Chapter 10
Volatile Fatty Acid Platform: Concept and Application
Nag-Jong Kim, Seong-Jin Lim and Ho Nam Chang
10.1 Concept of Volatile Fatty Acid Platform
10.1.1 Platforms for Biofuel Production
Various technologies to convert biomass to usable fuels have been developed and many processes have been commercialized up to recently. The typical platforms mostly researched are (i) sugar, (ii) thermochemical (syngas), (iii) carbon-rich chains, and (iv) biogas platform (Figure 10.1). Syngas and sugar are two major platform actively researched for liquid fuel production [1].
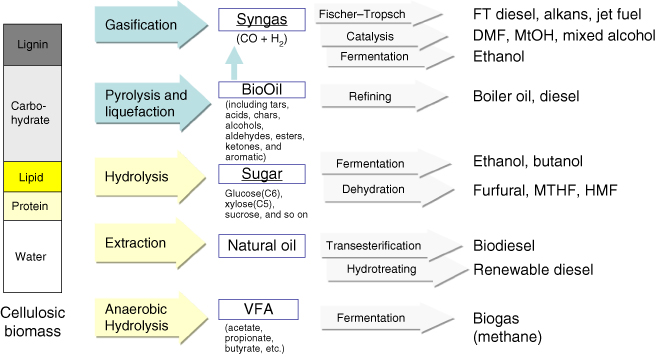
Figure 10.1 Platforms for biofuel production. Two major platforms using sugar and syngas were underlined.
The sugar platform uses hexose and pentose sugars extracted or converted from plant body. The thermochemical (syngas) platform involves a chemical or biological conversion process using pyrolysis or gasification of plant mass to produce biofuels. The carbon-rich chains platform is used to produce biodiesel from long-chain fatty acids or glycerides. These platforms, respectively, have unique advantages and disadvantages.
Finally, the biogas platform producing methane gas from municipal solid wastes (MSWs) through an anaerobic digestion (AD) process exploits rapid acidogenesis and slow methanogenesis. The acidogenesis stage is the production step of volatile fatty acids (VFAs), which are short-chain fatty acids composed mainly of acetate and butyrate and are rapidly produced from nonwoody biomass by the natural consortia of mixed anaerobic bacteria. The AD process, which converts all parts of biomass (carbohydrates, lipids, and proteins) except for lignin to VFAs, is suitable for organic wastes treatment and does not need a high cost pretreatment step or additional hydrolysis enzymes.
10.1.1.1 Sugar Platform
The sugar platform for converting lignocellulose to ethanol via sugars contains four steps: (i) pretreatment to change structural features to make cellulose accessible to enzymes, (ii) formation of fermentable sugars, called saccharification, (iii) fermentation of these sugars to ethanol, and (iv) separation and purification (S&P) of the ethanol. Separate hydrolysis and fermentation (SHF) allows each step to operate at its optimal temperature. Combining saccharification and fermentation into a single step is called simultaneous saccharification fermentation (SSF). The primary advantage of SSF is that the immediate consumption of sugars by microorganisms reduces the glucose and cellobiose concentrations in the fermentor, which significantly reduces enzyme inhibition to improve the kinetics and economics of biomass conversion [2, 3]. Combining hydrolysis, saccharification, and fermentation into a single step is called consolidated bioprocessing (CBP), which use a super microorganism to do all these processes.
10.1.1.2 Syngas Platform
Thermochemical conversion of biomass to syngas is an attractive route to extract the oxygen from carbohydrate structures to produce intermediate compounds having C1 (CO and CH4), which can be further synthesized into hydrocarbons by catalysis or fermentation (Figure 10.3). Other thermochemical schemes of decarboxylation (CO2 removal) and dehydration (H2O removal) from carbohydrates result in higher hydrocarbons (higher than C2) having undesired properties that require further conversion to be compatible with transportation fuels. Thermochemical conversion technologies have certain advantages and disadvantages over biochemical conversion technologies. The main advantages are that the feedstock for thermochemical conversion can be any type of dry biomass including agricultural residues, forestry residues, non-fermentable by-products from biorefineries, by-products of food industry, by-products of any bioprocessing facility, and even organic municipal wastes; the product gases can be converted to a variety of fuels (H2, Fischer–Tropsch (FT) diesels, synthetic gasoline) and chemicals (methanol, urea) as substitutes for petroleum-based chemicals and the products are more compatible with existing petroleum refining operations. The major disadvantages are the high cost associated with cleaning the product gas from tar and undesirable contaminants like alkali compounds, inefficiency due to the high temperatures required, and the unproven use of products (syngas and bio-oil) as transportation fuels. So, research on the optimization of gasifier operating conditions and heat recovery, syngas cleaning, bio-oil stabilization, and efficient product utilization is needed for sustainable production of biofuels [4].
10.1.2 Development of Volatile Fatty Acid Platform
10.1.2.1 Anaerobic Digestion Process
Initially employed mainly for food and beverage production, anaerobic conversions are among the oldest biological process technologies utilized by mankind. They have been applied and developed over many centuries, although the most dramatic advances have been achieved in the last few decades with the introduction of various forms of high-rate treatment processes, particularly for industrial wastewater.
AD involves a transformation of organic matter by a mixed culture bacterial ecosystem without oxygen. It is a natural process that produces a gas principally composed of methane and carbon dioxide. AD takes place in several steps, as shown in Figure 10.2.

Figure 10.2 COD flux for a particulate composite comprised of 10% inerts and 30% each of carbohydrates, proteins, and lipids (in terms of COD) [5]. All organic parts of living things are broken down to volatile fatty acids (a mixture of C2–C6 acids including acetic acid, propionic acid/lactic acid, butyric acid, valeric acid, and caproic acid), which turn into methane and CO2 gas by anaerobic hydrolysis. Methane will be chemically or biologically oxidized into CO2 and H2O. Nitrogen and phosphate compounds in living things (animals, plants, and microbial cell mass) can be recycled eventually into N2 gas and phosphate salts, through a series of chemical or biological reactions. Sulfur compounds digested anaerobically are converted into H2S gas. Propionic acid (10%), butyric acid (12%), and valeric acid (7%) are grouped in the figure for simplicity. Abbreviations include LCFA (long-chain fatty acids), HPr (propionic acid), Hbu (butyric acid), and HVa (valeric acid).
The first step of AD is the hydrolysis of animal or plant matter. This step breaks down biopolymers and other organic material to smaller molecules:
- Lipids → fatty acids
- Polysaccharides → monosaccharides
- Protein → amino acids
- Nucleic acids → purines and pyrimidines
The second step is the conversion, by acetogenic bacteria, of products of the first step to organic acids, carbon dioxide, and hydrogen. Acetogenic bacteria produce acetic acid; however other organic acids are also produced. The principal organic acids produced are acetic acid (CH3COOH), propionic acid (CH3CH2COOH), and butyric acid (CH3CH2CH2COOH). Ethanol (CH3CH2OH) and other products are also produced. The final step is methanogenesis. Methane and carbon dioxide are produced from acetate, ethanol, and other intermediates:
- CH3COOH → CH4 + CO2
- 2CH3CH2OH + CO2 → CH4 + 2CH3COOH
- CO2 + 4H2 → CH4 + 2H2O
The advantage of this method is that it couples the treatment of waste with energy production (methane).
10.1.2.2 Mixed VFAs Fermentation
Biological processes typically need a sterilization process to culture a specific microorganism. However, the mixed VFAs fermentation process does not require a sterilization process and instead uses a mixed microbial community. Hence, the mixed culture can provide energy savings and is an economical process [6]. In anaerobic mixed acid fermentation, the composition of the formed VFAs is different.
Depending on the composition of the VFAs, the mass balance may also vary. At high temperature the typical composition is the ratio of acetate/propionate/butyrate = 6 : 1 : 3 or 7 : 1 : 2. For a ratio of 7 : 1 : 2, the theoretical mass balance is given as follows:

10.1.2.3 VFA Platform Development
Homoacetogenic bacteria including Moorella thermoacetica were isolated as an obligate heterotroph and were observed to convert glucose to acetate without carbon loss because the CO2 produced via oxidation was subsequently utilized in the synthesis of acetate; the stoichiometry of this process is approximated by the following reaction:
Based on this high carbon yield of acetate from glucose without any loss, three moles of ethanol can be produced from one mole of glucose by a hydrogenation reaction as delineated below:
Because of low release of CO2 during fermentation, mixed VFAs fermentation provides a higher carbon yield than direct ethanol fermentation using yeast or Zymomonas mobilis [7]. AD process also converts proteins and lipids as well as carbohydrates to mixed VFAs. Thus, if VFAs can be converted to fuels and chemicals such as ethanol and butanol via economical processes, mixed VFAs fermentation could provide a new platform with versatile applications for the production of biofuels and biochemicals (Figure 10.3).
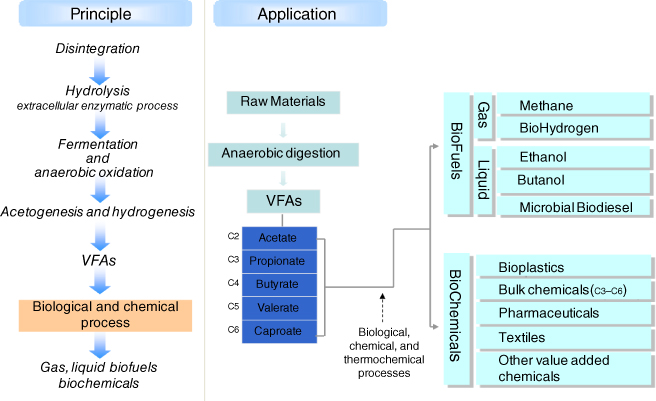
Figure 10.3 Concept of VFA platform.
10.1.3 Comparison of Biofuel Production Platforms
10.1.3.1 Theoretical Comparison of Major Platforms for Ethanol Production
Holtzapple and Granda [8] theoretically compared three major platforms for bioethanol production from ideal biomass with 31.7% lignin (represented as CH1.12O0.377) and 68.3% polysaccharides (collectively represented as C6H10O5) on an ash-free basis, which is a typical composition of hardwoods, such as poplar. To form products, polysaccharides can be processed thermochemically or biologically under anaerobic conditions. In contrast, lignin is not biologically reactive under anaerobic conditions and can only be processed thermochemically. Different moles of ethanol are formed via three major platforms using the ideal biomass (C6H10O5 + 3.93CH1.12O0.377) as follows:
- 2.5H3CCH2OH (thermochemical platform)
- 3H3CCH2OH (sugar platform)
- 3H3CCH2OH (volatile fatty acid platform)
The lower yield of the thermochemical platform is a result of the gasification partially oxidizing the biomass, which reduces the yields. From the ideal biomass, it has been claimed that the maximum potential yield of ethanol is 0.582 kg ethanol/kg ash-free biomass (175 gal/ton) using the two biological routes and 0.485 kg ethanol/kg ash-free biomass (145 gal/ton) using the thermochemical route.
10.1.3.2 Biomass Properties Needed for Each Platform
The carbohydrate part of biomass is converted to the target product with the sugar platform. Biomass with high carbohydrate content, especially hexose, is suitable for this process, because microorganisms including yeast Saccharomyces cervisiae used in the ethanol industry generally cannot utilize hexose and pentose sugars simultaneously.
The thermochemical platform needs good quality biomass to control the syngas composition and avoid catalyst poisoning by contaminants such as sulfur. Hence, dried and well-defined biomass such as woody lignocellulose must be selected or purified from raw materials. An additional advantage of this platform is the usability of biologically nondegradable plastic wastes including waste tires.
Meanwhile, a VFA platform such as a biogas platform can use organic biomass mixtures including MSW, manure, lignocelluloses, and marine biomass with high water content. If stable control methods can be realized for the VFA platform, thereby enabling high productivity and conversion to valuable products, a new gate will be opened for biorefining for biofuels and biochemicals.
10.1.3.3 Advantages and Disadvantages of Three Major Platforms
Global biofuel production tripled from 4.8 billion gallons in 2000 to about 16.0 billion gallons in 2007, but still accounts for less than 3% of the global transportation fuel supply. Feedstock costs are the most significant cost of biofuel production, ranging from 37% for sugarcane-based ethanol in Brazil in 2003–2004 to 40–50% for corn-based ethanol in the United States. Sugar beets represented 34% of the cost of sugar-based ethanol production in the EU [9]. Accordingly, the production cost should be reduced in order to increase the market position of biofuels. Lignocellulosic ethanol production is attractive because the non-food portion of the plant can be used to produce ethanol; hence, there is no competition for feedstock with the food industry. Indeed a key trend in the market today is a move away from food crops to non-food crops. However, lignocellulose conversion to fuels involves costly processes such as pretreatment and enzyme hydrolysis in the sugar platform.
Advantages and disadvantages of the three major platforms are summarized in Table 10.1. Compared with the sugar platform approach mentioned previously, the VFA process offers several advantages such as adaptability to a wide variety of feedstock, non-aseptic process conditions, inexpensive fermentation, no addition of expensive enzymes, and high fuel yield [10].
Table 10.1 Comparison of three major platforms
| Platforms | Advantages | Disadvantages | Companies or institutes |
| Sugar | High yield, low energy requirement | High enzyme price, high cost pretreatment, need of high sugar content biomass | Many companies including DuPont, DSM-Poet, BP, Abengoa, Renmatix |
| SynGas | Diverse use of raw materials, simple pretreatment | Low theoretical yield, huge facility, need of high catalytic technology | Standard AlcoholLanzaTechNRELPNNL |
| VFA | Highest yield, easy application to wastes and marine biomass, no additional enzyme, no sterilization | Hydrogen need for alcohol production, mixed alcohol production | Earth Energy RenewablesZeaChemMITKAISTTexas A&M University |
10.2 Application of VFA Platform
10.2.1 Pure and Mixed Acids as Chemicals
The VFAs production studies conducted by previous researchers are summarized in Table 10.2. Typical values of VFA production were around 30 g/l of concentration, not less than 7 g/l per day of productivity, and not less than 0.6 g acid/g VS fed of yield. The composition of VFAs was complexly varied depending on the kinds and loading rate of raw material, origin of inoculums, pH, temperature, salt concentration, and so on.
Table 10.2 Studies for volatile fatty acid production
| Studies (reference) | Agbogdo [11] | Thanakoses et al. [12] | Moody [13] | Lim et al. [14] | Chang et al. [1] | |
| Raw material | Rice straw/chicken manure | Corn stover/pig manure | Office paper/chicken manure | Food waste | Food waste | |
| Pretreatment | Lime | Lime | Lime | Lime | Grinding | Grinding |
| Number of stages | 4 | 4 | 4 | 4 | 1 | 4 |
| Average pH in fermentation | 5.8 | 6.4 | 6.0 | 5.8 | 5.5 | 7.0 |
| Temperature (°C) | 40 | 40 | 40 | 40 | 35 | 40 |
| Productivity (g/l/day) | 1.69 | 0.172 | 0.87 | 2.65 | 3.1 | 6.92 |
| TVFA (g/l) | 32.4 | 42.3 | 16.0 | 34.5 | 25 | 34.6 |
| Yield (g acid/g VS fed) | 0.286 | 0.61 | 0.55 | 0.118 | 0.37 | 0.49 |
| Acetic acid (wt%) | 44.0 | 73.6 | 39.8 | 39.6 | 49.2 | 44.2 |
| Propionic acid (wt%) | 5.46 | 4.12 | 20.4 | 8.9 | 23.5 | 7.2 |
| Butyric acid (wt%) | 29.9 | 10.3 | 16.6 | 26.8 | 20.7 | 46.9 |
| Others (wt%) | 20.64 | 11.98 | 23.2 | 24.7 | 6.6 | 1.7 |
Mixed VFAs itself is basically a little valuable, unless it is converted to value-added chemicals or separated to pure form of each component. But the separation of the mixed acids into each component is difficult, because of the azeotropic mixture formation with H2O, which needs to be solved prior to the application of the chemicals platform [15].
However, hydrogenated mixtures of C2–C4 acids and mixed alcohols can substitute for fuel ethanol [10]. And fractionated solutions of mixed esters produced by the esterification reaction with C2–C6 acids and alcohols may be used in perfumes and cosmetics.
Pure carboxylic acids are very important industrial chemicals [16]. For example, acetic acid, about 75% of which is chemically produced from methanol carbonylation, is an important chemical product. Its derivatives have reached several hundred varieties that are extensively used in many industries, such as chemicals, light industry, textiles, pharmaceuticals, printing/dyeing, rubber, pesticides, photographic chemicals, electronics, and food processing. Because acetic acid is under severe pressure from the high cost of methanol and producers are seeking price increases, the recovered acetic acid from mixed VFAs can be an additional supply source. Butyric acid and propionic acid are also important industrial chemicals in plastics, flavors, and pharmaceutical industry as shown in Figure 10.4. Lactic acid is also a useful high volume product, which is mainly used for poly(lactic acid) production although it is not our first target in the VFAs fermentation. Valeric acid and caproic acid are also used for industrial chemicals. Its primary use is in the synthesis of its esters. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors [17].

Figure 10.4 Useful carboxylates with high volume market potential to be produced in VFAs fermentation.
10.2.2 VFA Conversion to Value-Added Products
The VFAs can be converted to useful chemicals such as ketones, esters, 1-alcohlols and 2-alcohols (Figure 10.5), and polymers using various routes (Figure 10.6). Some microorganism can use VFAs as substrate for growth and product formation. Some bacteria, yeast, and fungi can produce lipid as the form of triglycerides or free fatty acids that can be converted to biodiesel by transesterification or esterification with alcohols such as methanol or ethanol [18–26]. Some microbes such as Ralstonia eutropha can produce polyhydroxy alkanoate (PHA), a biodegradable polymer, from mixed VFAs [27–29]. Polyhydroxybutyrate, one of PHAs, can be converted to ethyl 3-ethoxybutyrate, which was recently characterized as a novel fuel oxygenate with high cetane values and reduced pollutant emissions [30]. Moreover, VFAs can be thermally converted to syngas or hydrogen [31]. The produced hydrogen can be used in hydrogenation reaction to produce 1-alcohols/2-alcohols.
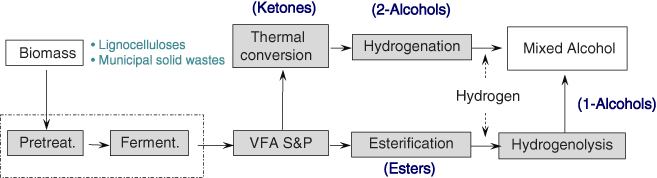
Figure 10.5 MixAlco® process developed at Texas A&M University.

Figure 10.6 Extended VFA platform.
10.2.2.1 Mixed Alcohols, Esters, and Ketones
Holtzapple et al. was developed the MixAlco® process [10]. In the MixAlco® process, biomass is first pretreated with suitable chemicals to enhance digestibility and is then fermented to produce VFAs, such as acetic, propionic, and butyric acids.
To prevent the pH from decreasing as the acids are formed, a neutralizing agent (CaCO3, NaHCO2, or NH4HCO3) is added to the fermentor; thus VFAs salts will exit the fermentor. After S&P of VFAs followed by thermal conversion steps, the salts are converted into ketones and finally hydrogenated to 2-alcohols. After the esterification of VFAs with alcohol, 1-alcohols are produced by hydrogenolysis reaction (Figure 10.5).
10.2.2.2 Microbial Lipids and Polyhydroxyalkanoate (PHA)
Biodiesel is one of the most promising alternatives to fossil fuels as its production is nontoxic, sustainable, and energy efficient. The world's consumption of biodiesel was 24 billion liters per year in 2011 according to the EIA international energy statistics (http://www.eia.gov). Biodiesel has been traditionally obtained using crops that store high quantities of oils such as soy, jatropha, and palm. The microbial lipids/oil from oleaginous microbes, which are the class of microbes that store more than 20% of their cell mass as lipids, can be an alternative for the crop-based oil. The advantages in transitioning from crop-based oil to microbial oil production for the oil feedstock include adaptability to diverse feedstocks, reduced land requirements, efficient process cycle turnover, and ease of scale-up [32, 33].
The oil stored by such microbes, referred to as single cell oil, is similar in quality to plant oils (triglycerides). For example, the major fatty acids of the lipids accumulated by Cryptococcus albidus were similar to those of soybean oil and jatropha oil [18, 19, 25].
To date, most studies on lipid production on oleaginous microorganisms (microalgae, yeast, bacteria, etc.) have been carried out with glucose as the sole carbon source. However, the high cost of biodiesel from oleaginous microorganisms mainly stems from the high cost of glucose, which is estimated to be about 80% of the total medium cost. Because VFAs are easily produced from waste organics and precursors of long-chain fatty acid [1, 14, 34], VFAs can be promising alternative carbon sources for lipid accumulation by oleaginous microorganisms.
Oleaginous cells can directly convert some organic acids into acetyl coenzyme A (CoA) via fatty acid degradation, a central intermediate in lipid synthesis, by CoA synthetase; this CoA is then used for biosynthesis of polyunsaturated fatty acids and lipid accumulation in oleaginous cells [35]. The stoichiometry and production yield of tripalmitin as a model lipid component on glucose and VFAs was analyzed by Chakraborty [33] and summarized in Table 10.3.
Table 10.3 Summary of the stoichiometry of lipid production and lipid yield on glucose and VFAs
| Substrate | Stoichiometric equation | Lipid yield (g/g) |
| Glucose (C6) | 14.1 Glucose → 33.5CO2 + tripalmitin | 0.32 |
| Acetate (C2) | 50.1 Acetate → 49.2CO2 + tripalmitin | 0.27 |
| Propionate (C3) | 29.2 Propionate → 36.7CO2 + tripalmitin | 0.38 |
| Butyrate (C4) | 19.6 Butyrate → 27.4CO2 + tripalmitin | 0.47 |
| Pentanoate (C5) | 15.3 Pentanoate → 25.4CO2 + tripalmitin | 0.52 |
| Hexanoate (C6) | 12.2 Hexanoate → 22.1CO2 + tripalmitin | 0.57 |
Although many batch or fed-batch cultures have been carried out for lipid production [20, 23, 36], bioreactor productivity was not high enough for viable commercial production. Recent studies achieved a lipid content of 55% (w/w) using VFAs as a carbon source in two-stage cultivation [18, 21] as shown in Table 10.4.
Table 10.4 Studies for microbial lipid production using VFAs
| Studies (reference) | Fei et al. [18] | Chi et al. [20] | Fontanill et al. [21] | Gong et al. [23] | |
| Raw material | Mixed VFAs | Acetic acid | Glucose + VFAs | Acetic acid | |
| Fermentation mode | Two-stage fed-batch | pH-Stat fed-batch | Two-stage fed-batch | Fed-batch | |
| Microbe | Cryptococcus albidus | Cryptococcus curvatus | Yarrowia lipolytica | Cryptococcus curvatus | |
| Cell concentration (g/l) | 26.4 | 16.9 | 41.0 | 8.1 | |
| Lipid | Concentration (g/l) | 14.5 | 8.3 | 16.50 | 4.04 |
| Contents (wt%) | 55.1 | 49.2 | 40.2 | 49.9 | |
| Yield (g lipid/g acid) | 0.125 | 0.288 | 0.20 | 0.15 | |
| Productivity (g/l/h) | — | — | 0.33 | 0.10 | |
Multistage continuous high cell density culture (MSC-HCDC) is recently considered to be a fermentation technology that can lead to high productivity and titer for both extracellular and intracellular products [37]. High cell density culture fermentation gives a very high productivity because of its high cell density achieved by membrane cell recycling, or a moderate cell density attained by using a packed-bed combined with gravity settling [38–40]. The multistage culture mode could be modified to provide high lipid content by using nutrient limitation strategies [19, 36].
Park et al. [22] analyzed the production cost for economic assessment of microbial lipids for biodiesel production in a MSC-HCDC process. A production cost of USD 1.048/kg−1 lipid was predicted with raw material costs of USD 0.2/kg−1 for wood hydrolyzates and USD 0.15/kg−1 for VFAs, 9 g/l/h bioreactor productivity, 100 000 MT/year production capacity, and 75% lipids content. The variables having the highest impact on microbial lipid production costs were the cost of VFAs and lipid yield, followed by lipid content, fermenter cost, and lipid productivity. The cost of raw materials accounted for 66.25% of total operating costs. As comparing experimental result (Table 10.4) with simulation input, the enhancing low productivity of lipid is one of the major tasks for cost reduction.
This analysis of the entire biofuel production process from microbial lipids will allow us to design a more efficient method of microbial lipid production and to evaluate the manufacturing costs of microbial lipids and biodiesel produced at a commercial scale [22].
Many heterotrophic microbial cells can utilize acids as their carbon and energy sources for PHA polymers [19, 20] that are linear polyesters and used in as a packaging material and an additive of polyvinylchloride. Yun et al. [28] used pH-stat fed-batch culture to add VFAs to the fermentor and obtained a copolymer of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] with dry cell weight of 8.1 g/l, PHA content of 50%, and 3HV fraction of 20 mol% from mixed VFAs (acetic acid: propionic acid: butyric acid = 1 : 2 : 2) after 40 h cultivation. The PHA polymer production using VFAs can enhance an economic value of product in waste treatment systems.
10.2.3 VFA Use as a Carbon Source of Denitrification Process
Nitrogen and phosphorus nutrient removal in wastewater can be made by means of various physicochemical methods. However, these methods are expensive and create secondary pollution by adding chemicals to wastewater treatment systems. For this reason biological nutrient removal of nitrogen and phosphorus is favored over physicochemical methods. Since the organic carbon present in the wastewater is often quite limited, a large amount of an external carbon source is required for the complete removal of nitrate from wastewaters that contain a high nitrogen concentration. VFAs appear to be an attractive alternative source of carbon required for heterotrophic denitrification [14, 34].
VFAs can be produced on-site via anaerobic acidogenesis of organic wastes such as food wastes [14]. VFAs derived from food waste were used as an alternative carbon source in biological
nutrient removal. The pH profiles were monitored during the nutrient removal in an Na-acetate fed sequencing batch reactor (SBR). Effluent N, P, and soluble chemical oxygen demand (SCOD) concentrations of 0.5 and 0.1 mg/l were achieved with 5.5 h of hydraulic retention time(HRT) when influent concentrations of NH4+-N, PO4 3−, and SCOD were 42.5, 5.92, and 180 mg/l, respectively. Then the SBR was fed with four solutions of VFAs produced under different acidogenesis conditions of food wastes. VFAs-added SBR showed similar specific nitrification rates (3.0–3.9 mg-N/g MLSS·h) to that of acetate, but specific denitrification rates (3.2–4.2 mg NO3−-N/g MLSS·h) were slightly lower than with acetate of 4.67 mg NO3−-N/g MLSS·h. VFAs-introduced SBR efficiently removed phosphorus except when the SBR was fed with a VFA solution containing high amounts of valerate and caproate [34].
10.2.4 Cost Analysis of Mixed Alcohol Produced from Various Raw Materials
The fuel alcohol cost is highly dependent on the biomass price [41, 42]. We roughly estimated the production cost of mixed alcohol from various biomass materials, which include lignocelluloses, marine biomass, and organic wastes, using the VFA platform (Figure 10.7a), and compared the cost from lignocelluloses with the sugar cane ethanol and cellulosic ethanol produced by sugar platform (Figure 10.7b). VFA platform showed the lower production cost than sugar platform because of mainly higher alcohol yield if the same biomass was used as a raw material.

Figure 10.7 Cost estimation of fuel alcohols. (a) Mixed alcohol production costs from various raw materials depending on the biomass price. The biomass materials include (i) lignocelluloses, (ii) marine biomass cultivated in huge scale, (iii) organic wastes, and (iv) Korean food waste. Hydrogen price was assumed as $2.0/kg−1. The plant size was 500 dry biomass tons per day or 180 000 dry biomass tons per year. (b) Comparison of ethanol costs in typical production processes. Values were estimated by (1) IEA/OECD, 2006 and (2) by our group. The mixed alcohols from VFA platform was converted to ethanol equivalent amount based on the energy equivalence and compared with other ethanol costs.
For example, typical food wastes have 20% dry matter and 80% water [34], and the price range of Korean food wastes is $80–130/dry tones waste (personal communication data from Gwangjin-gu, Seoul, Korea). Based on our experimental data, the VFA yield from Korean food waste was about 0.33–0.6 g/g depending on the waste composition.
The VFA composition varied in terms of the ratio of acetate, propionate, and butyrate (6 : 1 : 3–5 : 1 : 5, weight basis). So, the mixed alcohol yield from food waste may be 0.25–0.46 g/g compared with 0.2–0.3 g/g of ethanol yield in the sugar platform. Although additional operation costs are considered, the mixed alcohol production process using the VFA platform is likely to give high profits in excess of $100/dry tone to production companies and local governments if the process is scaled up to an appropriate production size larger than 500 dry biomass tons per day.
10.3 Tasks for Commercialization
10.3.1 Technical Bottlenecks in Industrialization of the VFA Platform
Although the VFA platform has several advantages over other platforms, the VFA platform is still in a demonstration plant stage involving a mixed VFA production process. Some unresolved problems in the VFA platform are listed in Figure 10.8. The VFA fermentation and purification process is the most important step, as other steps are already well developed in other platforms or conventional industries.
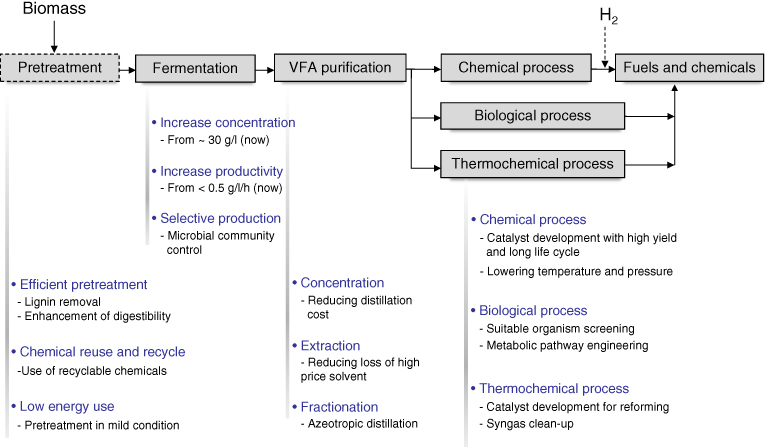
Figure 10.8 Research topics needed in VFA platform.
KAIST team has been developing a high efficiency VFA fermentation process operated in a multistage continuous high cell density mode, in which VFAs are extracted continuously with a solvent mixture. It was confirmed that VFAs could be produced in a yield higher than 0.5 g/g from the volatile solids of various biomass resources including marine seaweed (e.g., Laminaria), terrestrial biomass (e.g., reed), and MSW (e.g., food waste) [11–14].
Because the hydrogen production required for alcohol synthesis in the VFA platform and residual lignin treatment in the sugar platform are connected with the syngas platform, the future figure of an efficient biofuel production plant is likely to be a consolidated form synergistically combined with different platforms.
10.3.2 Commercialization Activities of VFA Platform
Some companies such as earth energy renewables (EER) in the United States and Aquiris in the EU are trying to commercialize the VFA platform.
EER (Bryan, TX, USA, www.ee-renewables.com) is developing a hybrid biological/chemical process to make cheap VFAs from biodegradable raw materials such as food wastes and MSW. EER was formed in 2012 with the acquisition of the intellectual property, data, and assets (including an operational demonstration plant) of Terrabon, Inc., using the technology that was originally developed at Texas A&M University. EER use liquid extraction for recovering VFAs, which can be chemically converted into other desired products. If the demonstration is finished at a scale of 3-tpd (tons per day), the EER's next plan is to build an 8-tpd facility to serve the fragrance and flavor industry and eventually move on to a 30-tpd plant to cater to agricultural and industrial markets. According to EER, “their technology can produce these acids at a price of less than $500 per ton, compared with $2000 for traditional methods [43].”
The first kilogram of PHA, which is made from VFAs derived from sewage by bacteria, was recently presented as the result of a pilot project in the Netherlands. The Dutch water boards (local government bodies responsible for water management in their areas) and their partners filtered VFAs from the sludge and mixed with bacteria that convert them into biopolymers. At present, several kilograms per week are produced, but this can be scaled up to 2000 tons per year.
A similar project has been running for some time in Brussels. Belgium-based wastewater treatment company Aquiris, a subsidiary of the conglomerate Veolia, has a pilot project at its Brussels-North wastewater treatment plant to turn wastewater into PHA since 2010. The plant claims that the sewage from the 1.1 million people of Brussels could potentially produce some 20 000 ton of bioplastics per year. After the pilot project Aquiris will work with investors to scale up the project [44].
References
- 1. Chang, H.N., Kim, N.J., Kang, J.W., and Jeong, C.M. (2010) Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioproc. Eng., 15 (1), 1–10.
- 2. Takagi, M., Abe, S., Suzuki, S., Emert, G.H. et al. (1997) A Method for Production of Alcohol Directly from Cellulose Using Cellulase and Yeast, Indian Institute of Technology, Delhi, p. 571.
- 3. Wright, J.D., Wyman, C.E., and Grohmann, K. (1988) Simultaneous saccharification and fermentation of lignocellulose. Appl. Biochem. Biotechnol., 18, 75–90.
- 4. Kumar, A., Jones, D.D., and Hanna, M.A. (2009) Thermochemical biomass gasification: a review of the current status of the technology. Energies, 2, 556–581.
- 5. Batstone, D.J., Keller, J., Angelidaki, I., Kalyuzhnyi, S.V. et al (2002) Anaerobic Digestion Model No. 1, IWA task group for mathematical modelling of anaerobic digestion processes staff, London.
- 6. Datta, R. (1981) Acidogenic fermentation of corn stover. Biotechnol. Bioeng., 23, 61–77.
- 7. Chang, H.N., Kim, B.J., Kang, J.W., Jeong, C.M. et al. (2008) High cell density ethanol fermentation in an upflow packed-bed cell recycle bioreactor. Biotechnol. Bioprocess Eng., 13, 123–135.
- 8. Holtzapple, M.T. and Granda, C.B. (2008) Carboxylate platform: the MixAlco process part 1: comparison of three biomass conversion platforms. Appl. Biochem. Biotechnol., 156, 95–106.
- 9. Coyle, W. (2007) The future of biofuels: a global perspective. AMBER Waves, 5, 24–29.
- 10. Holtzapple, M., Ross, M., Chang, N., Chang, V. et al (1999) Biomass conversion to mixed alcohol fuels using the MixAlco process. Appl. Biochem. Biotechnol., 79, 609–631.
- 11. Agbogdo, F.K. (2005) Anaerobic fermentation of rice straw and chicken manure to carboxylic acids. PhD thesis. Texas A&M University, Texas, USA.
- 12. Thanakoses, P., Black, A.S., and Holtzapple, M.T. (2003) Fermentation of corn stover to carboxylic acids. Biotechnol. Bioeng., 83, 191–200.
- 13. Moody, A.G. (2006) Pilot-scale fermentation of office paper and chicken manure to carboxylic acids. M.S. thesis. Texas A&M University, Texas, USA.
- 14. Lim, S.J., Kim, B.J., Jeong, C.M., Choi, J., Ahn, Y.H., and Chang, H.N. (2008) Anaerobic organic acid production of food waste in once-a-day feeding and drawing off bioreactor. Bioresour. Technol., 99, 7866–7874.
- 15. Chang, H.N., Lee, S.Y., Yang, S.M., Kim, N.J., et al. (2011) Method for producing organic acids from biomass Patent WO2011084002.
- 16. Rpgers, P., Chen, J.-S., and Zidwick, M.J. (2006) Organic acid and solvent production, in Prokaryotes, 3rd edn (eds M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt), Springer, pp. 511–671.
- 17. Wikipedia, the free encyclopedia, https://en.wikipedia.org/wiki/Valeric_acid (accessed 28 March 2016).
- 18. Fei, Q., Chang, H.N., Shang, L., and Choi, J.-d.-r. (2011) Exploring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol. Bioprocess Eng., 16 (3), 482–487.
- 19. Fei, Q., Chang, H.N., Shang, L., Kim, N.J., and Kang, J.W. (2011) The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol., 102 (3), 2695–2701.
- 20. Chi, Z., Zheng, Y., Ma, J., and Chen, S. (2011) Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrogen Energy, 36 (16), 9542–9550.
- 21. Fontanille, P., Kumar, V., Christophe, G., Nouaille, R., and Larroche, C. (2012) Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol., 114, 443–449.
- 22. Park, G.W., Fei, Q., Jung, K., Chang, H.N. et al. (2014) Volatile fatty acids derived from waste organics provide an economical carbon source for microbial lipids/biodiesel production. Biotechnol. J., 9 (12), 1536–1546.
- 23. Gong, Z., Shen, H., Zhou, W., Wang, Y. et al. (2015) Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels, 8, 189–197.
- 24. Fei, Q., Fu, R., Shang, L., Brigham, C.J., and Chang, H.N. (2015) Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst. Eng., 38 (4), 691–700.
- 25. Li, X., Xiong, L., Chen, X., Huang, C. et al. (2015) Effects of acetic acid on growth and lipid production by Cryptococcus albidus. J. Am. Oil Chem. Soc., 92 (8), 1113–1118.
- 26. Huang, X.F., Liu, J.N., Lu, L.J., Peng, K.M. et al. (2016) Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour. Technol., 206, 141–149.
- 27. Chakraborty, P., Gibbons, W., and Muthukumarappan, K. (2009) Conversion of volatile fatty acids into polyhydroxyalkanoate by Ralstonia eutropha. J. Appl. Microbiol., 106, 1996–2005.
- 28. Yun, J.H., Sawant, S.S., and Kim, B.S. (2013) Production of polyhydroxyalkanoates by Ralstonia eutropha from volatile fatty acids. Korean J. Chem. Eng., 30 (12), 2223–2227.
- 29. Cerrone, F., Choudhari, S.K., Davis, R., Cysneiros, D. et al (2014) Medium chain length polyhydroxyalkanoate (mcl-PHA) production from volatile fatty acids derived from the anaerobic digestion of grass. Appl. Microbiol. Biotechnol., 98 (2), 611–620.
- 30. Buncea, M.P., Storey, J.M.E., Edmonds, J.W., Findlay, R.H. et al. (2015) Ethyl 3-ethoxybutyrate, a new component of the transportation renewable fuel portfolio. Fuel, 161 (1), 262–268.
- 31. Jeong, C.M., Park, G.W., Kang, J.W., Kim, S.M. et al. (2011) Steam reforming of volatile fatty acids (VFAs) over supported Pt/Al2O3 catalysts. Int. J. Hydrogen Energy, 36 (13), 7505–7515.
- 32. Beopoulos, A., Nicaud, J.-M., and Gaillardin, C. (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol., 90, 1193–1206.
- 33. Chakraborty, S. (2015) Exploring volatile fatty acids (VFAs) as a novel substrate for microbial oil production. PhD thesis. Massachusetts Institute of Technology.
- 34. Lim, S.J., Kim, E.Y., Ahn, Y.H., and Chang, H.N. (2008) Biological nutrient removal with volatile fatty acids from food wastes in sequencing batch reactor. Korean J. Chem. Eng., 25 (1), 129–133.
- 35. Ratledge, C. and Wynn, J.P. (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol., 51, 1–51.
- 36. Li, Y.H., Zhao, Z.B., and Bai, F.W. (2007) A high-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol., 41, 312–317.
- 37. Chang, H.N., Kim, N.J., Kang, J.W., Jeong, C.M. et al. (2011) Multi-stage high cell continuous fermentation for high productivity and titer. Biotechnol. Biosyst. Eng., 34, 419–431.
- 38. Lee, C.W. and Chang, H.N. (1987) Kinetics of ethanol fermentations in membrane cell recycle fermentors. Biotechnol. Bioeng., 29, 1105–1112.
- 39. Chang, H.N. and Furusaki, S. (1991) Membrane bioreactors: Present and prospects. Adv. Biochem. Eng. Biotechnol., 44, 27–64.
- 40. Chang, H.N., Yoo, I.K., and Kim, B.S. (1994) High density cell culture by membrane-based cell recycle. Biotech. Adv., 12, 467–487.
- 41. Bain, R.L. (2007) World Biofuels Assessment Worldwide Biomass Potential: Technology Characterizations. NREL milestone report. NREL/MP-510-42467.
- 42. Kann, B., Gruber, P.R., and Kamm, M. (2007) Biorefineries – Industrial Processes and Products: Status Quo and Future Directions, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
- 43. Chemical Weekly (11 August 2015), pp. 217–224.
- 44. http://blog.sirris.be/blog/bioplastics-sewage (accessed 30 March 2016).
