract Ratio, and Reaction Rate
Nanoparticle synthesis was found to increase with increase in reaction time [35, 39]. Metal precursor to plant extract is significant to obtain AgNPs with varying shape, size, and stability [38]. AgNP synthesis increased with increasing garlic extract ratio to constant AgNO3 concentration. Twenty percent A. indica (neem) leaf extract synthesized more AgNPs than the 40% extract [39]. The ability of nanoparticle synthesis varies with plant varieties. The plants that can synthesize stable and uniform AgNPs with use at lower quantity of plant extract are good. Song and Kim [28] studied extracellular synthesis of metallic AgNPs using five plant leaf extracts (pine, persimmon, ginkgo, magnolia, and platanus). Magnolia leaf broth was the best reducing agent in terms of synthesis rate and conversion to AgNPs of 15–500 nm size.
32.4.3 Effect of Temperature
Increase in temperature was found to increase nanoparticle synthesis [14, 28, 40, 41]. Only 11 min was required for more than 90% conversion at the reaction temperature of 95 °C using magnolia leaf broth [28]. Temperature change was found to alter the shape of nanoparticles [42, 43]. Physicochemical environment may be changing due to temperature enhancing nucleation and controlling aggregation leading to synthesis of stable nanoparticles. Detection of the presence of biomolecules at higher temperature is not much studied. Presumption that proteins are thermolabile suggests that other biomolecules in plants with thermostability may have an active role at higher temperature.
32.5 Mechanism of AgNP Synthesis
Different researchers have correlated synthesis of AgNPs with the presence of bioactive metabolites in plant extracts. Plant metabolites such as terpenoids, flavonoids, polyphenols [44], amines [45], saponins [46], aldehydes, ketones [47], arabinose, galactose [48], and starch [49] have been reported to have an active role in the synthesis of AgNPs, which involves proteins and enzymes. Carbonyl groups of amino acid of peptide and protein have a strong affinity to bind metal. Curcacyclin A (octapeptide), curcacyclin B (nonapeptide), and curcain (enzyme) present in the latex of Jatropha curcas were responsible for the synthesis of AgNPs [50]. Using FTIR analyses, authors reported that decrease in intensity of band at 1537 cm−1 after reduction confirms role of amines and shift of band from 1618 to 1604 cm−1 attributed to binding of (NH)CO group with nanoparticles. Patil et al. [26] also predicted the role of proteins from the latex of Euphorbian plants in the formation of AgNPs. The actual mechanism of reduction and capping using isolated pure compounds has not been much investigated. The course of AgNP synthesis may be happening like this. The metal precursor AgNO3 may be dissociated into Ag+ and NO3−. The bioactive phytochemicals such as phenolic compounds have hydroxyl and ketonic groups that have the ability to bind to metals and reduce the metal salt and provide stability against agglomeration. Plant extract gives protein and enzyme to the AgNO3 solution in which Ag+ ions may combine with the enzyme to form enzyme–substrate complex. The enzyme released from the plant extract may be acting on the silver ions and AgNPs may be released from this enzyme. These released AgNPs may be combining with the proteins from the plant extract thereby leading to the production of protein-capped AgNPs.
32.6 Applications of AgNPs
AgNPs are promising for a number of beneficial applications due to their unique properties. Different accredited bodies such as the United States Food and Drug Administration (US FDA), US Environmental Protection Agency (EPA), the Society of International sustaining growth for Antimicrobial Articles (SIAA) of Japan, Korea's Testing and Research Institute for the chemical industry, and FITI Testing and Research Institute have approved some products made with AgNPs [51–55]. Textile, health industry, food storage, and environmental sectors make use of AgNPs for diverse applications. They are valuable as antimicrobial agents for disinfecting medical devices, home appliances, and water treatment [56–60]. AgNPs were integrated in fibers to produce silver nanocomposite fabric [10]. Their enhanced antibacterial activity against Escherichia coli was observed for the cotton fibers [10, 61, 62]. AgNPs show antiviral activity against HIV-1 at noncytotoxic concentrations. However, the mechanism of HIV-inhibitory activity is not exactly understood yet [63]. Special interest has been directed at providing enhanced biomolecular diagnostics, including single-nucleotide polymorphism detection gene expression profiles and biomarker characterization. These strategies have been focused on the development of nanoscale devices and platforms that can be used for single-molecule characterization of nucleic acid, DNA or RNA, and protein at an increased rate when compared with traditional techniques [64]. AgNPs are useful to be used in nanoscale sensors as they show faster response times and lower detection limits due to their electrochemical properties [65]. AgNPs enhanced the bleaching of the organic dyes by the application of potassium peroxodisulfate in aqueous solution at room temperature [66]. Chemiluminescence from the luminol–hydrogen peroxide system showed better catalytic activity in the presence of AgNPs than Au and Pt colloid [67]. AgNP-supported halloysite nanotubes with Ag content of about 11% were able to exhibit improved reduction of 4-nitrophenol with NaBH4 in alkaline aqueous solutions [68]. AgNPs and their composites display catalytic activities such as dye reduction. The reduction of methylene blue by arsine in the presence of AgNPs was successful [69]. AgNPs showed catalytic activity by the reduction of phenosafranine dye [70].
32.7 Conclusion
There is an increasing awareness toward the development of environmentally friendly techniques for the synthesis of AgNPs using green methods. The use of plants can be more advantageous than other biological entities as the time-consuming process of employing microbes and maintaining their culture is not needed. Plants have diverse chemical compounds like proteins, carbohydrates, alkaloids, tannins, phenolics, oils, and saponins that have medicinal value and can act as reducing and capping agents for AgNP synthesis. The approach of using plant extracts for the synthesis of AgNPs is economical, energy efficient, and cost-effective. This approach will also provide avenue for healthier workplaces and communities, protecting human health and environment and producing safer products with lesser waste. AgNPs have many applications in textile, health industry, food storage, and environmental sectors. Large-scale synthesis study, finding mechanism of nanoparticle formation, isolation, purification, and genetic engineering of plant for production of active major compounds catalyzing reduction and capping are promising areas of research.
References
- 1. Sergeev, G.B. and Shabatina, T.I. (2008) Cryochemistry of nanometals. Colloids Surf. A, 313, 18–22.
- 2. Jiang, K. and Pinchuk, A.O. (2015) Noble metal nanomaterials: synthetic routes, fundamental properties, and promising applications. Solid State Phys., 66, 131–211.
- 3. Williams, D. (2008) The relationship between biomaterials and nanotechnology. Biomaterials, 29 (12), 1737–1738.
- 4. Borase, H.P., Salunke, B.K., Salunkhe, R.B., Patil, C.D., Hallsworth, J.E., Kim, B.S., and Patil, S.V. (2014) Plant extract: a promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol., 173 (1), 1–29.
- 5. Lee, S.H., Salunke, B.K., and Kim, B.S. (2014) Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts. Biotechnol. Bioprocess Eng., 19 (1), 169–174.
- 6. Wang, Y., Fu, X., and Chen, L. (2014) Novel Optical Nanoprobes for Chemical and Biological Analysis, Springer.
- 7. Polte, J. (2015) Fundamental growth principles of colloidal metal nanoparticles – a new perspective. CrystEngComm, 17 (36), 6809–6830.
- 8. Sun, S., Murray, C.B., Weller, D., Folks, L., and Moser, A. (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science, 287 (5460), 1989–1992.
- 9. Vilchis-Nestor, A.R., Sánchez-Mendieta, V., Camacho-López, M.A., Gómez-Espinosa, R.M., Camacho-López, M.A., and Arenas-Alatorre, J.A. (2008) Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett., 62 (17), 3103–3105.
- 10. Yeo, S.Y., Lee, H.J., and Jeong, S.H. (2003) Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci., 38 (10), 2143–2147.
- 11. Chimentao, R.J., Kirm, I., Medina, F., Rodriguez, X., Cesteros, Y., Salagre, P., and Sueiras, J.E. (2004) Different morphologies of silver nanoparticles as catalysts for the selective oxidation of styrene in the gas phase. Chem. Commun., 7, 846–847.
- 12. He, B., Tan, J.J., Liew, K.Y., and Liu, H. (2004) Synthesis of size controlled Ag nanoparticles. J. Mol. Catal. A Chem., 221 (1), 121–126.
- 13. Zhang, W., Qiao, X., Chen, J., and Wang, H. (2006) Preparation of silver nanoparticles in water-in-oil AOT reverse micelles. J. Colloid Interface Sci., 302 (1), 370–373.
- 14. Salunke, B.K., Shin, J., Sawant, S.S., Alkotaini, B., Lee, S., and Kim, B.S. (2014) Rapid biological synthesis of silver nanoparticles using Kalopanax pictus plant extract and their antimicrobial activity. Korean J. Chem. Eng., 31 (11), 2035–2040.
- 15. Salunke, B.K., Sawant, S.S., Lee, S.I., and Kim, B.S. (2015) Comparative study of MnO2 nanoparticle synthesis by marine bacterium Saccharophagus degradans and yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 99 (13), 5419–5427.
- 16. Kalishwaralal, K., Deepak, V., Pandian, S.R.K., Kottaisamy, M., BarathManiKanth, S., Kartikeyan, B., and Gurunathan, S. (2010) Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B, 77 (2), 257–262.
- 17. Suja, E., Nancharaiah, Y.V., and Venugopalan, V.P. (2012) p-Nitrophenol biodegradation by aerobic microbial granules. Appl. Biochem. Biotechnol., 167 (6), 1569–1577.
- 18. Klaus-Joerger, T., Joerger, R., Olsson, E., and Granqvist, C.G. (2001) Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol., 19 (1), 15–20.
- 19. Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M.I., Kumar, R., and Sastry, M. (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B, 28 (4), 313–318.
- 20. Kirubaharan, C.J., Kalpana, D., Lee, Y.S., Kim, A.R., Yoo, D.J., Nahm, K.S., and Kumar, G.G. (2012) Biomediated silver nanoparticles for the highly selective copper(II) ion sensor applications. Ind. Eng. Chem. Res., 51 (21), 7441–7446.
- 21. Vankar, P.S. and Shukla, D. (2012) Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci., 2 (2), 163–168.
- 22. Rai, M. and Ingle, A. (2012) Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol., 94 (2), 287–293.
- 23. Patil, C.D., Patil, S.V., Borase, H.P., Salunke, B.K., and Salunkhe, R.B. (2012) Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res., 110 (5), 1815–1822.
- 24. Suri, S.S., Fenniri, H., and Singh, B. (2007) Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol., 2 (1), 16.
- 25. Jain, K.K. (2010) Advances in the field of nanooncology. BMC Med., 8 (1), 1.
- 26. Patil, S.V., Borase, H.P., Patil, C.D., and Salunke, B.K. (2012) Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl. Biochem. Biotechnol., 167 (4), 776–790.
- 27. Anisimova, Y.V., Gelperina, S.I., Peloquin, C.A., and Heifets, L.B. (2000) Nanoparticles as antituberculosis drugs carriers: effect on activity against Mycobacterium tuberculosis in human monocyte-derived macrophages. J. Nanopart. Res., 2 (2), 165–171.
- 28. Song, J.Y. and Kim, B.S. (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng., 32 (1), 79–84.
- 29. Choi, Y., Ho, N.H., and Tung, C.H. (2007) Sensing phosphatase activity by using gold nanoparticles. Angew. Chem. Int. Ed., 46 (5), 707–709.
- 30. Salunke, B.K., Sawant, S.S., and Kim, B.S. (2014) Potential of Kalopanax septemlobus leaf extract in synthesis of silver nanoparticles for selective inhibition of specific bacterial strain in mixed culture. Appl. Biochem. Biotechnol., 174 (2), 587–601.
- 31. Rai, A., Chaudhary, M., Ahmad, A., Bhargava, S., and Sastry, M. (2007) Synthesis of triangular Au core–Ag shell nanoparticles. Mater. Res. Bull., 42 (7), 1212–1220.
- 32. Andreescu, D., Eastman, C., Balantrapu, K., and Goia, D.V. (2007) A simple route for manufacturing highly dispersed silver nanoparticles. J. Mater. Res., 22 (9), 2488–2496.
- 33. Shukla, V.K., Singh, R.P., and Pandey, A.C. (2010) Black pepper assisted biomimetic synthesis of silver nanoparticles. J. Alloys Compd., 507 (1), L13–L16.
- 34. Badawy, A.M.E., Luxton, T.P., Silva, R.G., Scheckel, K.G., Suidan, M.T., and Tolaymat, T.M. (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol., 44 (4), 1260–1266.
- 35. Daniel, S.K., Vinothini, G., Subramanian, N., Nehru, K., and Sivakumar, M. (2013) Biosynthesis of Cu, ZVI, and Ag nanoparticles using Dodonaea viscosa extract for antibacterial activity against human pathogens. J. Nanopart. Res., 15 (1), 1–10.
- 36. Velmurugan, P., Lee, S.M., Iydroose, M., Lee, K.J., and Oh, B.T. (2013) Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl. Microbiol. Biotechnol., 97 (1), 361–368.
- 37. Tripathi, A., Chandrasekaran, N., Raichur, A.M., and Mukherjee, A. (2009) Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta Indica (Neem) leaves. J. Biomed. Nanotechnol., 5 (1), 93–98.
- 38. Gan, P.P. and Li, S.F.Y. (2012) Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev. Environ. Sci. Biotechnol., 11, 169–206.
- 39. Krishnaraj, C., Jagan, E.G., Rajasekar, S., Selvakumar, P., Kalaichelvan, P.T., and Mohan, N. (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B, 76 (1), 50–56.
- 40. Antony, J.J., Sivalingam, P., Siva, D., Kamalakkannan, S., Anbarasu, K., Sukirtha, R., Krishnan, M., and Achiraman, S. (2011) Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B, 88 (1), 134–140.
- 41. Ghosh, S., Patil, S., Ahire, M., Kitture, R., Kale, S., Pardesi, K., Cameotra, S.S., Bellare, J., Dhavale, D.D., Jabgunde, A., and Chopade, B.A. (2012) Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed., 7, 483–496.
- 42. Lin, L., Wang, W., Huang, J., Li, Q., Sun, D., Yang, X., Wang, H., He, N., and Wang, Y. (2010) Nature factory of silver nanowires: plant-mediated synthesis using broth of Cassia fistula leaf. Chem. Eng. J., 162 (2), 852–858.
- 43. Lukman, A.I., Gong, B., Marjo, C.E., Roessner, U., and Harris, A.T. (2011) Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J. Colloid Interface Sci., 353 (2), 433–444.
- 44. Marimuthu, S., Rahuman, A.A., Rajakumar, G., Santhoshkumar, T., Kirthi, A.V., Jayaseelan, C., Bagavan, A., Zahir, A.A., Elango, G., and Kamaraj, C. (2011) Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res., 108, 1541–1549.
- 45. Prasad, K.S., Pathak, D., Patel, A., Dalwadi, P., Prasad, R., Patel, P., and Selvaraj, K. (2011) Biogenic synthesis of silver nanoparticles using Nicotiana tobaccum leaf extract and study of their antibacterial effect. Afr. J. Biotechnol., 10 (41), 8122–8130.
- 46. Elavazhagan, T. and Arunachalam, K.D. (2011) Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed., 6, 1265–1278.
- 47. Chandran, S.P., Chaudhary, M., Pasricha, R., Ahmad, A., and Sastry, M. (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog., 22 (2), 577–583.
- 48. Kora, A.J., Beedu, S.R., and Jayaraman, A. (2012) Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett., 2 (1), 1–10.
- 49. Vigneshwaran, N., Nachane, R.P., Balasubramanya, R.H., and Varadarajan, P.V. (2006) A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res., 341 (12), 2012–2018.
- 50. Bar, H., Bhui, D.K., Sahoo, G.P., Sarkar, P., De, S.P., and Misra, A. (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A, 339 (1), 134–139.
- 51. Zhong, L.S., Hu, J.S., Cui, Z.M., Wan, L.J., and Song, W.G. (2007) In-situ loading of noble metal nanoparticles on hydroxyl-group-rich titania precursor and their catalytic applications. Chem. Mater., 19 (18), 4557–4562.
- 52. Deng, Z., Chen, M., and Wu, L. (2007) Novel method to fabricate SiO2/Ag composite spheres and their catalytic, surface-enhanced Raman scattering properties. J. Phys. Chem. C, 111 (31), 11692–11698.
- 53. Wang, J.X., Wen, L.X., Wang, Z.H., and Chen, J.F. (2006) Immobilization of silver on hollow silica nanospheres and nanotubes and their antibacterial effects. Mater. Chem. Phys., 96 (1), 90–97.
- 54. Wei, H., Li, J., Wang, Y., and Wang, E. (2007) Silver nanoparticles coated with adenine: preparation, self-assembly and application in surface-enhanced Raman scattering. Nanotechnology, 18 (17), 175610.
- 55. Jia, X., Ma, X., Wei, D., Dong, J., and Qian, W. (2008) Direct formation of silver nanoparticles in cuttlebone-derived organic matrix for catalytic applications. Colloids Surf. A, 330 (2), 234–240.
- 56. Bosetti, M., Masse, A., Tobin, E., and Cannas, M. (2002) Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials, 23 (3), 887–892.
- 57. Cho, M., Chung, H., Choi, W., and Yoon, J. (2005) Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol., 71 (1), 270–275.
- 58. Gupta, A. and Silver, S. (1998) Molecular genetics: silver as a biocide: will resistance become a problem? Nat. Biotechnol., 16 (10), 888.
- 59. Jain, P. and Pradeep, T. (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng., 90 (1), 59–63.
- 60. Li, Q., Mahendra, S., Lyon, D.Y., Brunet, L., Liga, M.V., Li, D., and Alvarez, P.J. (2008) Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res., 42 (18), 4591–4602.
- 61. Duran, N., Marcato, P.D., De Souza, G.I., Alves, O.L., and Esposito, E. (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol., 3 (2), 203–208.
- 62. Chen, C.Y. and Chiang, C.L. (2008) Preparation of cotton fibers with antibacterial silver nanoparticles. Mater. Lett., 62 (21), 3607–3609.
- 63. Lara, H.H., Ayala-Nuñez, N.V., Ixtepan-Turrent, L., and Rodriguez-Padilla, C. (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol., 8 (1), 1–8.
- 64. Goyal, R.N., Oyama, M., Bachheti, N., and Singh, S.P. (2009) Fullerene C60 modified gold electrode and nanogold modified indium tin oxide electrode for prednisolone determination. Bioelectrochemistry, 74 (2), 272–277.
- 65. Manno, D., Filippo, E., Di Giulio, M., and Serra, A. (2008) J. Non-Cryst. Solids, 354 (52-54), 5515–5520.
- 66. Köhler, J.M., Abahmane, L., Wagner, J., Albert, J., and Mayer, G. (2008) Preparation of metal nanoparticles with varied composition for catalytical applications in microreactors. Chem. Eng. Sci., 63 (20), 5048–5055.
- 67. Guo, J., Wang, X., Miao, P., Liao, X., Zhang, W., and Shi, B. (2012) One-step seeding growth of controllable Ag@Ni core–shell nanoparticles on skin collagen fiber with introduction of plant tannin and their application in high-performance microwave absorption. J. Mater. Chem., 22 (24), 11933–11942.
- 68. Liu, P. and Zhao, M. (2009) Silver nanoparticle supported on halloysite nanotubes catalyzed reduction of 4-nitrophenol (4-NP). Appl. Surf. Sci., 255 (7), 3989–3993.
- 69. Kundu, S., Ghosh, S.K., Mandal, M., and Pal, T. (2002) Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in micellar medium. Bull. Mater. Sci., 25 (6), 577–579.
- 70. Mallick, K., Witcomb, M., and Scurrell, M. (2006) Silver nanoparticle catalysed redox reaction: an electron relay effect. Mater. Chem. Phys., 97 (2), 283–287.
