Chapter 23
Biopolymers Based on Raw Materials from Biomass
Jonggeon Jegal
23.1 Introduction
Plastics produced from petroleum have been used for more than a century in a wide range of applications. However, conventional petroleum-based plastics now pose two major problems: the production of substantial amounts of carbon dioxide (CO2) during their degradation and their lack of biodegradability. Both of these problems relate to the environmental effect of plastics. Production of CO2 is correlated with climate change, while lack of biodegradability is associated with environmental pollution. To solve both of these problems, researchers have attempted to use biomass that grows readily and consumes CO2 to produce plastics, which are known as bioplastics, instead of petroleum [1–9]. It is believed that the concentration of CO2 in the atmosphere could be reduced by switching from petroleum to produce plastics. Bioplastics may also help to alleviate the current heavy reliance on our finite petroleum resources.
Numerous bioplastics have been produced from different types of biomass-based materials. To produce bioplastics, monomeric compounds are first prepared from biomass. Many different monomeric compounds such as lactic acid, succinic acid (SA) and 1,4-butanediol (BD) have been prepared by fermentation using suitable microorganisms such as Lactobacillus and Clostridium. The bioplastics that have been prepared from the various monomers derived from biomass can be divided into five categories: polyesters, polyamides, polyurethanes, polyolefins, and others. Some biopolyesters, like poly(lactic acid) (PLA) and poly(trimethylene terephthalate), polyamides, including nylon-11 and nylon-10,10, and biopolyethylene have already been commercialized. PLA is the most well-known bioplastic and has been widely used for a variety of applications [1, 2]. However, PLA has some limitations in terms of its physical properties and moldability. The mechanical and thermal properties of PLA are also poorer than those of conventional plastics currently in use.
Therefore, it remains important to develop and improve the physical and chemical properties of bioplastics so that they may become comparable with those of conventional petroleum-based plastics. Manufacturing price is another factor that has to be considered seriously to produce bioplastics suitable for commercialization. A consideration of the challenges facing the development of bioplastics reveals that polymers based on SA have good potential. SA is economically favorable because it can be produced inexpensively from glucose by fermentation. SA is a monomer of poly(butylene succinate) (PBS), which possesses desirable physical and chemical properties as well as good thermal processability using conventional techniques. An advantage of PBS is that the mechanical and physical properties required for a specific application can be readily obtained by modification using methods such as copolymerization, blending, and composite formation. Another appeal of PBS is its biodegradability. To minimize the environmental pollution caused by plastics, they need to be biodegradable. Copolymers of PBS such as poly(butylene succinate terephthalate) (PBST) and poly(butylene succinate adipate) (PBSA) possess good biodegradability in addition to desirable physical properties, which makes them attractive bioplastic candidates.
In this chapter, we review the development of bioplastics including PBS and its copolymers PBST and PBSA, along with composites of PBS with various natural fibers and PBS modified with different organic fillers. We examine the thermal, mechanical, and biodegradation properties of these materials to highlight their advantages and limitations with respect to future commercial use as bioplastics.
23.2 Poly(butylene succinate)
PBS is a commercially available biodegradable synthetic polyester produced from SA and BD with properties similar to those of polyethylene (PE) [10, 11]. The monomers SA and BD are currently prepared from petroleum by chemical processes [12, 13].
However, recently technologies to produce SA and BD from biomass have been developed. One method is fermentation using microorganisms [14–20]. With the improvement of biotechnology, the efficiency of SA and BD production by fermentation has increased. SA and BD can also be prepared by the oxidation of furfural followed by sequential hydrogenation using different catalysts. Tachibana et al. [21] produced SA and BD from furfural by chemical reactions. They first prepared fumaric acid (FA) from furfural by oxidation. Then, by sequential hydrogenation using different catalysts, SA and BD were synthesized, as shown in Figure 23.1.

Figure 23.1 Synthesis of succinic acid (SA) and 1,4-butanediol (BD) from furfural [19].
23.2.1 Synthesis
PBS can be synthesized by polycondensation of SA and BD or BD and dimethyl succinate (DA) or by enzymatic polymerization of SA and BD using lipase. Production of PBS by polycondensation has already been used in industry worldwide. PBS can be polymerized by the direct polycondensation of SA and BD using titanium tetraisopropoxide (Ti(i-PrO)4) as a catalyst at 243 °C under 1 mm Hg for 2 h. However, transesterification polycondensation of DS and BD with Ti(i-PrO)4 catalyst can also be used to produce PBS. When transesterification polycondensation was carried out at 215 °C under 10 Pa for 4 h, PBS with high molecular weight can be obtained in a yield of over 90% [19].
In the case of enzymatic polymerization, Azim et al. [22] used Candida antarctica lipase B to polymerize DS and BD. They obtained PBS with an average molecular weight of 38 000 g/mol using the temperature–variable two-stage polymerization method. They reported that the slow diffusivity of the substrates and growing polymer chains resulting from polymer precipitation, which limited further increases in molecular weight after 5–10 h of reaction time, was overcome by increasing the polymerization temperature in the two-stage polymerization process (Figure 23.2).
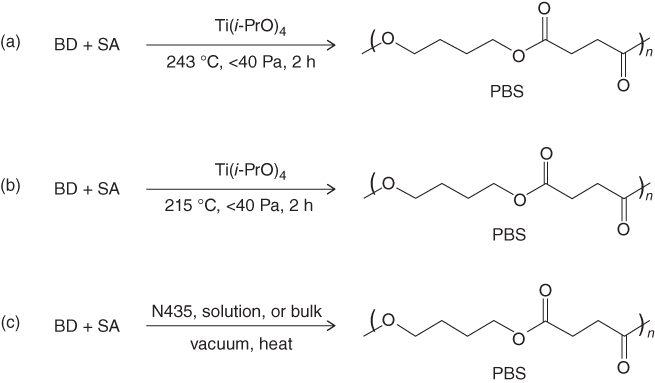
Figure 23.2 Polymerization of PBS: (a) direct polycondensation, (b) transesterification polycondensation, and (c) enzymatic polymerization.
23.2.2 Physical Properties
23.2.2.1 Thermal Properties
PBS is white semicrystalline thermoplastic polymer with an equilibrium melting point of 132 °C and glass transition temperature of −38 °C [23]. However, when PBS samples were isothermally crystallized at temperatures of 80–93 °C, four melting points, Tm1, Tm2, Tm3, and Tm4, appear because of the coexistence of melt–recrystallization and dual thermal stability distribution. The strongest melting peak, Tm1, at 114.6 °C does not depend on crystallization conditions, but the other three melting points, which appear at much lower temperature than Tm1, show very weak peaks; Tm3 and Tm4 disappear for some samples when cooled rapidly from their melting point [24].
23.2.2.2 Mechanical Properties
PBS has excellent mechanical properties and good processability. It can be processed in the temperature range of 160–200 °C to prepare injected, extruded, or blow-molded products using conventional polyolefin equipment. The properties of biomass-based PBS should be the same as those of the petroleum-based equivalent because the properties of BD and SA are independent of their source. The mechanical properties of PBS with a molecular weight Mw of 3.0 × 105 g/mol are presented in Table 23.1 [25].
Table 23.1 Mechanical properties of PBS.a, b)
| Mechanical property | Value |
| Tensile modulus (GPa) | 0.5 |
| Tensile strength (MPa) | 33 |
| Bending modulus (GPa) | 2.5 |
| Bending strength (MPa) | 32 |
| Elongation at break (%) | 126 |
a Weight average molecular weight (Mw) of PBS: 3.0 × 105 g/mol.
b Tm of PBS: 114 °C.
These data reveal that PBS is more ductile than l-PLLA because the elongation at break of PBS is much higher (126%) than that of the PLLA (4%) [26].
PBS is well known for its biodegradability and recyclability. One of the best ways to reduce the problem of waste plastic in the environment is to reuse plastics [27, 28]. Recycling biodegradable materials would prevent environmental pollution by plastics (Figure 23.3). To recycle a plastic, it has to be reprocessable. Therefore, typical semicrystalline polymers such as PE and poly(ethylene terephthalate) (PET) are regarded as promising reprocessable materials. PBS has also proved to be a good reprocessable material.
Figure 23.3 Schematic diagram of biodegradable polymer recycling [29].
Kanemura et al. [29] examined the variation of the mechanical properties of PBS following reprocessing. In particular, the recovery of the mechanical properties of PBS by reprocessing PBS samples damaged by treatment with water is interesting. In their study, they used PBS resin (Bionolle 1020) supplied by Showa Highpolymer Co., Ltd. They prepared a PBS film by molding PBS pellets at 140 °C for 30 min. The film was then cut to dimensions of 70 mm × 10 mm × 3 mm for testing. The resulting PBS samples were immersed in water for a certain time at a given temperature to study their degradation behavior. The PBS samples were then reprocessed and crushed into pieces in a plastic mill at 0 °C before remolding at 140 °C for 30 min to study the effect of reprocessing on the properties of PBS films. To test the mechanical properties of the PBS films, three-point bending tests were performed following Japanese Industrial Standard (JIS) K 7055. By immersion in water at different temperatures for a certain time, the bending strength and molecular weight of the PBS sample were decreased by hydrolysis. When a PBS sample was immersed in water for about 75 h, its molecular weight and tensile strength were almost half (0.42 × 105 g/mol and 23 MPa, respectively) those of a fresh PBS sample (1.0 × 105 g/mol and 43 MPa, respectively). However, when the immersed samples were reprocessed, their molecular weight and tensile strength recovered to 0.63 × 105 g/mol and 32 MPa, respectively, which is attributed to the resynthesis of PBS by reprocessing. In other words, the PBS molecules hydrolyzed by water reacted with each other again at the high reprocessing temperature of 140 °C under dry conditions (Figure 23.4).

Figure 23.4 Hydrolysis and dehydration reactions of PBS [29].
This behavior was not found for PLA even though it is a typical biodegradable aliphatic polyester, because the molecular weight of PLA decreases by thermal degradation during melt processing [30–32]. Kanemura et al. [29] reported that PLA is degraded by hydrolysis in the presence of water, causing its molecular weight to decrease. However, reprocessing of PLA at 140 °C did not recover its molecular weight and mechanical properties like those of PBS; the molecular weight of PLA was decreased even more by molding. This is because PLA generates lactic acid upon hydrolysis rather than lactide, as shown in Figure 23.5. PLA of high molecular weight is prepared by the ring-opening polymerization of lactide, not by the direct polycondensation of lactic acid. Therefore, the lactic acid generated by the hydrolysis of PLA cannot be polymerized into PLA; instead it accelerates the thermal degradation of PLA during reprocessing at high temperature.

Figure 23.5 Hydrolysis and polymerization of PLA.
23.2.2.3 Hydrophilicity
The hydrophilicity of PBS was determined by measuring the contact angle of PBS films using the sessile drop method at 25 °C with a contact angle goniometer (SZ10-JC2000A, Shanghai, China) and compared with those of other biodegradable polymers [25, 33, 34]. These results are summarized in Table 23.2.
Table 23.2 Water contact angles of PBS and other biodegradable polymers [25, 33, 34]
| Sample | Water contact angle (°) |
| PBS | 59 |
| PDLLAa | 67 |
| PHBVb | 66 |
a Poly(d/l-lactic acid).
b Poly(hydroxyl butyrate–valerate).
23.2.3 Biodegradability
Biodegradability is a characteristic feature of PBS, and it has been studied extensively. Li et al. investigated the biodegradation properties of PBS films immersed in water [25, 35–37]. They found that the degradation behavior of PBS was similar to that of poly(α-hydroxyesters) such as PLA, poly(glycolic acid) (PGA) and their copolymer (PLGA). PBS was observed to acidify the solution during degradation [38, 39]. The degradation mechanism of PBS was found to be hydrolysis, the rate of which strongly depends on solution pH because of autocatalysis, as explained by Göpferich and Pitt et al. [40, 41]. Consequently PBS might be degraded through random chain scission by ester hydrolysis in a process autocatalyzed by the generation of carboxylic acid end groups.
According to Li et al. [25, 35–37], when five pieces of PBS (5 mm × 6 mm × 0.2 mm) were immersed in PBS solution, the initial degradation rate was very slow so that after 9 weeks, around 90% of the samples remained. However, the degradation rate accelerated from this point, and only about 35% of the sample remained after 15 weeks. The molecular weight of the PBS sample decreased with increasing degradation time more rapidly than the degradation rate increased. They found that the molecular weight of the PBS films decreased immediately after incubation in PBS solution and continued to decrease throughout the reaction period. At the end of week 15, the molecular weight of the PBS films was only 12.5% of the initial value. The pH of the PBS solution varied with degradation time. Solution pH decreased slowly at first, but more rapidly after 9 weeks, eventually becoming about 5.5 after 15 weeks (Table 23.3).
Table 23.3 Biodegradation data for PBS films [25]
| Degradation time (weeks) | Percent weight remaining (%) | Percent Mw remaining (%) | pH of the PBS solution |
| 0 | 100 | 100 | 7.5 |
| 3 | 97 | 97 | 7.4 |
| 6 | 94 | 80 | 7.3 |
| 9 | 90 | 70 | 6.9 |
| 12 | 55 | 42 | 6.0 |
| 15 | 37 | 13 | 5.5 |
23.2.3.1 Biodegradation in Compost
PBS is known as an environmentally friendly biodegradable material. It is very important to study the biodegradability of PBS in nature. One of the best ways to do this is to study the biodegradability of PBS in compost, because composting is a promising technology to deal with plastic waste [42]. Zhao et al. [43] investigated the biodegradability of PBS film in compost, studying the effect of sample form on biodegradation and the best microorganisms to degrade PBS films.
The effect of PBS form on biodegradation was investigated using compost derived from municipal solid waste following the compositing conditions of ISO 14 855 [44] at 58 °C. They determined the biodegradation percentage (Dt) using the following equation:
where (CO2)t is the accumulated amount of carbon dioxide released by each composting vessel containing test material, (CO2)b is the accumulated amount of carbon dioxide released by the control, and ThCO2 is the theoretical amount of carbon dioxide in the test material in the test vessel. Zhao et al. [43] used PBS samples in three different forms – powder, film, and granule – and found that the degradation percentages after 90 days of incubation were 71.9%, 60.7%, and 14.1%, respectively. The molecular weight of the sample decreased from 8 × 104 to 1.4 × 104 g/mol after 90 days of composting (Table 23.4).
Table 23.4 Biodegradation of different forms of PBS after 90 days of composting [43]
| Biodegradation percentage (%) | |
| Sample form | Degradation percentage (%) |
| Powder | 71.9 |
| Film | 60.7 |
| Granule | 14.1 |
The high biodegradation percentage obtained by composting compared with biodegradation in water is caused by the microorganisms present in the compost aiding degradation. The microorganisms synergistically promoted the biodegradation of PBS powder. Among the four strains isolated from the compost – Aspergillus versicolor, Penicillium, Bacillus, and Thermopolyspora – A. versicolor was found to be the best at degrading PBS [43].
23.2.4 Modification of PBS
PBS is a commercially available biodegradable aliphatic thermoplastic polyester with good mechanical properties, melt processability, and thermal properties. Because of its good melt processability, it has often been used in different types of fibers such as multifilament, monofilament, nonwoven, and flat and split yarns. PBS has also been used to make various types of products by injection molding. However, its physical and chemical properties need to be modified for it to be suitable for various potential applications. Three methods are commonly used to modify PBS: copolymerization, blending, and compositing [45–57]. Lactic acid, dioxanone, and ethanediol are frequently used to form PBS copolymers with good biodegradability [58–62]. PBS can also be blended with other polymers such as PLA, PHB, and poly(butylene adipate) (PBA) [53–67].
PBS is often modified by compositing with different types of materials such as lignocellulose fibers and inorganic fillers because it is the most convenient way to modulate its properties. The useful natural fibers used to modify PBS include silk fiber [68, 69], sisal fiber [70], red algae fiber [71], coir fiber [72], henequen [68], abaca fiber [73], and jute fiber [74]. Using such natural fibers, PBS composites with good mechanical properties and biodegradability can be prepared. PBS has been modified with numerous organic fillers including silicate [75], montmorillonite [76–78], nanoclays [79–82], nanotubes [83–85], zeolites [86], ZnO [83, 87], graphene [88], and hollow glass microspheres [89].
23.2.4.1 Modification with Inorganic Fillers
Organically modified layered silicates (OMLS) such as octadecylammonium-modified montmorillonite (C18-MMT), octadecyltrimethylammonium-modified montmorillonite (qC18-MMT), and hexadecyltributylphosphonium-modified saponite (qC16-SAP) have often been used to modify the properties of aliphatic polyesters such as PLA and PBS. Physical properties of PBS such as softness, tensile strength, gas barrier, and melt viscosity can easily be modified by making PBS/layered silicate nanocomposites.
PBS/layered silicate nanocomposites can be prepared by extrusion using a twin extruder at 150 °C. Before making nanocomposite pellets, a PBS/OMLS master batch has to be prepared by adding OMLS powder through the window of the extruder barrel into a PBS melt. The prepared master batch is then dry mixed with PBS pellets using a bag and added to the twin extruder, which makes PBS/layered silicate nanocomposite pellets at 150 °C.
Okamoto et al. [75, 90] found from wide-angle X-ray diffraction (WAXD) analysis that the d-spacing of the pure C18-MMT (2.31 nm) became larger in the corresponding nanocomposite PBS/C18-MMT (2.82 nm), indicating the formation of an intercalated structure in the PBS/OMLS nanocomposite. The specifications of the clay they used are provided in Table 23.5.
Table 23.5 Specifications of OMLS
| OMLS | Pristine clay | Particle size (nm) | CEC (mol equiv/100 g) | Organic salts to modify the pristine clay | Supplier |
| C18-MMT | Montmorillonite | 150–200 | 110 | Octadecylammonium cation | Nancor Inc. |
| qC18-MMT | Montmorillonite | 100–130 | 90 | Octadecyltriammonium cation | Hojun Yoko Co. |
| qC16-SAP | Saponite | 50–60 | 86.6 | Hexadecyltributylphosphonium cation | CO-OP Chemicals |
They reported that for all nanocomposites, marked enhancement of G′ and G″ was observed in the given temperature range (Table 23.6). This behavior was attributed to the effect of intercalation with OMLS on the elastic properties of the PBS matrix. The clay particles provide the composite with mechanical reinforcement. It was also found that above the glass transition temperature (Tg), the reinforcement effect of the clay particles becomes prominent. This phenomenon is caused by the clay restricting the movement of the polymer chains. The essential factor governing the enhancement of mechanical properties is the aspect ratio of the dispersed intercalated clay particles.
Table 23.6 G′ of PBS and nanocomposites at different temperatures [90]
| Sample | G′/GPa | |||
| −50 °C | 0 °C | 50 °C | 100 °C | |
| PBSa | 1.6 | 0.34 | 0.2 | 0.19 |
| PBS/C18-MMTb | 4.3 | 1.10 | 0.69 | 0.27 |
| PBS/qC18-MMTc | 3.9 | 1.05 | 0.63 | 0.28 |
| PBS/qC16-SAPd | 3.2 | 0.79 | 0.43 | 0.22 |
a Mw of PBS: 101 000 g/mol.
b Clay content: 3.6wt%, Mw of PBS: 104 000 g/mol.
c Clay content: 3.6wt%, Mw of PBS: 112 000 g/mol.
d Clay content: 3.8wt%, Mw of PBS: 91 000 g/mol.
Table 23.7 compares some of the properties of neat PBS and its nanocomposites. The marked increase in the tensile modulus of the nanocomposites compared with that of PBS is caused by the high aspect ratio of dispersed clay particles in the nanocomposites. The tensile strength of the nanocomposites was lower than that of PBS. This is caused by the lack of interaction between PBS polymer matrix and OMLS.
Table 23.7 Tensile properties and O2 gas permeability of neat PBS and nanocomposites [90]
| Property | PBS | PBS/C18-MMT | PBS/qC18-MMT | PBS/qC16-SAP |
| Tensile yield strength (MPa) | 35 | 34.2 | 32.5 | 31.6 |
| Tensile modulus (GPa) | 0.53 | 0.88 | 0.82 | 0.71 |
| O2 gas permeability coefficient (ml mm/m2 day MPa) | 87.3 | 42.2 | 69.0 | 71.2 |
In the case of O2 gas permeability, the nanocomposites containing inorganic clay generally have lower permeability than the polymer matrix itself because clay in its crystalline state restricts the permeability of gas molecules. Table 23.7 reveals that the nanocomposites have lower O2 gas permeability than PBS itself.
Another interesting property of the PBS/OMLS nanocomposites is their biodegradability. Okamoto et al. [90] studied the biodegradability of their PBS/OMLS nanocomposites. The results are presented in Table 23.8, in which the molecular weight of PBS in different nanocomposites after 35 days of biodegradation is shown. They used biodegradation conditions of incubation at 58 °C in custom-made compost.
Table 23.8 Molecular weight of PBS in various samples after 35 days of biodegradation in compost [90]
| Sample | Mw × 10−3 (g/mol) | Mn × 10−3 (g/mol) | Mwo × 10−3 (g/mol) | Mw/Mwo |
| PBS | 16 | 3.8 | 101 | 0.16 |
| PBS/C18-MMT | 17 | 6.6 | 104 | 0.16 |
| PBS/C18-MMT | 17 | 4.4 | 112 | 0.15 |
| PBS/qC16-SAP | 8.7 | 1.2 | 91 | 0.096 |
Mwo is the molecular weight before composting.
Table 23.8 reveals that the biodegradability of nanocomposites is dependent on the clay used. The degree of biodegradation of PBS/OMLS nanocomposites is more or less the same as that of neat PBS except for PBS/qC16-SAP. This suggests that montmorillonite and alkylammonium cations do not affect the biodegradability of PBS. The increased biodegradation of the PBS/qC16-SAP nanocomposite is because of the alkylphosphonium surfactant it contained rather than the montmorillonite. Similar behavior has also been reported for other PLA/OMLS nanocomposites [91, 92].
Carbon nanotubes (CNTs) have become famous nanomaterials because of their excellent mechanical properties such as high tensile strength, modulus, and electrical and thermal conductivity since their discovery by Iijima in 1991 [93, 94]. Recently, CNTs have been widely used to form nanocomposites. They consist of concentric cylinders of graphite layers and can be classified into two different types: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). With their high aspect ratio, nanoscale diameter, very low density, and excellent physical properties, CNTs are considered ideal reinforcing fillers in polymer nanocomposites. The formation of PBS/CNT nanocomposites is one of the best ways to improve the mechanical and thermal properties of PBS for engineering plastic applications, similar to other biodegradable polymer/CNT nanocomposites.
Table 23.9 Thermal properties of PBS and PBS/CNT nanocomposites [83]
| Samplea | Tm1 (°C) | Tm2 (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
| PBS | 103.7 | 112.8 | 70.3 | 89.9 | 69.2 |
| PBS/CNT 0.1 | 103.7 | 112.8 | 69.7 | 90.0 | 68.9 |
| PBS/CNT 0.5 | 103.9 | 123.1 | 70.1 | 90.5 | 69.8 |
| PBS/CNT 1 | 104.1 | 112.9 | 69.5 | 90.8 | 69.1 |
| PBS/CNT 3 | 104.7 | 113.2 | 70.2 | 92.7 | 69.7 |
| PBS/CNT 5 | 105.2 | 113.0 | 70.0 | 95.8 | 69.5 |
a CNT contents of PBS/CNT nanocomposites: 0.1 phr for PBS/CNT 0.1, 0.5 phr for PBS/CNT 0.5, 1.0 phr for PBS/CNT 1, 3.0 phr for PBS/CNT 3, and 5.0 phr for PBS/CNT 5.
There are three different ways to make polymer/CNT nanocomposites: solution casting [95–98], melt blending [99–102], and in situ polymerization of monomers in the presence of CNTs [103–105]. Among these methods, melt blending is used the most often in the industry because it is the simplest and the most compatible with current industrial practice. Using melt blending, it is possible to prepare high-performance polymer nanocomposites at low cost. Even though it is less effective than solution blending for the dispersion of CNTs throughout the polymer matrix, it is the method most suited for commercial applications.
Ali and Mohan [83] prepared PBS/CNT nanocomposites by melt mixing PBS (density 1.22 g/cm3, Ire Chemical Co., Seoul Korea) with MWCNTs (Nanocyl-700, average diameter 9.5 nm, length 1.5 mm, specific surface area 250–300 m2/g, Nano Best Co., South Korea) using a mixer (Haake Rheomix 600p).
Table 23.9 lists the thermal properties of PBS and the PBS/CNT nanocomposites. The recrystallization temperature (Tc) of the PBS/CNT nanocomposites increased with nanotube content, and all values are higher than that of neat PBS. This result is strongly related to the role of CNTs as nucleating agents, because they increase the crystallization rate of PBS. However, the two melting points of pristine PBS were not changed substantially in the PBS/CNT nanocomposites, indicating the size and size distribution of the crystals formed in the different PBS/CNT nanocomposites were similar.
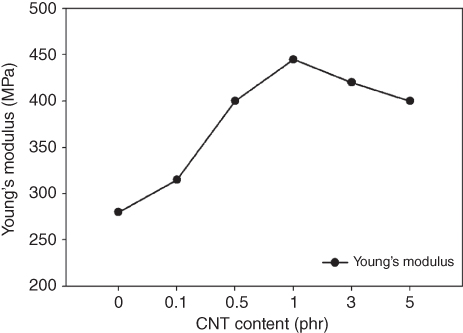
Figure 23.6 Young's modulus of PBS/CNT nanocomposites as a function of CNT content.
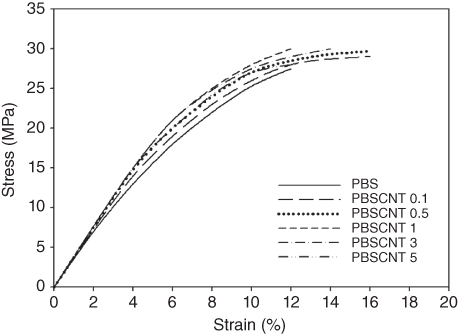
Figure 23.7 Tensile properties of PBS/CNT nanocomposites with different CNT contents.
Figures 23.6 and 23.7 show Young's modulus and tensile properties of the PBS/CNT nanocomposites, respectively [83]. Figure 23.6 reveals that Young's modulus of the PBS/CNT nanocomposites increased with CNT content up to 1.0 phr, and beyond that point, it decreased. This behavior can be explained by the extent of CNT dispersion throughout the nanocomposites. Up to a CNT content of 1 phr, CNTs were well dispersed, and because of the good interaction of PBS molecules with each CNT with high aspect ratio, Young's modulus of the nanocomposites was increased. However, with further increase in CNT content, because of the strong van der Waals interaction between CNTs, they began to agglomerate, decreasing their dispersion in the corresponding nanocomposites. The agglomeration of CNTs beyond a CNT content of 1.0 phr hindered further improvement in Young' modulus of the nanocomposites, but it was still higher than that of pristine PBS.
Similar behavior was also observed for the tensile strength of the nanocomposites (Figure 23.7). With increasing CNT content, tensile strength at break was improved. The maximum improvement was obtained for a CNT content of 1 phr. The elongation at break of the PBS/CNT nanocomposites showed different behavior. Up to a CNT content of 0.5 phr, elongation at break increased, but beyond this CNT content, there was no improvement.
Figure 23.8 illustrates the electrical conductivity of the PBS/CNT nanocomposites. One anticipates improvement of the electrical conductivity of the PBS/CNT nanocomposites compared with that of PBS because CNTs are highly conductive. As expected, the PBS/CNT nanocomposites exhibited much higher electrical conductivity as the CNT content increased. Unlike their mechanical properties, the PBS/CNT nanocomposites showed the highest electrical conductivity at higher CNT content, and at 5 phr, the conductivity was about 10−4 S/cm. This is a substantial increase considering that the conductivity of neat PBS is 10−12 S/cm. The high conductivity obtained at high CNT content is caused by the interconnection between CNTs [106–109]. The CNTs dispersed throughout the polymer matrix connect to one another to form conductive pathways. When an electric field is applied to the PBS/CNT nanocomposite, electrons readily move through its interconnecting conductive channels, resulting in high conductivity.
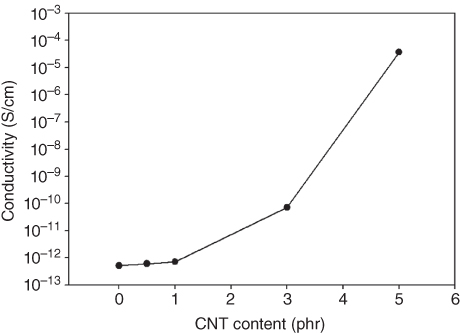
Figure 23.8 Electrical conductivities of PBS/CNT nanocomposites as a function of CNT content.
Graphene is a flat two-dimensional monolayer of Sp2-bonded carbon atoms that is as well known as CNTs. Like CNTs, graphene possesses high conductivity and excellent mechanical and thermal properties [110–112]. Recently, formation of nanocomposites using graphene sheets has produced polymeric materials with high performances for a variety of potential applications including thermally stable and mechanically reinforced materials [113, 114], conductive composites [115], ultrasensitive sensors [116], and supercapacitor electrodes [117].
Wang et al. [118, 119] investigated the morphology and mechanical and thermal properties of graphene-reinforced PBS nanocomposites prepared via a solution-based processing method. They tried to improve the mechanical and thermal properties of PBS by using graphene as a reinforcing material. Graphene has a strong tendency to agglomerate because of its high surface area and hydrophobic nature, so they treated graphene oxide with hydrazine to prepare a graphene nanosheet (GNS) solution before synthesizing PBS/graphene nanocomposites.
Table 23.10 lists the physical properties of their PBS/graphene nanocomposites. The mechanical properties and electrical conductivity of PBS were substantially improved by the inclusion of a small amount of graphene (<2.0 wt%) because of the strong interaction between PBS and GNS. The electrical conductivity of PBS increased from 2.5 × 10−12 to 2.08 × 10−7 S/cm by including just 2.0 wt% graphene. These results indicate that the GNS are well dispersed and form effective conductive channels throughout the PBS matrix. However, GNS do not strongly influence the crystallinity and Tg of PBS.
Table 23.10 Physical properties of PBS/graphene nanocomposites [118]
| Sample | GNS (wt%) | Tensile strength (MPa) | Elongation at break (%) | Conductivity (S/cm) |
| PBS | 0 | 30.8 | 17.9 | 2.50 × 10−12 |
| PBS/GNS-0.5 | 0.5 | 34.3 | 18.5 | 1.36 × 10−11 |
| PBS/GNS-1.0 | 1.0 | 36.4 | 18.5 | 2.61 × 10−9 |
| PBS/GNS-2.0 | 2.0 | 37.2 | 19.0 | 2.08 × 10−7 |
23.2.4.2 Modification with Natural Fibers
In the past decade, many different types of natural fibers have been used to fabricate composites with petroleum-based polymers. Among the commonly used natural fibers are henequen, hemp, jute, kenaf, sisal, flax, bamboo, coir, banana, palm, silk, cotton, and wood. Compared with petroleum-based fibers, these natural fibers have many advantages such as low cost, low density, low pollutant emissions, acceptable specific properties, renewable characteristics, enhanced energy recovery, and complete biodegradability [120–123]. Therefore, they are considered strong candidates for the formation of PBS/ fiber composites with desirable properties.
Table 23.11 shows the chemical composition and structural parameters of some natural fibers, while Table 23.12 compares the properties of some natural fibers with conventional man-made fibers.
Table 23.11 Chemical composition and structural parameters of some natural fibers [120]
| Type of fiber | Cellulose (wt%) | Lignin (wt%) | Hemicellulose (wt%) | Pectin (wt%) | Wax (wt%) | Microfibrillar/spiral angle (°) | Moisture content (wt%) |
| Jute | 61–71.5 | 12–13 | 13.6–20.4 | 0.2 | 0.5 | 8.0 | 12.6 |
| Flax | 71 | 2.2 | 18.6–20.6 | 2.3 | 1.7 | 10.0 | 10.0 |
| Hemp | 70.2–74.4 | 3.7–5.7 | 17.9–22.4 | 0.9 | 0.8 | 6.2 | 10.8 |
| Ramie | 68.6–76.2 | 0.6–0.7 | 13.1–16.7 | 1.9 | 0.3 | 7.5 | 8.0 |
| Kenaf | 31–39 | 15–19 | 21.5 | — | — | — | — |
| Sisal | 67–78 | 8.0–11.0 | 10.0–14.2 | 10.0 | 2.0 | 20.0 | 11.0 |
| PALFa | 70–82 | 5–12 | — | — | — | 14.0 | 11.8 |
| Henequen | 77.6 | 13.1 | 4–8 | — | — | — | — |
| Cotton | 82.7 | — | 5.7 | — | 0.6 | — | — |
| Coir | 36–43 | 41–45 | 0.15–0.25 | 3–4 | — | 41–45 | 8.0 |
a PALF is pineapple leaf fiber.
Table 23.12 Properties of some natural and conventional man-made fibers [120]
| Fiber | Density (g/cm3) | Diameter (μm) | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) |
| Cotton | 1.5–1.6 | — | 287–800 | 5.5–12.6 | 7.0–8.0 |
| Jute | 1.3–1.45 | 25–100 | 393–773 | 13–26.5 | 1.16–1.5 |
| Flax | 1.50 | — | 345–1100 | 27.6 | 2.7–3.2 |
| Hemp | — | — | 690 | — | 1.6 |
| Ramie | 1.50 | — | 400–938 | 61.4–128 | 1.2–3.8 |
| Sisal | 1.45 | 50–200 | 468–640 | 9.4–22.0 | 3–7 |
| PALFa | — | 20–80 | 413–1627 | 34.5–82.51 | 1.6 |
| Coir | 1.15. | 100–450 | 131–175 | 4–6 | 15–40 |
| E-glass | 2.5 | — | 2000–3500 | 70 | 2.5 |
| S-glass | 2.5 | — | 4570 | 86 | 2.8 |
| Aramid | 1.4 | — | 3000–3150 | 63–67 | 3.3–3.7 |
| Carbon | 1.7 | — | 4000 | 230–240 | 1.4–1.8 |
a PALF is pineapple leaf fiber.
Coir is lignocellulosic fiber extracted from coconut palm that has low cellulose and hemicellulose contents, high lignin content, and high microfibril angle compared with other natural fibers (see Table 23.11). The tensile strength and Young's modulus of coir are relatively low; however, its elongation at break is the highest among the fibers shown in Table 23.12. Such mechanical properties are favorable for the fabrication of automobile seat cushions, so it has been widely used in the industry [124]. The combination of coir fibers and PBS resin can produce environmentally friendly biodegradable composites.
Nam et al. [72] examined the mechanical properties of PBS/coir fiber composites. They prepared PBS/coir fiber composite sheets by the compression molding method using a PBS sheet and coir fibers and studied the effect of alkali treatment and fiber content on the mechanical properties of the resulting PBS/coir fiber composites. They treated the coir fibers with 5% NaOH solution for different periods to modify the surface of the coir fibers and improve the compatibility between PBS and coir fibers. Figures 23.9 23.10, and 23.11 illustrate the tensile properties of the coir fiber-reinforced PBS composites.

Figure 23.9 Tensile strength of untreated and alkali-treated coir fiber/PBS biodegradable composites (mean value and standard deviation).
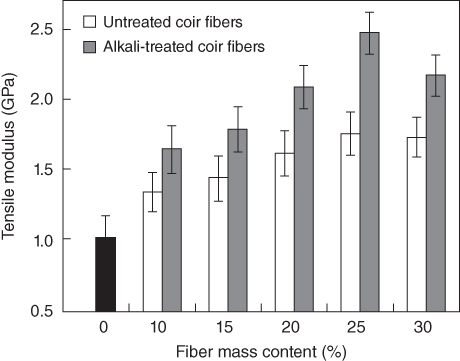
Figure 23.10 Tensile modulus of untreated and alkali-treated coir fiber/PBS biodegradable composites (mean value and standard deviation).

Figure 23.11 Elongation at break of untreated and alkali-treated coir fiber/PBS biodegradable composites (mean value and standard deviation).
As shown in Figures 23.9 and 23.10, the tensile strength and tensile modulus of the samples improved with increasing coir fiber content up to 25% for both untreated and alkali-treated coir fibers, but beyond that point, they started to decrease. The increase of tensile strength and modulus of the composites probably results from reinforcement by the coir fibers in the PBS matrix in the direction of external load, because the strength and modulus of coir fiber are higher than those of the PBS matrix. Generally, fiber-reinforced composites have higher tensile modulus than the pure resin itself, because the factors affecting the tensile modulus of fiber-reinforced composites are the modulus of the fiber and matrix, fiber content, and orientation. The modulus of a composite strongly depends on that of the fibers used.
The elongation at break of the PBS/coir fiber composites exhibited the opposite behavior, decreasing with increasing content of coir fiber. Figure 23.11 shows the elongation at break of the PBS/coir composite sheets as a function of fiber content. This behavior is closely related to the interface between the PBS matrix and coir fibers. Poor fiber–matrix adhesion caused by the poor compatibility between the fiber and PBS matrix is the main reason for the decrease in the elongation at break of PBS/coir fiber composites compared with that of PBS.
Figures 23.9–23.11 reveal that every physical property of the PBS/coir fiber composites is better when alkali-modified coir fibers are used instead of untreated ones because alkali treatment improved the compatibility between the fiber and PBS matrix. Treatment with alkaline chemicals causes the surface of natural fibers to become more hydrophobic, improving their compatibility with PBS. In fact, most natural fibers possess very hydrophilic surfaces with hydrophilic functional groups such as −OH and −COOH. Such hydrophilic functional groups can be removed by alkali treatment.
Jute fiber is a natural fiber that is often used to form fiber-reinforced polymer composites. It is a lignocellulosic fiber with strongly polarized hydroxyl groups and hydrophilic characteristics such as high moisture absorption and poor wettability and compatibility with polymer matrices. As a result, it has to be chemically treated to make it more hydrophobic and compatible with polymer matrices.
Liu et al. [74] studied the mechanical properties of PBS biocomposites reinforced with surface-modified jute fibers. They treated jute fibers in three different ways before the formation of composites: (i) treatment with a solution of 2% NaOH, 0.2% Na2SiO3, 0.2% Na2P3O10, 0.15% Na2SO3, and 0.2% penetrate JFC at 100 °C for 1 h, (ii) treatment of the fibers from the treatment (i) with 5% NaOH solution, and (iii) treatment of the fibers from the treatment (i) with coupling agent (CH2=C(CH3)COO(CH2)3Si(OH)3). After treatment of the jute fibers, they chopped them into shorter fibers, and then PBS/jute fiber composites were prepared by extrusion.
The characteristics of the jute fibers treated under different conditions are presented in Table 23.13. Treatment with NaOH caused the moisture regain of jute fibers to substantially decrease depending on treatment conditions, suggesting improved compatibility of jute fibers and PBS. The diameter of the fibers also decreased following treatment. These changes in the characteristics of the jute fibers mean that PBS/jute fiber composites with better mechanical properties might be obtained by pretreatment of jute fibers with NaOH.
Table 23.13 Characteristics of jute fibers following surface modification
| Jute fiber | Moisture regain (%) | Diameter (μm) | Main compositions (%) | |||
| Cellulose | Hemicellulose | Lignin | Residual pectin | |||
| No treatment | 11.9 | 60 | 67.4 | 14.0 | 14.8 | 24.8 |
| 2% NaOH treatment | 9.7 | 48 | 76.7 | 11.6 | 10.1 | 6.3 |
| 2 + 5% NaOH treatment | 8.8 | 45 | 79.6 | 10.1 | 9.0 | 5.4 |
| Coupling agent treatment | 8.5 | 46 | 80.3 | 9.5 | 9.2 | 5.2 |
Figure 23.12 shows the tensile properties of the PBS/jute fiber composites containing jute fibers modified under different conditions. When the jute fibers modified with coupling agent were used, the mechanical properties of the resulting composite were the best, indicating improved compatibility between the jute fibers and PBS (see Figure 23.13 for SEM images of fracture surfaces of the composites). The dependence of the tensile properties of the PBS/jute fiber composites on fiber content is very similar to that of PBS composites containing other natural fibers such as coir (see Figures 23.9–23.11) whose tensile properties are very closely related to the compatibility between PBS and the natural fibers and the dispersion of the fiber throughout the PBS matrix. Better compatibility and dispersion tend to result in better tensile properties. Liu et al. [125] also studied biodegradability of PBS/jute fiber composites by burial in compost soil. The biodegradability of the composites strongly depended on fiber content, and the degree of weight loss followed the order PBS/10% jute composite > PBS/20% jute composite > PBS/30% jute composite > pure PBS film > bulk jute fiber. The weight loss of the PBS/10% jute composite after 180 days was 62.5%. Surface modification of the fiber also affected biodegradation, with the samples showing weight loss with the order PBS/untreated jute > PBS/alkali-treated jute > PBS/coupling agent-treated jute, indicating that improved hydrophobicity (i.e., decreased hydrophilicity of the jute fibers and improved compatibility with PBS) is not favorable for biodegradation by microorganisms.
Cotton is another very well-known natural fiber that is widely used in fabrics. However, as a natural fiber, cotton is not an exception with respect to undesirable properties such as moisture absorption and poor compatibility with hydrophobic polymer matrices.

Figure 23.12 Tensile properties of PBS/jute fiber composites [113].
Calabia et al. [126] studied the effect of cotton fiber content on the mechanical properties and biodegradability of PBS/cotton fiber composites containing cotton fibers with and without silane treatment [126]. Before formation of the composites, they treated the cotton fibers with silane coupling agents such as 3-aminopropyltriethoxysilane (APTES), 3-aminopropyltrimethoxysilane (APTMS), and N1-3-trimethoxysilylpropyldiethylene triamine (TMSPDET) to overcome the difficulties encountered with natural fibers. Then they used compression molding to produce PBS/cotton fiber composites from PBS powder and the cotton fibers.
Figure 23.14 shows the effect of treating cotton fibers with silane coupling agents on the mechanical properties of the resulting PBS/cotton fiber composites according to cotton fiber content. As expected, the tensile properties of the PBS/cotton fiber composites are very similar to those of other PBS composites with different fibers such as jute and coir. The biodegradability of the PBS/cotton fiber composites is better than that of neat PBS, like the other PBS/natural fiber composites.
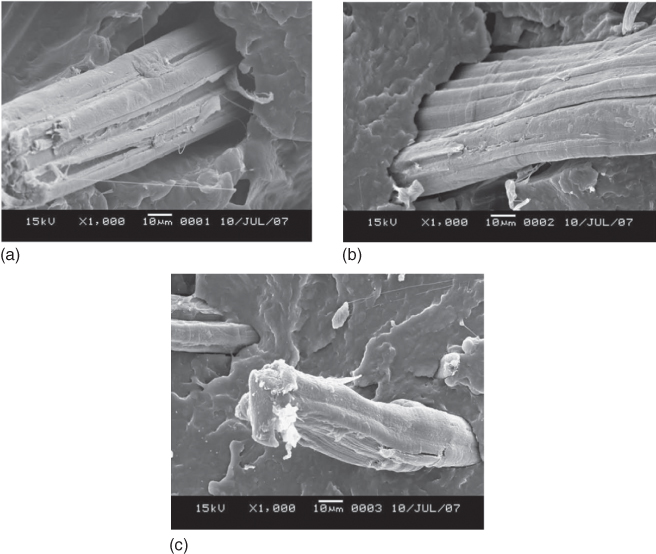
Figure 23.13 Fracture surfaces of PBS composites reinforced with (a) untreated, (b) 2% NaOH-treated, and (c) coupling agent-treated jute fibers.
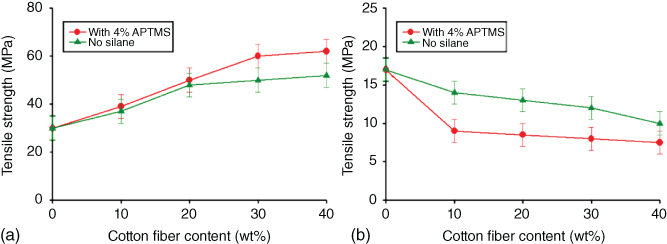
Figure 23.14 Effect of silane treatment (with 3% APTMS) on the (a) tensile strength and (b) elongation at break of PBS/CF composites.
23.3 Conclusion
With improvement of biotechnology, it has become possible to produce various monomers not only from petroleum but also from biomass, mainly by fermentation using suitable microorganisms. It is also possible to prepare monomers directly from biomass by chemical processes using appropriate chemical catalysts. SA and BD are examples of monomers that can be prepared in high efficiency from biomass through fermentation using glucose as a feed source. They also can be prepared from furfural obtained from biomass by a chemical process involving oxidation and sequential hydrogenation. Using these monomers, PBS can be prepared as a bioplastic by chemical polycondensation or transesterification. Under suitable polymerization conditions, PBS of high molecular weight can be produced, usually using Ti(i-PrO)4 as a catalyst. PBS has good thermal and physical properties; in particular, its processability is suitable for fiber formation or injection molding. PBS shows good biodegradability, making it more environmentally friendly than petroleum-based polymers.
However, sometimes PBS has to be modified to suit various potential applications because its physical and chemical properties do not fit the specific requirements. Three different methods are commonly used to modify PBS: copolymerization, blending, and compositing. Copolymerization with terephthalic acid or adipic acid produces PBST or PBSA, respectively. PBST has much better mechanical and thermal properties than PBS because of the presence of aromatic rings in its main chain, so PBST is better suited for applications that require high mechanical strength than PBS. PBSA also has better flexibility than PBS, because of the longer alkyl chains in its main chain. Compositing allows more diverse modification of PBS. PBS composites have been formed using many different types of materials. Natural fibers such as silk, sisal, red algae, coir, henequen, abaca, and jute fibers have been used to produce PBS composites with good mechanical properties and biodegradability. Inorganic fillers can also be used to prepare PBS composites with high mechanical strength and improved heat resistance. Among the inorganic fillers most often combined with PBS are silicate, montmorillonite, nanoclay, nanotube, zeolite, ZnO, graphene, and hollow glass microspheres.
With its good physical and chemical properties and ease of modification, PBS is a promising bioplastic. The low cost of producing SA by fermentation reduces the production cost of biomass-based PBS, so it shows good economic feasibility to be commercialized.
References
- 1. Lunt, J. (1998) Large-scale production, properties and commercial application of polylactic and polymers. Polym. Degrad. Stab., 59 (1–3), 145–152.
- 2. Vink, E.T.H., Rábago, K.R., Glassner, D.A., and Gruber, P.R. (2003) Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab., 80 (3), 403–419.
- 3. Sudesh, K., Abe, H., and Doi, Y. (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci., 25 (10), 1503–1555.
- 4. Sudesh, K. and Doi, Y. (2000) Molecular design and biosynthesis of biodegradable polyesters. Polym. Adv. Technol., 11 (8–12), 865–872.
- 5. Kim, S. and Dale, B.E. (2008) Energy and greenhouse gas profiles of polyhydroxybutyrates derived from corn grain: a life cycle perspective. Environ. Sci. Technol., 42 (20), 7690–7695.
- 6. Jones, R.F. (ed.) (2009) The Future of the Chemical Industry, ACS Symposium Series 1026, American Chemical Society, Washington, DC, pp. 1–17.
- 7. Rincones, J., Zeidler, A.F., Grassi, M.C.B., Carazzolle, M.F. et al. (2009) The golden bridge for nature: the new biology applied to bioplastics. Polym. Rev., 49 (2), 85–106.
- 8. Petrović, Z.S. (2008) Polyurethanes from vegetable oils. Polym. Rev., 48 (1), 109–155.
- 9. Dwan'isa, J.P.L., Mohanty, A.K., Misra, M., Drza, L.T. et al. (2004) Biobased polyurethane and its composite with glass fiber. J. Mater. Sci., 39 (6), 2081–2087.
- 10. Ba, C., Yang, J., Hao, Q., Liu, X. et al. (2003) Syntheses and physical characterization of new aliphatic triblock poly(l-lactide-b-butylene succinate-b-l-lactide)s bearing soft and hard biodegradable building blocks. Biomacromolecules, 4 (6), 1827–1834.
- 11. Shirahama, H., Kawaguchi, Y., Aludin, M., and Yasuda, H. (2001) Synthesis and enzymatic degradation of high molecular weight aliphatic polyesters. J. Appl. Polym. Sci., 80 (3), 340–347.
- 12. Furia, T.E. (1973) Handbook of Food Additives, CRC Press.
- 13. Kunioka, M. (2010) Possible incorporation of petroleum-based carbons in biochemicals produced by bioprocess. Appl. Microbiol. Biotechnol., 87 (2), 491–497.
- 14. McKinlay, J.B., Vielle, C., and Zeikus, J.G. (2007) Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol., 76 (4), 727–740.
- 15. Song, H. and Lee, S.Y. (2006) Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol., 39 (3), 352–361.
- 16. Carnahan, J.E., Ford, T.A., Gresham, W.F., Grigsby, W.E. et al. (1955) Ruthenium-catalyzed hydrogenation of acids to alcohols. J. Am. Chem. Soc., 77 (14), 3766–3768.
- 17. Xu, J. and Guo, B.H. (2010) Plastics from bacteria, in Natural Functions and Applications (ed. G.G.Q. Chen), Springer, Heiderberg, pp. 347–388.
- 18. Mizukoshi, T. (2006) Bionolle-Approach to the More Environmentally-Friendly Green Plastics. Proceedings of the 2nd International Conference on Technology and Application of Biodegradable and Biobased Plastics (ICTABP2), October 13–15, 2006, Hangzhou, China. China Plastics Processing Industry Association, Beijing, pp. 123.
- 19. Kato, S.,Tsukahara, T., Kishimoto, M., and Nagaya, I. (2006) Development of Green Sustainable Plastic (CS Pla). Proceedings of the 2nd International Conference on Technology and Application of Biodegradable and Biobased Plastics (ICTABP2), October 13–15, 2006, Hangzhou, China. China Plastics Processing Industry Association, Beijing, pp. 96.
- 20. Shitani, N. and Kato, S. (2007) GSC-AON, vol. 235, Japan Chemical Innovation Institute, Tokyo.
- 21. Tachibana, Y., Masuda, T., Funabashi, M., and Kunioka, M. (2010) Chemical synthesis of fully biomass-based poly(butylene succinate) from inedible-biomass-based furfural and evaluation of its biomass carbon ratio. Biomacromolecules, 11 (10), 2760–2765.
- 22. Azim, H., Dekhterman, A., Jiang, Z., and Gross, R.A. (2006) Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): shorter chain building blocks also work. Biomacromolecules, 7 (11), 3093–3097.
- 23. Miyata, T. and Masuko, T. (1998) Crystallization behaviour of poly(tetramethylene succinate). Polymer, 39 (6–7), 1399–1404.
- 24. Wang, X., Zhou, J., and Li, L. (2007) Multiple melting behavior of poly(butylene succinate). Eur. Polym. J., 43 (8), 3163–3170.
- 25. Li, H., Chang, J., Cao, A., and Wang, J. (2005) In vitro evaluation of biodegradable poly(butylene succinate) as a novel biomaterial. Macromol. Biosci., 5 (5), 433–440.
- 26. Södergard, A. and Stolt, M. (2002) Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci., 27 (6), 1123–1163.
- 27. Kartalis, C.N., Papaspyrides, C.D., Pfaendner, R., Hoffmann, K. et al. (1999) Mechanical recycling of postused high-density polyethylene crates using the restabilization technique. I. Influence of reprocessing. J. Appl. Polym. Sci., 73 (9), 1775–1785.
- 28. Noll, K.E., Haas, C.N., Schmidt, C., and Kodukula, P. (1985) in Recovery, Recycle, and Reuse of Industrial Waste (ed. K.E. Noll), Lewis Publishers Inc..
- 29. Kanemura, C., Nakashima, S., and Hotta, A. (2012) Mechanical properties and chemical structures of biodegradable poly(butylene succinate) for material reprocessing. Polym. Degrad. Stab., 97 (6), 972–980.
- 30. Södergård, A. and Näsman, J.H. (1994) Stabilization of poly(l-lactide) in the melt. Polym. Degrad. Stab., 46 (1), 25–30.
- 31. Signori, F., Coltelli, M.B., and Bronco, S. (2009) Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab., 94 (1), 74–82.
- 32. Pillin, I., Montrelay, N., Bourmaud, A., and Grohens, Y. (2008) Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polym. Degrad. Stab., 93 (2), 321–328.
- 33. Li, H. and Chang, J. (2004) Preparation and characterization of bioactive and biodegradable wollastonite/poly(d,l-lactic acid) composite scaffolds. J. Mater. Sci. Mater. Med., 15 (10), 1089–1095.
- 34. Li, H. and Chang, J. (2004) Fabrication and characterization of bioactive wollastonite/PHBV composite scaffolds. Biomaterials, 25 (24), 5473–5480.
- 35. Wu, L. and Ding, J. (2004) In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials, 25 (27), 5821–5830.
- 36. Martin, C., Winet, H., and Bao, J.Y. (1996) Acidity near eroding polylactide–polyglycolide in vitro and in vivo in rabbit tibial bone chambers. Biomaterials, 17 (24), 2373–2380.
- 37. Lu, L., Peter, S.J., Lyman, M.D., Lai, H.L. et al. (2005) In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials, 21 (18), 1837–1845.
- 38. Agrawal, C.M. and Athanasiou, K.A. (1997) Technique to control pH in vicinity of biodegrading PLA-PGA implants. J. Biomed. Mater. Res., 38 (2), 105–114.
- 39. Van der Meer, S.A.T., de Wijn, J.R., and Wolke, J.G.C. (1996) The influence of basic filler materials on the degradation of amorphous d- and l-lactide copolymer. J. Mater. Sci. Mater. Med., 7 (6), 359–361.
- 40. Göpferich, A. (1996) Mechanisms of polymer degradation and erosion. Biomaterials, 17 (2), 103–114.
- 41. Pitt, C.G., Jeffcoat, A.R., Zweidinger, R.A., Schindler, A. et al. (1979) Sustained drug delivery systems. I. The permeability of poly(ϵ-caprolactone), poly(dl-lactic acid), and their copolymers. J. Biomed. Mater. Res., 13 (3), 497–507.
- 42. Tokiwa, Y., Iwamoto, A., Koyama, M., Kataoka, N. et al. (1992) Biological recycling of plastics containing ester bonds. Makromol. Chem. Macromol. Symp., 57 (1), 273–279.
- 43. Zhao, J.H., Wang, X.Q., Zeng, J., Yang, G. et al. (2005) Biodegradation of poly(butylene succinate) in compost. J. Appl. Polym. Sci., 97 (6), 2273–2278.
- 44. ISO 14855 (1999) Determination of the ultimate aerobic biodegradability and disintegration of plastic materials under controlled composting conditions–method by analysis of evolved carbon dioxide.
- 45. Sun, Y., Wu, L., Bu, Z. et al (2014) Synthesis and thermomechanical and reheological properties of biodegradable long chain branched poly(butylene succinate-cobutylene terephthalate) copolyesters. Ind. Eng. Chem. Res., 53, 10380–10386.
- 46. Genovese, L., Lotti, N., Gazzano, M. et al. (2016) Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging application. Polym. Degrad. Stab., 132, 191–201.
- 47. Sheikholeslami, S., Rafizadeh, M., Taromi, F.-A. et al. (2016) Material properties of degradable poly(butylene-succinate-co-fumarate) copolymers networks synthesized by polycondensation of pre-homopolyesters. Polymer, 98, 70–79.
- 48. Totaro, G., Sisti, L., Celli, A. et al. (2016) Poly(butylene succinate) bionanocomposites: a novel bio-organo modified layered double hydroxide for superior mechanical properties. RSC Adv, 6, 4780–4791.
- 49. Wu, W., Cao, X.-W., Lin, H. et al. (2015) Preparation of biodegradable poly(butylene succinate)/halloysite nanotube nanocomposite foaming using supercritical CO2 as blowing agent. J. Polym. Res., 22, 177–188.
- 50. Snowdon, M., Mohanty, A., Misra, M. et al. (2015) Melt processing and characterization of biocomposites made from poly(butylene succinate) bioplastic and carbon black. Mater. Eng., 300, 118–126.
- 51. Jacquel, N., Sain-Loup, R., Pascault, J. et al. (2014) Structure–properties relationship of in situ synthesized poly(butylene succinate)/silica nanocomposites : Application in extrusion blowing of films. Macromol. Mater. Eng., 299, 977–989.
- 52. Bourmaud, A., Corre, Y.-M., and Baley, C. (2015) Fully biodegradable composites: use of poly(butylene succinate) as matrix and to plasticize PLA-flax blends. Ind. Crops Prod., 64, 251–257.
- 53. Liu, Y., Shao, J., Sun, J. et al. (2015) Toughening effect of poly(d-lactide)-b-poly(butylene succinate)-b-poly(d-lactide) copolymers on poly(l-lactic acid) by solution casting method. Mater. Lett., 155, 94–96.
- 54. Bertoldo, M., Coltelli, M.-B., Messina, T. et al. (2016) Emulsion blending approach for the preparation of gelatin/poly(butylene succinate-co-adipate) films. ACS Biomater. Sci. Eng., 2, 677–686.
- 55. Weraporn, P.-A., Kazunori, F., Keiichiro, N. et al. (2016) Isothermal crystallization kinetics of talc-filled poly(lactic acid)and poly(butylene succinate) blends. J. Polym. Res., 23, 144–152.
- 56. Zhang, Z., Zhang, J., Tian, T-S. (2016) Utilizing biodegradable poly(butylene succinate) to synergistically toughen polymer blends without sacrificing stiffness, RSC Adv, 6, 73853-73858.
- 57. Voznyak, Y., Morawiec, J., and Galwski, A. (2016) Ductility of polylactide composites reinforced with poly(butylene succinate) nanofibers. Compos. A Appl. Sci. Manuf., 90, 218–224.
- 58. Tan, L., Chen, Y., Zhou, W., Nie, H. et al. (2010) Novel poly(butylene succinate-co-lactic acid) copolyesters: synthesis, crystallization, and enzymatic degradation. Polym. Degrad. Stab., 95 (9), 1920–1927.
- 59. Zeng, J.B., Li, Y.D., Zhu, Q.Y., Yang, K.K. et al. (2009) A novel biodegradable multiblock poly(ester urethane) containing poly(l-lactic acid) and poly(butylene succinate) blocks. Polymer, 50 (5), 1178–1186.
- 60. Zhang, Y.H., Wang, X.L., Wang, Y.Z., Yang, K.K. et al. (2005) A novel biodegradable polyester from chain-extension of poly(p-dioxanone) with poly(butylene succinate). Polym. Degrad. Stab., 88 (2), 294–299.
- 61. Zhu, Q.Y., He, Y.S., Zeng, J.B., Huang, Q. et al. (2011) Synthesis and characterization of a novel multiblock copolyester containing poly(ethylene succinate) and poly(butylene succinate). Mater. Chem. Phys., 130 (3), 943–949.
- 62. Jiao, L., Huang, C.L., Zeng, J.B., Wang, Y.Z. et al. (2012) Miscibility, crystallization and mechanical properties of biodegradable blends of poly(l-lactic acid) and poly(butylene succinate-b-ethylene succinate) multiblock copolymer. Thermochim. Acta, 539, 16–22.
- 63. Shibata, M., Inoue, Y., and Miyoshi, M. (2006) Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylenes succinate-co-l-lactate) and poly(butylene succinate). Polymer, 47 (10), 3557–3564.
- 64. Yokohara, T. and Yamaguchi, M. (2008) Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J., 44 (3), 677–685.
- 65. Ma, P., Hristova-Bogaerds, D.G., Lemstra, P.J., Zhang, Y. et al. (2012) Toughening of PHBV/PBS and PHB/PBS blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng., 297 (5), 402–410.
- 66. Wang, H., Gan, Z., Schultz, J.M., and Yan, S. (2008) A morphological study of poly(butylene succinate)/poly(butylene adipate) blends with different blend ratios and crystallization processes. Polymer, 49 (9), 2342–2353.
- 67. Yang, J., Pan, P., Hua, L., Xie, Y. et al. (2011) Fractionated crystallization, polymorphic crystalline structure, and spherulite morphology of poly(butylene adipate) in its miscible blend with poly(butylene succinate). Polymer, 52 (15), 3460–3468.
- 68. Han, S.O., Ahn, H.J., and Cho, D. (2010) Hygrothermal effect on henequen or silk fiber reinforced poly(butylene succinate) biocomposites. Compos. B Eng., 41 (6), 491–497.
- 69. Lee, S.M., Cho, D., Park, W.H., Lee, S.G. et al. (2005) Novel silk/poly(butylene succinate) biocomposites: the effect of short fibre content on their mechanical and thermal properties. Compos. Sci. Technol., 65 (3–4), 647–657.
- 70. Feng, Y., Shen, H., Qu, J., Liu, B. et al. (2011) Preparation and properties of PBS/sisal-fiber composites. Polym. Eng. Sci., 51 (3), 474–481.
- 71. Lee, M.W., Han, S.O., and Seo, Y.B. (2008) Red algae fibre/poly(butylene succinate) biocomposites: the effect of fibre content on their mechanical and thermal properties. Compos. Sci. Technol., 68 (6), 1266–1272.
- 72. Nam, T.H., Ogihara, S., Tung, N.H., and Kobayashi, S. (2011) Effect of alkali treatment on interfacial and mechanical properties of coir fiber reinforced poly(butylenes succinate) biodegradable composites. Compos. B Eng., 42 (6), 1648–1656.
- 73. Teramoto, N., Urata, K., Ozawa, K., and Shibata, M. (2004) Biodegradation of aliphatic polyester composites reinforced by abaca fiber. Polym. Degrad. Stab., 86 (3), 401–409.
- 74. Liu, L., Yu, J., Cheng, L., and Qu, W. (2009) Mechanical properties of poly(butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos. A Appl. Sci. Manuf., 40 (5), 669–674.
- 75. Ray, S.S., Okamoto, K., and Okamoto, M. (2003) Structure property relationship in biodegradable poly(butylene succinate)-layered silicate nanocomposites. Macromolecules, 36 (7), 2355–2367.
- 76. Hwang, S.Y., Yoo, E.S., and Im, S.S. (2009) Effect of the urethane group on treated clay surfaces for high-performance poly(butylene succinate)/montmorillonite nanocomposites. Polym. Degrad. Stab., 94 (12), 2163–2169.
- 77. Phua, Y.J., Chow, W.S., and Mohd Ishak, Z.A. (2011) The hydrolytic effect of moisture and hygrothermal aging on poly(butylene succinate)/organo-montmorillonite nanocomposites. Polym. Degrad. Stab., 96 (7), 1194–1203.
- 78. Phua, Y.J., Lau, N.S., Sudesh, K., Chow, W.S. et al. (2012) Biodegradability studies of poly(butylene succinate)/organo-montmorillonite nanocomposites under controlled compost soil conditions: effects of clay loading and compatibilizer. Polym. Degrad. Stab., 97 (8), 1345–1354.
- 79. Chen, G.X. and Yoon, J.S. (2005) Thermal stability of poly(l-lactide)/poly(butylenes succinate)/clay nanocomposites. Polym. Degrad. Stab., 88 (2), 206–212.
- 80. Chen, G.X., Kim, H.S., Kim, E.S., and Yoon, J.S. (2005) Compatibilization-like effect of reactive organoclay on the poly(l-lactide)/poly(butylene succinate) blends. Polymer, 46 (25), 11829–11836.
- 81. Hwang, S.Y., Ham, M.J., and Im, S.S. (2010) Influence of clay surface modification with urethane groups on the crystallization behavior of in situ polymerized poly(butylene succinate) nanocomposites. Polym. Degrad. Stab., 95 (8), 1313–1320.
- 82. Ojijo, V., Malwela, T., Ray, S.S., and Sadiku, R. (2012) Unique isothermal crystallization phenomenon in the ternary blends of biopolymers polylactide and poly[(butylene succinate)-co-adipate] and nano-clay. Polymer, 53 (2), 505–518.
- 83. Ali, F.B. and Mohan, R. (2010) Thermal, mechanical, and rheological properties of biodegradable polybutylene succinate/carbon nanotubes nanocomposites. Polym. Compos., 31 (8), 1309–1314.
- 84. Song, L. and Qiu, Z. (2009) Crystallization behavior and thermal property of biodegradable poly(butylene succinate)/functional multi-walled carbon nanotubes nanocomposite. Polym. Degrad. Stab., 94 (4), 632–637.
- 85. Tan, L., Chen, Y., Zhou, W., Ye, S. et al. (2011) Novel approach toward poly(butylenes succinate)/single-walled carbon nanotubes nanocomposites with interfacial induced crystallization behaviors and mechanical strength. Polymer, 52 (16), 3587–3596.
- 86. Hwang, S.Y., Yoo, E.S., and Im, S.S. (2011) Effects of TS-1 zeolite structures on physical properties and enzymatic degradation of poly(butylene succinate) (PBS)/TS-1 zeolite hybrid composites. Polymer, 52 (4), 965–975.
- 87. Liu, W.G., Zhang, X.C., Li, H.Y., and Liu, Z. (2012) Effect of surface modification with 3-aminopropyltriethyloxy silane on mechanical and crystallization performances of ZnO/poly(butylene succinate) composites. Compos. B Eng., 43 (5), 2209–2216.
- 88. Wang, X., Song, L., Yang, H., Lu, H. et al. (2011) Synergistic effect of graphene on antidripping and fire resistance of in tumescent flame retardant poly(butylenes succinate) composites. Ind. Eng. Chem. Res., 50 (9), 5376–5383.
- 89. Li, J., Luo, X., and Lin, X. (2013) Preparation and characterization of hollow glass microsphere reinforced poly(butylene succinate) composites. Mater. Des., 46, 902–909.
- 90. Okamoto, K., Ray, S.S., and Okamoto, M. (2003) New poly(butylene succinate)/layered silicate nanocomposites. II. Effect of organically modified layered silicates on structure, properties, melt rheology, and biodegradability. J. Polym. Sci. Part B Polym. Phys., 41 (24), 3160–3172.
- 91. Ray, S.S., Yamada, K., Okamoto, M., and Ueda, K. (2003) Control of biodegradability of polylactide via nanocomposite technology. Macromol. Mater. Eng., 288 (3), 203–208.
- 92. Ray, S.S., Yamada, K., Okamoto, M., Fujimoto, Y. et al. (2003) New polylactide/layered silicate nanocomposites. 5. Designing of materials with desired properties. Polymer, 44 (21), 6633–6646.
- 93. Iijima, S. (1991) Helical microtubules of graphitic carbon. Nature, 354, 56–58.
- 94. Iijima, S. and Ichihashi, T. (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature, 363, 603–605.
- 95. Li, Y. and Shimizu, H. (2007) High-shear processing induced homogenous dispersion of pristine multiwalled carbon nanotubes in a thermoplastic elastomer. Polymer, 48 (8), 2203–2207.
- 96. Wang, S.F., Shen, L., Zhang, W.D., and Tong, Y.J. (2005) Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules, 6 (6), 3067–3072.
- 97. Zhang, D., Kandadai, M.A., Cech, J., Roth, S. et al. (2006) Poly(l-lactide) (PLLA)/multiwalled carbon nanotube (MWCNT) composite: characterization and biocompatibility evaluation. J. Phys. Chem. B, 110 (26), 12910–12915.
- 98. Levi, N., Czerw, R., Xing, S., Iyer, P. et al. (2004) Properties of polyvinylidene difluoride–carbon nanotube blends. Nano Lett., 4 (7), 1267–1271.
- 99. Wu, C.S. and Liao, H.T. (2007) Study on the preparation and characterization of biodegradable polylactide/multi-walled carbon nanotubes nanocomposites. Polymer, 48 (15), 4449–4458.
- 100. Liu, T., Phang, I.Y., Shen, L., Chow, S.Y. et al. (2004) Morphology and mechanical properties of multiwalled carbon nanotubes reinforced nylon-6 composites. Macromolecules, 37 (19), 7214–7222.
- 101. Zhang, W.D., Shen, L., Phang, I.Y., and Liu, T. (2004) Carbon nanotubes reinforced nylon-6 composite prepared by simple melt-compounding. Macromolecules, 37 (2), 256–259.
- 102. Ray, S.S., Vaudreuil, S., Maazouz, A., and Bousmina, M. (2006) Dispersion of multi-walled carbon nanotubes in biodegradable poly(butylene succinate) matrix. J. Nanosci. Nanotechnol., 6 (7), 2191–2195.
- 103. Du, F., Guthy, C., Kashiwagi, T., Fischer, J.E. et al. (2006) An infiltration method for preparing single-wall nanotube/epoxy composites with improved thermal conductivity. J. Polym. Sci. B Polym. Phys., 44 (10), 1513–1519.
- 104. Moniruzzaman, M., Du, F., Romero, N., and Winey, K.I. (2006) Increased flexural modulus and strength in SWNT/epoxy composites by a new fabrication method. Polymer, 47 (1), 293–298.
- 105. Zhu, J., Kim, J.D., Peng, H., Margrave, J.L. et al. (2003) Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization. Nano Lett., 3 (8), 1107–1113.
- 106. Bai, J.B. and Allaoui, A. (2003) Effect of the length and the aggregate size of MWNTs on the improvement efficiency of the mechanical and electrical properties of nanocomposites–experimental investigation. Compos. A Appl. Sci. Manuf., 34 (8), 689–694.
- 107. Hu, G., Zhao, C., Zhang, S., Yang, M. et al. (2006) Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer, 47 (1), 480–488.
- 108. Sandlera, J., Shaffera, M.S.P., Prasseb, T., Bauhoferb, W. et al. (1999) Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer, 40 (21), 5967–5971.
- 109. Zhang, Q., Rastogi, S., Chen, D., Lippits, D. et al. (2006) Low percolation threshold in single-walled carbon nanotube/high density polyethylene composites prepared by melt processing technique. Carbon, 44 (4), 778–785.
- 110. Geim, A.K. and Novoselov, K.S. (2007) The rise of graphene. Nat. Mater., 6, 183–191.
- 111. Li, D., Muller, M.B., Gilje, S., Kaner, R.B. et al. (2008) Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol., 3, 101–105.
- 112. Fan, X., Peng, W., Li, Y., Li, X. et al. (2008) Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to grapheme preparation. Adv. Mater., 20 (23), 4490–4493.
- 113. Ramanathan, T., Abdala, A.A., Stankovich, S., Dikin, D.A. et al. (2008) Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol., 3, 327–331.
- 114. Liang, J., Huang, Y., Zhang, L., Wang, Y. et al. (2009) Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater., 19 (14), 2297–2302.
- 115. Zhang, H.B., Zheng, W.G., Yan, Q., Yang, Y. et al. (2010) Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer, 51 (5), 1191–1196.
- 116. Schedin, F., Geim, A.K., Morozov, S.V., Hill, E.W. et al. (2007) Detection of individual gas molecules adsorbed on graphene. Nat. Mater., 6, 652–655.
- 117. Zhang, L.L., Zhou, R., and Zhao, X.S. (2010) Graphene-based materials as supercapacitor electrodes. J. Mater. Chem., 20 (29), 5983–5992.
- 118. Wang, X., Yang, H., Song, L., Hu, Y. et al. (2011) Morphology, mechanical and thermal properties of graphene-reinforced poly(butylene succinate) nanocomposites. Compos. Sci. Technol., 72 (1), 1–6.
- 119. Stankovich, S., Dikin, D.A., Piner, R.D., Kohlhaas, K.A. et al. (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 45 (7), 1558–1565.
- 120. Mohanty, A.K., Misra, M., and Hinrichsen, G. (2000) Biofibres, biodegradable polymers and biocomposites: an overview. Macromol. Mater. Eng., 276–277 (1), 1–24.
- 121. Monteiro, S.N., Lopes, F.P.D., Ferreira, A.S., and Nascimento, D.C.O. (2009) Natural-fiber polymer-matrix composites: cheaper, tougher, and environmentally friendly. J. Minerals Metals Mater. Soc., 61 (1), 17–22.
- 122. Satyanarayana, K.G., Arizaga, G.G.C., and Wypych, F. (2009) Biodegradable composites based on lignocellulosic fibers – an overview. Prog. Polym. Sci., 34 (9), 982–1021.
- 123. Zhang, M.Q., Rong, M.Z., and Lu, X. (2005) Fully biodegradable natural fiber composites from renewable resources: all plant fiber composites. Compos. Sci. Technol., 65 (15–16), 2514–2525.
- 124. Monteiro, S.N., Terrones, L.A.H., Lopes, F.P.D., and d'Almeida, J.R.M. (2005) Mechanical strength of polyester matrix composites reinforced with coconut fiber wastes. Rev. Mater., 10 (4), 571–576.
- 125. Liu, L., Yu, J., Cheng, L., and Yang, X. (2009) Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab., 94 (1), 90–94.
- 126. Calabia, B.P., Ninomiya, F., Yagi, H., Oishi, A. et al. (2013) Biodegradable poly(butylene succinate) composites reinforced by cotton fiber with silane coupling agent. Polymers, 5, 128–141.
