Chapter 24
Bacterial Biofertilizers: High Density Cultivation
S. Mutturi and Virendra S. Bisaria
24.1 Introduction
Sustainable agricultural practices are being sought due to increasing concerns of the harmful effect of the usage of chemical fertilizers globally. Low-input agriculture has invoked interest in understanding the microorganisms present in the rhizosphere and their synergetic effect on the host plant. Plants are natural habitats for microbial growth as the presence of moisture and nutrients on their surfaces and tissues are amenable for the proliferation of microorganisms [1]. The interest in plant–microbe interactions is not just pertinent to naturally available microorganisms in the rhizosphere but also upon external addition of beneficial microorganisms [2]. This external addition via a formulated product containing live bacteria/fungi or spores that improves the overall growth of the plant are termed as biofertilizers. Vessey [3] defined a biofertilizer as “a substance which contains living microorganisms, which, when applied to seed, plant surfaces, or soil, colonizes the rhizosphere or the interior of the plant and promotes growth by increasing the supply or availability of primary nutrients to the host plant.” Here in this chapter, we assume biofertilizers to be synonymous with bio-inoculants.
According to Berg [4], biofertilizers could be potential substitutes to chemical fertilizers and pesticides due to the following reasons: (i) biologically safe and can cause lower environmental damage, (ii) increased targeted activity, and (iii) highly effective in smaller dosages. The microbial interactions with plants in the rhizosphere can improve the plants' health in general by the following three mechanisms: (i) directly affecting the availability of nutrients present (e.g., nitrogen fixation, phosphorus solubilization, etc.), (ii) indirectly stimulating the growth by lowering the effect of plant borne pathogens in the rhizosphere, and (iii) improving the plant growth by production of phytohormones and volatiles [5, 6].
Punja [7] details the information required for development of a microbial biocontrol agent as a commercial product, and these prerequisites certainly also apply to any bio-inoculant for commercialization. Among the various process operations, the scale-up for mass production of the bio-inoculant prior to formulation is a key rate limiting step in the supply chain. Most of the experimental studies to enumerate the efficacy of biofertilizers are restricted to shake flask cultivation of the organism. The sequence of events pertinent to high cell density cultivation of bio-inoculants is depicted in Figure 24.1. Development of synthetic medium in case of submerged cultivation of axenic organisms is essential to eliminate the traces of unutilized complex sources to prevent contamination of bio-inoculant during supply chain until delivery. This chapter focuses on cultivation strategies for some of the important microorganisms used as bio-inoculant formulations and provides pointers for mass cultivation techniques for production of other bio-inoculants also.
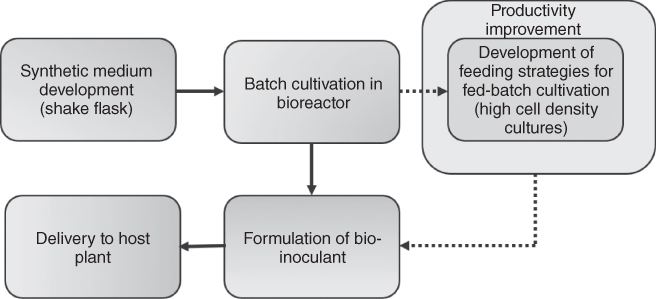
Figure 24.1 Sequence of process operations during production of bio-inoculant.
24.2 Cultivation Strategies for a Few Important Bacterial Inoculants
24.2.1 Azospirillum sp
Azospirillum sp. is one of the widely studied rhizobacteria that confers plant growth promotion properties due to the release of growth regulators such as auxins, cytokinins, gibberellins, ethylene, abscisic acid, nitric oxide, and polyamines such as cadaverine [8–12]. Azospirillum sp. are Gram-negative diazotrophic bacteria belonging to class of α-proteobacteria, which grow well on organic acids such as malate and succinate but variedly on other sugars and amino acids as carbon and energy sources [13].
They can grow anaerobically using NO3− as final electron acceptor, micro-aerobically using N2 or NH3 as nitrogen sources, and aerobically using combined nitrogen sources (NH3, NO3−, and amino acids). Azospirillum brasilense, Azospirillum lipoferum, Amazonense amazonense, Amazonense halopraeferens, and Amazonense irakense are some of the prominent species in this genus, among which A. brasilense is extensively studied for plant growth promotion both in glasshouse and field trials [14]. Some of the commercial products derived from A. brasilense for plant growth promotion include ACCOLADETM-L (1 × 109 cfu/g formulation, Verdesian Life Sciences, NC, USA) and Bactofil A 10 (net consortium: 4.3 × 109 cell/ml, AgroBio, Hungary).
Azospirillum strains are usually maintained on yeast extract–peptone–agar (YPA) plates and are cultivated micro-aerobically (dissolved oxygen (DO) in the range of 1–5%) or aerobically using malic acid or sodium malate as carbon source and NH4Cl as nitrogen source [11, 15]. A. brasilense was cultivated in bioreactor using both batch and fed-batch modes [16, 17], and it was observed that maximum cell growth was using malic acid (5%) as the carbon source and NH4Cl as nitrogen source, with optimal growth at 30 °C. There was no significant difference in biomass concentration (OD600 ∼1.9–2.2) when cultivated at microaerophilic (3%) or aerobic conditions (30%). However, it was also observed that indole-3-acetic acid (IAA) production (which depends on tryptophan supplementation) was found only under microaerophilic conditions, and fed-batch mode of cultivation did not improve cell density [17]. A. brasilense strains were cultivated in 10 l bioreactor and scaled-up to 1000 l using volumetric oxygen transfer coefficient (kLa) as the criterion. The specific growth rate of the A. brasilense strains ranged between 0.20 and 0.25 h−1. The viable cell count was found to be in the range of 3.5–7.5 × 108 CFU/ml [18].
24.2.2 Azotobacter spp
Azotobacter sp. are soil-dwelling, nitrogen-fixing, aerobic microorganisms that possess diverse metabolic capabilities such as synthesis of alginates, polyhydroxybutyrate (PHB), polyhydroxyalkanoates (PHA), IAA, gibberellic acid (GA3), cytokinin, and vitamins [19–21]. This genus possessing such interesting phenotypes along with phosphate-mineralization has a major role in the promotion of plant growth and has been extensively studied as biofertilizer. Some of the commercial bio-inoculants based on Azotobacter sp. include Bactofil A10, Microbion UNC, and Phylazonit-M from Hungary.
Nitrogen-free modified Burk's medium containing sucrose (2%) as the carbon source is usually used for cultivating Azotobacter sp. [22]. Strains belonging to this genus grow optimally in bioreactor in the temperature range of 28–32 °C, pH 7.0–7.5, and DO ≥5% saturation. Maximum biomass production of 7.5 g/l and specific growth rate of 0.13 h−1 were obtained at the end of 24 h of batch cultivation with DO maintained at 10% in bioreactor cultivation of A. vinelandii [23]. Damir et al. [24] cultivated Azotobacter chroococcum strain in bioreactor using chemically defined medium (2% glucose as carbon source) and complex medium (4% molasses) both in batch and fed-batch modes. In their study, DO was maintained at 30%, and the fed-batch was initiated with three times concentrated medium used for batch when the residual substrate dropped below 2 g/l. In shake flask cultivation using chemically defined medium, 5 × 107–2 × 08 CFU/ml was observed with biomass yield ranging from 0.103 to 0.166 g/g using initial glucose concentration in the range of 10–40 g/l, whereas in the case of complex medium (2−8% molasses corresponding to 9.5–38.1 g/l sucrose), the biomass yield ranged between 0.126 and 0.180 g/g. Fed-batch cultivation of this strain in defined medium (2% glucose) and complex medium (4% molasses) resulted in maximum biomass concentration of 5.4 g/l and CFU of 6.4 × 108 ml−1 and 15.6 g/l and 7.6 × 108 ml−1, respectively. Repeated-batch cultivation increased the biomass yield and productivity, but CFU counts were maximum during fed-batch cultivation.
24.2.3 Bacillus spp
Bacillus spp. have been well established for their plant growth promotion and biocontrol activities and hence considered as potential bio-inoculants to improve the plant health. Some of the modes of action for the species in this genus include [25] phosphate solubilization by Bacillus megaterium; potassium solubilization by Bacillus mucilaginosus; nitrogen fixation, IAA synthesis, iso-pentenyladenine synthesis, and cell wall-degrading enzyme production by Paenibacillus polymyxa (also known as Bacillus polymyxa); lipopeptides synthesis by Bacillus amyloliquefaciens; gibberellins synthesis by Bacillus pumilus and Bacillus licheniformis; biocontrol activities by Bacillus subtilis, Bacillus thuringiensis, and Bacillus sphaericus; and insecticidal activity of Lysinibacillus sphaericus. Bacillus spp. improve plant health by one of the three mechanisms [26]: (i) antagonism of phytopathogens, (ii) stimulation of plant host defenses using induced systemic resistance (ISR), and (iii) promotion of their nutrition and growth.
One of the major advantages of Bacillus spp. over other plant growth-promoting bacteria is their ability to form spores under stress conditions, which in turn can simplify their formulation for delivery to the plants. The spores of Bacillus spp. are resistant to extreme heat, radiation, and chemicals [27]. Sporulation is induced by exhaustion of medium component or by shifting the cells from a nutrient-rich medium to a poor medium and also by addition of inhibitor molecule, decoyinine. Among bacterial biocontrol agents, Bacillus spp.-based products represent more than 50% of commercial market share among which B. thuringiensis alone occupies 70% share [28]. El-Bendary [29] reviewed various cultivation strategies for production of B. thuringiensis and B. sphaericus. Posada Uribe et al. [30] studied the effect of medium components for cultivation of B. subtilis EA-CB0575 in order to improve the spore count and sporulation efficiency. They observed that by varying components, glucose and MgSO4·7H2O, a maximum spore count of 1.37 × 109 CFU/ml and spore efficiency of 93.5% could be achieved. Spore yields of some of the Bacillus spp. in liquid cultures are shown in Table 24.1.
Table 24.1 Spore yields during cultivation of Bacillus spp. in various studies
| S. no | Species | Spores/ml | Carbon and nitrogen source | Mode of cultivation | References |
| 1 | B. thuringiensis | 1.25 × 1010 | Glucose, yeast extract | Fed-batch (bioreactor) | [31] |
| 2 | B. sphaericus | 1.64 × 1010 | Acetate, yeast extract + CSL | Fed-batch (bioreactor) | [32] |
| 3 | B. subtilis R14 | 1.00 × 109 | Molasses, yeast extract | Batch (shake flask) | [33] |
| 4 | B. subtilis MB24 | 7.40 × 109 | Glucose, bacto-nutrient broth | Fed-batch (bioreactor) | [34] |
| 5 | B. subtilis WHK-Z12 | 1.56 × 1010 | Soy bean flour, yeast extract + CSL | Batch (bioreactor) | [35] |
| 6 | B. subtilis EA-CB0575 | 8.78 × 109 | Glucose + meat extract, peptone | Batch (bioreactor) | [30] |
CSL: Corn steep liquor.
24.2.4 Pseudomonas spp
Fluorescent pseudomonads are considered as rhizobacteria, which rigorously colonize the roots and exert positive influence on the host plants [36]. They are known to synthesize a variety of metabolites, iron chelator (siderophore), plant growth hormone IAA, a range of organic acids needed for solubilizing insoluble phosphates, and compounds that exert toxicity against pathogenic and deleterious microorganisms in rhizosphere such as butyrolactones, 2,4-diacetylphloroglucinol (DAPG), kanosamine, oligomycin A, oomycin A, phenazine-1-carboxylic acid, pyoluteorin, pyrrolnitrin, viscosinamide, xanthobaccin, and zwittermycin A [37–39]. There are very few reports on the cultivation of Pseudomonas spp. for the production of bio-inoculant under high cell density cultivation. Since the complex medium-based cultivation leads to the presence of residual sugars and salts at the time of harvest, this provides scope for contamination when formulated as bio-inoculant. Therefore a synthetic medium and a cultivation strategy were developed in the authors' laboratory to build a robust bio-inoculant process technology for potential Pseudomonas strains [40].
It has been observed that the Pseudomonas' culture broth containing siderophore and DAPG could limit the proliferation of contaminants and also improved the shelf storage period [41]. Therefore, studies were carried to improve biomass along with such useful metabolites in the culture broth using batch cultivation. However, it was observed that beyond 15 g/l of initial glycerol concentration, the specific growth decreased, indicating the onset of substrate inhibition [40]. Hence in studies carried out by Sarma et al. [40], both open-loop and closed-loop controlled feeding strategies were used for two pseudomonad strains to produce high cell density cultures containing DAPG and siderophore. In the case of open-loop feeding strategy, the authors adopted exponential feeding using 800 g/l of glycerol feed concentration and dosing exponentially at a predefined specific growth rates of 0.1 and 0.2 h−1. It was observed that even at 0.1 h−1 of specific growth rate, there was accumulation of glycerol in the bioreactor indicating the dosage rate was significantly higher than the substrate consumption rate. Hence closed-loop fed-batch strategies were designed using DO signals and pH signals as feedback parameters to understand the physiology of the cells and dose the bioreactor accordingly. In case of DO-based feeding, an algorithm was designed by online calculation of infinitesimal change in the rate of DO between two time points, and if the function value was greater than zero, the feed pump was switched on, and similarly if it was zero, the feed pump was switched off. This way the feeding of glycerol ensured that there was no substrate inhibition due to overfeeding. The rationale behind such strategy was that the DO-signal reaches saturation value if the carbon source in the reactor is exhausted and will fall below critical value for the culture if the demand from growing cells is higher. Using this strategy 25 g/l of biomass and 250 mg/l of DAPG was achieved using pseudomonad strain R81 with specific growth rate reaching 0.02 h−1 during fed-batch phase. However, the productivity was 0.29 g/(l·h), which was higher than that of the batch cultivation (0.19 g/(l·h)). It was observed that along with the shoot-up of DO signal during glycerol exhaustion, the pH signal was also affected and rose above the set value of 6.90 at every instance of glycerol exhaustion during DO-based fed-batch cultivation. Hence, the authors used pH signal as a feedback parameter, and an algorithm was designed to dose the reactor.
The rationale behind the sensitivity of pH signal toward carbon exhaustion was that whenever there is enough carbon source in the bioreactor, the pH falls sharply due to its consumption and rises sharply whenever the same carbon source becomes limiting due to production of ammonium ions associated with protein catabolism. The algorithm developed exploited the deadband region for any standard pH controller. Here 6.90 was selected as set value for the pH controller along with ±0.05 pH units as the deadband. This indicates that the pH controller will not function between 6.85 and 6.95, and this region of one unit pH was selected to design control algorithm that operated the feed pump. Another user-defined pH set point of 6.92 was created within the deadband region; whenever the pH rose above 6.92, the feed pump was switched on for a stipulated time. This strategy was repeated in cyclic fashion until the maximum possible working volume of the bioreactor had reached. This strategy improved the productivity to 0.54 g/(l·h), which was 1.86-fold higher than that of DO-based fed-batch culture. Also the biomass and DAPG concentrations were found to be 27 g/l (corresponding to 2.4 × 1010 cfu/ml) and 342 mg/l, respectively, which were the maximum among all fed-batch cultivations employed. This cell count of 2.4 × 1010 ml−1 was about 20-fold higher than the cell counts obtained in a conventional batch culture. This translates to substantial savings in equipment as a much smaller bioreactor can be used to obtain the same cell counts in a fed-batch process. Thus fed-batch cultures with suitable feeding strategies can be used for getting high cell densities, which can make the bio-inoculant production process economical and industrially attractive.
24.2.5 Rhizobia spp
Nitrogen fixation by Rhizobia in legume plants pumps in nearly 40 million tones of nitrogen into agriculture via an environmentally benign process [42]. Some of these symbiotic nitrogen fixers include Rhizobium, Bradyrhizobium, Sinorhizobium, Mesorhizobium, Allorhizobium, and Azorhizobium [43]. Among these, fast-growing Rhizobium spp. and slow-growing Bradyrhizobium spp. have been well studied. Rhizobia inoculants for use on legume crops were commercially available as early as 1890s [44]. Rhizobium sp.-based bio-inoculant known as Nitragen was the first registered product for commercial exploitation [45]. Deaker et al. [46] describes various inoculation strategies of rhizobia in legume agronomy.
Optimal growth conditions include: temperature (25–30 °C), pH (6.0–7.0), aerobic, and microaerophilic conditions. Rhizobia are not fastidious microorganisms; however, the organisms are selective in their ability to utilize carbohydrate depending on the species and the plant they colonize. Starch is not utilized by rhizobia. Sucrose and mannitol (sometimes in combination) are usually chosen as both carbon and energy source, and yeast extract as nitrogen source. Cheese whey, malt sprouts, pea husk, molasses, water hyacinth, and jaggery have been used to substitute for expensive growth medium for mass cultivation of rhizobia [47]. Sludge generated by industrial and municipal wastewater treatment that contains both organic and inorganic waste sustains growth of various strains of rhizobia, reaching 1 × 109 CFU/ml in the case of fast-growing rhizobia and 1 × 108–109 CFU/ml in the case of Bradyrhizobium [47]. Singh et al. [48] utilized 60% diary sludge as the growth medium and found superior growth of strains belonging to Rhizobium trifolii MTCC905, R. trifolii MTCC906, and Rhizobium meliloti in comparison to standard media. In the studies carried out by Chang et al. [49], by optimizing fed-batch strategy (constant feeding) a net biomass of 69.10 g/l was achieved when R. trifolli was in cultivated 16 L bioreactor. Rhizobium leguminosarum was grown on peat in roller bottles and a rotating drum bioreactor in solid-state fermentation to achieve 1.3–1.9 × 109 CFU/g in 4D cultivation [50]. No studies have been reported on fed-batch cultivation for high cell density cultivation of these nitrogen-fixing bacteria.
Conflict of Interest
The authors have no conflict of interest.
References
- 1. Andrews, J.H. and Harris, R.F. (2000) The ecology and biogeography of microorganisms of plant surfaces. Annu. Rev. Phytopathol., 38, 145–180.
- 2. Elliott, L.F. and Lynch, J.M. (1995) The international workshop on establishment of microbial inocula in soils: cooperative research project on biological resource management of the organization for economic cooperation and development (OECD). Am. J. Alternat. Agric., 10 (02), 50–73.
- 3. Vessey, J. (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil, 255 (2), 571–586.
- 4. Berg, G. (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol., 84 (1), 11–18.
- 5. Podile, A.R. and Kishore, G.K. (2006) Plant growth promoting rhizobacteria, in Plant-associated Bacteria (ed. S.S. Gnanamanickam), Springer, Amsterdam, pp. 195–230.
- 6. Mutturi, S., Sahai, V., Sharma, S., and Bisaria, V.S. (2016) Strategies for high-density cultivation of bio-inoculants in submerged culture with special reference to pseudomonads, in Microbial Inoculants in Sustainable Agricultural Productivity, Vol. 1: Research Perspectives (eds D.P. Singh, H.B. Singh, and R. Prabha), Springer, Amsterdam, pp. 181–196.
- 7. Punja, Z.K. (1997) Comparative efficacy of bacteria, fungi, and yeasts as biological control agents for diseases of vegetable crops. Can. J. Plant Pathol., 19 (3), 315–323.
- 8. Steenhoudt, O. and Vandereyden, J. (2000) Azospirillum, fee-living nitrogen fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol. Rev., 24 (4), 487–506.
- 9. Perrig, D., Boiero, M.L., Masciarelli, O.A., Penna, C. et al. (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol., 75 (5), 1143–1150.
- 10. Bashan, Y., de-Bashan, L.E. (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth -a critical assessment. Adv. Agron. 108, 77–136.
- 11. Cohen, A.C., Bottini, R., Pontin, M., Berli, F.J. et al. (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant., 153 (1), 79–90.
- 12. Rivera, D., Revale, S., Molina, R., Gualpa, J. et al. (2014) Complete genome sequence of the model rhizosphere strain Azospirillum brasilense Az39, successfully applied in agriculture. Genome Announc., 2 (4), e00683-14.
- 13. Okon, Y. (1985) Azospirillum as a potential inoculant for agriculture. Trends Biotechnol., 3 (9), 223–228.
- 14. Mehnaz, S. (2015) Azospirillum: a biofertilizer for every crop, in Plant Microbes Symbiosis: Applied Facets (ed. N.K. Arora), Springer, pp. 297–314.
- 15. Vanstockem, M., Michiels, K., Vanderleyden, J., and Van Gool, A.P. (1987) Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl. Environ. Microbiol., 53 (2), 410–415.
- 16. Ona, O., van Impe, J., Prinsen, E., and Vanderleyden, J. (2005) Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol. Lett., 246, 125–132.
- 17. Cappuyns, A.M., Bernaerts, K., Smets, I.Y., Ona, O. et al. (2007) Optimal fed batch experiment design for estimation of monod kinetics of Azospirillum brasilense: from theory to practice. Biotechnol. Prog., 23 (5), 1074–1081.
- 18. Trujillo-Roldán, M.A., Valdez-Cruz, N.A., Gonzalez-Monterrubio, C.F., Acevedo-Sánchez, E.V. et al. (2013) Scale-up from shake flasks to pilot-scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. Appl. Microbiol. Biotechnol., 97 (22), 9665–9674.
- 19. Kennedy, C. and Toukdarian, A. (1987) Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu. Rev. Microbiol., 41, 227–258.
- 20. Mrkovacki, N. and Milic, V. (2001) Use of Azotobacter chroococcum as potentially useful in agricultural application. Ann. Microbiol., 51 (2), 145–158.
- 21. Gauri, S.S., Mandal, S.M., and Pati, B.R. (2012) Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl. Microbiol. Biotechnol., 95 (2), 331–338.
- 22. Peña, C., Campos, N., Galindo, E. (1997) Changes in alginate molecular mass distributions, broth viscosity and morphology of Azotobacter vinelandii cultured in shake flasks. Appl. Microbiol. Biotechnol., 48 (4), 510–515.
- 23. Mejía, M., Segura, D., Espín, G., Galindo, E., and Peña, C. (2010) Two stage fermentation process for alginate production by Azotobacter vinelandii mutant altered in poly-β-hydroxybutyrate (PHB) synthesis. J. Appl. Microbiol., 108, 55–61.
- 24. Damir, O., Mladen, P., Božidar, Š., and Srñan, N. (2011) Cultivation of the bacterium Azotobacter chroococcum for preparation of biofertilizers. Afr. J. Biotechnol., 10 (16), 3104–3111.
- 25. Lyngwi, N.A. and Joshi, S.R. (2014) Economically important Bacillus and related genera : a mini review, in Biology of Useful Plants and Microbes (ed. A. Sen), Narosa Publishing House, New Delhi, pp. 33–43.
- 26. McSpadden Gardener, B.B. (2004) Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology, 94 (11), 1252–1258.
- 27. Setlow, P. and Johnson, E.A. (2013) Spores and their significance, in Food Microbiology (eds M. Doyle and R. Buchanan), ASM Press, Washington, DC, pp. 45–79.
- 28. Ongena, M. and Jacques, P. (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol., 16 (3), 115–125.
- 29. El-Bendary, M.A. (2006) Bacillus thuringiensis and Bacillus sphaericus biopesticides production. J. Basic Microbiol., 46 (2), 158–170.
- 30. Posada Uribe, L.F., Romero Tabarez, M., and Villegas Escobar, V. (2015) Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst. Eng., 38 (10), 1879–1888.
- 31. Kang, B.C., Lee, S.Y., and Chang, H.N. (1992) Enhanced spore production of Bacillus thuringiensis by fed-batch culture. Biotechnol. Lett., 14, 721–726.
- 32. Sasaki, K., Jiaviriyaboonya, S., and Rogers, P.L. (1998) Enhancement of sporulation and crystal toxin production by cornsteep liquor feeding during intermittent fed-batch culture of Bacillus sphaericus 2362. Biotechnol. Lett., 20 (2), 165–168.
- 33. Luna, C.L., Mariano, R.L.R., and Souto-Maior, A.M. (2002) Production of a biocontrol agent for crucifers black rot disease. Braz. J. Chem. Eng., 19 (2), 133–140.
- 34. Monteiro, S.M., Clemente, J.J., Henriques, A.O., Gomes, R.J. et al. (2005) A procedure for high-yield spore production by Bacillus subtilis. Biotechnol. Prog., 21 (4), 1026–1031.
- 35. Chen, Z.M., Li, Q., Liu, H.M., Yu, N. et al. (2010) Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl. Microbiol. Biotechnol., 85 (5), 1353–1360.
- 36. Kluepfel, D.A. (1993) The behavior and tracking of bacteria in the rhizosphere. Annu. Rev. Phytopathol., 31, 441–472.
- 37. Dowling, D.N. and O'Gara, F. (1994) Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol., 12, 133–141.
- 38. Cook, R.J., Thomashow, L.S., Weller, D.M., Fujimoto, D. et al. (1995) Molecular mechanisms of defense by rhizobacteria against root disease. Proc. Nat. Acad. Sci. U.S.A, 92, 4197–4201.
- 39. Whipps, J.M. (2001) Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot., 52, 487–511.
- 40. Sarma, M.V.R.K., Gautam, A., Kumar, L., Saharan, K. et al. (2013) Bioprocess strategies for mass multiplication of and metabolite synthesis by plant growth promoting pseudomonads for agronomical applications. Process Biochem., 48, 1418–1424.
- 41. Saharan, K., Sarma, M.V.R.K., Prakash, A., Johri, B.N. et al. (2011) Shelf-life enhancement of bio-inoculant formulation by optimizing the trace metals ions in the culture medium for production of DAPG using fluorescent pseudomonad R62. Enzyme Microb. Technol., 48, 33–38.
- 42. Udvardi, M. and Poole, P.S. (2013) Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol., 64, 781–805.
- 43. Sessitsch, A., Howieson, J.G., Perret, X., Antoun, H., and Martinez-Romero, E. (2002) Advances in Rhizobium research. Crit. Rev. Plant Sci., 21, 323–378.
- 44. Fred, E.B., Baldwin, I.L., and McCoy, E. (1932) Root Nodule Bacteria and Leguminous Plants, University of Wisconsin, Madison, WI. 343 pp.
- 45. Nobbe, F. and Hiltner, L. (1896). Inoculation of the soil for cultivating leguminous plants, US Patent 570,813.
- 46. Deaker, R., Roughley, R.J., and Kennedy, I.R. (2004) Legume seed inoculation technology – a review. Soil Biol. Biochem., 36 (8), 1275–1288.
- 47. Ben Rebah, F., Prévost, D., Yezza, A., and Tyagi, R.D. (2007) Agro-industrial waste materials and wastewater sludge for rhizobial inoculant production: a review. Bioresour. Technol., 98 (18), 3535–3546.
- 48. Singh, A.K., Singh, G., Gautam, D., and Bedi, M.K. (2013) Optimization of dairy sludge for growth of Rhizobium cells. BioMed Res. Int., 2013, Article ID 845264. http://dx.doi.org/10.1155/2013/845264.
- 49. Chang, H.W., Then, C., Othman, N.Z., Malek, R.A., Peng, T., Sukumaran, S., Wan Mustapha, W.A., Ramli, S., Saat, M., Sarmidi, M.R., Aziz, R., El Enshasy, H.A. (2013) High cell density cultivation of Rhizobium trifolii in pilot scale stirred tank bioreactors for biofertilizer applications. AIChE Annual Meeting, November 3–8, San Francisco, CA, USA. http://www3.aiche.org/proceedings/content/Annual-2013/extended-abstracts/P330591.pdf (Accessed on 17th July 2017).
- 50. Jauhari, R., Gray, M., and Holloway, G. (1999) Growth of Rhizobium leguminosarum on peat in rotating bioreactors. Can. J. Chem. Eng., 77, 911–916.
