Chapter 27
Bioelectronic Nose
Hwi Jin Ko, Eun Hae Oh and Tai Hyun Park
27.1 Introduction
We use the olfactory sense in our daily life for determining the taste of food, as well as the freshness of food, for simply distinguishing malodor from fragrance, and sometimes for perceiving dangerous situations, such as fire. Since a Nobel Prize was awarded to Axel and Buck, who identified the mechanism of olfaction, more research groups have joined the study of an olfactory sensor mimicking olfaction, as well as the olfactory sense itself. Recently, more researches for the artificial sensor mimicking olfaction have been conducted, due to potential applications for diverse areas [1–7]. In particular, the study on a bioelectronic nose using olfactory receptors (OR), which play an important role in olfaction, has a significant meaning, in that human olfaction is expected to be realized in vitro, in addition to the development of an olfactory biosensor. The rapid growth in nanotechnology has also been playing a pivotal role in the progress of the bioelectronic nose. The bioelectronic nose consists of two parts. One is a primary transducer, and the other is a secondary transducer. The primary transducer is a biological part that recognizes odorant molecules and plays a key role in selectivity. The secondary transducer is a nonbiological part, which plays an important role in sensitivity. Primary transducers are biomaterials, such as cells [8], proteins, nanovesicles, or peptides. Various devices, such as surface plasmon resonance (SPR), quartz crystal microbalance (QCM), carbon nanotube-field effect transistor (CNT-FET), carboxylated polypyrrole nanotube-field effect transistor (CPNT-FET), and graphene-based field effect transistor (FET), have been developed as a secondary transducer [3–7, 9–12]. Thus, the performance of the bioelectronic nose has been significantly improved through the integration of biomaterials and nanomaterials; and eventually, bioelectronic noses will have great potential in the applications of a huge variety of fields, such as diagnosis, foods, fragrance and flavor, and the monitoring of environmental pollution.

Figure 27.1 Schematic diagram of bioelectronic nose, constructed by the combination of biomaterials and nanomaterial-based sensor elements. The specific binding of analytes to biomaterials can be measured by a nanomaterial-based sensing element. S and D represent “source” and “drain,” respectively, in the nanomaterial-based platform.
27.2 Concept of Bioelectronic Nose
27.2.1 Primary Transducer
Usually, the biosensor is a sensing device that detects analytes by the combination of a biological recognition part (primary transducer) with a physicochemical detection part (secondary transducer) (Figure 27.1). Various different biomaterials can be a primary transducer for the biosensor [13]. They are enzymes, antibodies, aptamers, and sensory receptors. Enzymes can be used as a primary transducer, due to the characteristics of their specific binding affinity and biocatalytic activity [14, 15]. Antibodies are efficient biological sensing elements for a biosensor, because of their ability to specifically bind to the target molecules [14]. Aptamers, which are nucleic acids or peptides, bind to specific target molecules and can also be used as a primary transducer for the biosensor [16]. The major role of a biological recognition part is to selectively discriminate target molecules, from a mixed variety of other molecules. Biosensors using sensory receptors as a sensing material have recently made rapid progress. In particular, OR among sensory receptors are useful sensing elements for olfactory biosensors, termed bioelectronic noses. They can effectively discriminate a specific target odor molecule from a large number of target molecules by enhancing the selectivity of the bioelectronic nose.
27.2.2 Secondary Transducer
As mentioned earlier, the biosensor contains a secondary transducer, which is a nonbiological part, in addition to the primary transducer, as a biological part (Figure 27.1). Specific interaction of analytes with biological recognition parts is measured by the sensing element, which produces an electrical signal. The secondary transducer utilizes a nanomaterial-based sensor platform, allowing a biosensor to obtain an extremely high sensitivity. Various nanomaterial-based sensor platforms are able to be utilized as a secondary transducer, such as QCM [10, 11, 17], SPR [18–21], and FET [3, 4, 22]. By combining nanomaterials and biomaterials, a bioelectronic nose can achieve high sensitivity and selectivity.
27.3 Primary Transducer for Bioelectronic Nose
27.3.1 Olfactory Receptor Protein
OR proteins belong to the family of G-protein-coupled receptors (GPCRs) and play a major role in recognizing and discriminating, different molecules [23]. The OR gene family provides the information for the sense of smell [24] and contains about 906 genes, including over 55% of pseudogenes in human, consisting of the largest gene superfamily in the mammalian genome [25–27]. The coding region of all OR genes is approximately 1 kb, without intron [28, 29]. OR proteins are seven-transmembrane proteins, and these structural features are important to their normal functions (Figure 27.2). Human can recognize odorant molecules at the concentration of 10−3 ppb and detect around 10 000 different odor molecules, even though the number of functional OR is about 390. Many research groups have been interested in the development of a biosensor mimicking the human olfactory sense and have tried heterologous expression of OR [10, 11, 30, 31]. Recently, several approaches have tried to develop a bioelectronic nose with high specificity and sensitivity by the combination of OR and a nanomaterial-based sensor platform [1]. The use of OR provides the bioelectronic nose with great improvement in the selectivity.

Figure 27.2 Proposed three-dimensional structure of the olfactory receptor. Each of the transmembrane regions is numbered.
27.3.2 Nanovesicle Containing Olfactory Receptor
If we want to make a cell-based bioelectronic nose, it would not be easy. Even though living cells are the best biological sensing materials – by providing an optimal environment for the binding of receptors to ligands and also by mimicking the olfactory signaling system with the closest proximity – problems with respect to stability and reusability still remain. A cell-based sensing system depends on the condition and viability of the cells, and it is difficult to maintain the cell condition and viability at a constant level. In addition, the size of the mammalian cell is too big to apply them to a nanomaterial-based sensor platform, and they generate noise signals when the binding of odorants to the OR is measured through the combination of cells and nanomaterial-based sensor platform, because various metabolic procedures occur in cells, and the senor platform is sensitive enough to generate noise. Therefore, nanovesicles containing an OR and simple signaling component molecules have been developed in order to overcome these limitations of a cell-based sensing system [6, 32, 33].
Since nanovesicles were derived from mammalian cells expressing ionotropic 5-HT3 receptor, and the ligand-specific receptor signaling was successfully measured with a whole-cell-like signaling process [34], nanovesicles have been derived from mammalian cells expressing OR for their application to a bioelectronic nose [6, 32, 33]. The nanovesicles containing OR were constructed by the treatment of mammalian cells expressing OR with cytochalasin B, and the treatment of cytochalasin B makes nanovesicles bud out of mammalian cell membrane. The average diameter of nanovesicles was around 200 nm, with a round shape, and this size is small enough to be immobilized on the nanomaterial-based sensor platform. Even though they are not alive – unlike mammalian cells – the function of nanovesicles can be measured by Ca2+ assay, because they have the components required in olfactory signaling. This characteristic of nanovesicles can exclude noises originating from many metabolic procedures in live cells. Nanovesicles were immobilized on a single-walled carbon nanotube-field effect transistors (SWNT-FET) sensor (Figure 27.3). The binding of odorant molecules to an OR was monitored by OR-mediated signaling on an SWNT-FET sensor platform, with high sensitivity and selectivity. Hexanal, which is one of the indicators of food oxidation, was detected by using nanovesicles containing the OR immobilized on SWNT-FET. This nanovesicle-based bioelectronic nose showed highly selective and sensitive detection of hexanal (Figure 27.4). Overall, nanovesicle-based bioelectronic noses are expected to be widely used for the detection of odor molecules in various industries because they can recognize a specific odor molecule with high sensitivity and selectivity and mimic the signaling pathway in the natural olfactory system; and additionally, they have the advantage of being much more stable than the cell-based system.

Figure 27.3 (a) Schematic diagram showing a method to prepare an olfactory-nanovesicle-fused carbon nanotube-transistor biosensor (OCB). A CNT-based transistor was coated with poly-d-lysine (PLD) for the stable adsorption of nanovesicles. Then, olfactory nanovesicles were immobilized on a CNT channel region in the transistor. (b) Schematic diagram showing the sensing mechanism for the detection of hexanal with an OCB. The binding of hexanal to ORs results in Ca2+ ions inside the nanovesicles, creating a positive gate potential in the vicinity of underlying CNTs, and the increased potential results in the decrease of conductance in the CNT channel.
(Reproduced by permission of The Royal Society of Chemistry.)
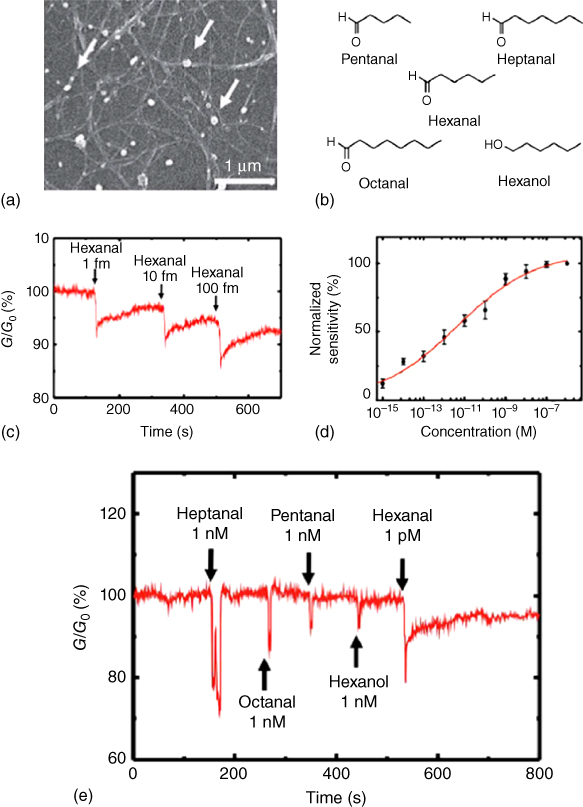
Figure 27.4 Highly selective and sensitive detection of hexanal with OCB devices. (a) SEM image of nanovesicles immobilized on a CNT channel. The white arrows indicate the nanovesicles. (b) Chemical structures of various odorant molecules. Hexanal is the specific ligand of cfOR5269 protein. Note that the odorants have similar structures, with slight differences in their alkyl chain length, or functional groups. (c) Real-time conductance measurement data obtained from an OCB after the introduction of hexanal. The conductance decreased after the introduction of hexanal solution with a femtomolar concentration. (d) Response curve of OCBs (n = 5) to hexanal with different concentrations. The responses of OCBs were fitted to the Langmuir isotherm curve (red solid curve). (e) Real-time conductance measurement data obtained from an OCB after the injection of different odorants. The addition of 1 nM heptanal, octanal, pentanal, and hexanol solutions had no effect on the conductance of the OCB, while the addition of 1 pM hexanal solution caused a sharp decrease in the conductance of the OCB.
(Reproduced by permission of The Royal Society of Chemistry.)
27.3.3 Peptide Derived from Olfactory Receptor Protein
OR-based bioelectronic noses have been developed to overcome the limitation of electronic and biomimetic noses. They exhibited high sensitivity and selectivity [3–6, 35]. The OR proteins are seven-transmembrane proteins, and have a regular structure and function, when they are incorporated into a lipid bilayer membrane. If we used the odorant-binding part, which is the peptide derived from the OR protein, it would be much easier to immobilize it on the nanomaterial-based sensor platform. Recently, Lim et al. have developed the bioelectronic nose that uses a peptide receptor derived from an OR protein [36]. The OR-derived peptides were functionalized on SWNT-FET by a single-step process, in which aromatic rings of peptides were immobilized by π–π stacking [36, 37] (Figure 27.5). This peptide receptor-based bioelectronic nose (PRBN) was used to determine the seafood quality in real time by quantitatively measuring trimethylamine (TMA), which is a major odorant molecule that is generated from spoiled seafood.

Figure 27.5 PRBN for the detection of TMA. Olfactory receptor peptides (ORPs) were self-assembled on the surface of SWNTs during the treatment of ORP-suspended deionized water solutions. The ORPs were immobilized by π–π stacking of aromatic rings of three phenylalanine sequences at their C-terminus and attracted TMA molecules very near to the SWNTs.
The PRBN detected TMA at an extremely low concentration of 10 fM and discriminated TMA from other odorant molecules with similar structures (Figure 27.6). Because TMA is an air pollutant and indicator of several diseases, as well as of spoiled seafood, the PRBN specifically detecting TMA has many applications. Much more recently, the bioelectronic nose has been developed in order to detect gaseous TMA for real-time on-site gas analysis [37]. Thus, the use of peptide receptors for the development of a bioelectronic nose is able to overcome the limitation of whole proteins and is expected to provide useful applications in various fields.
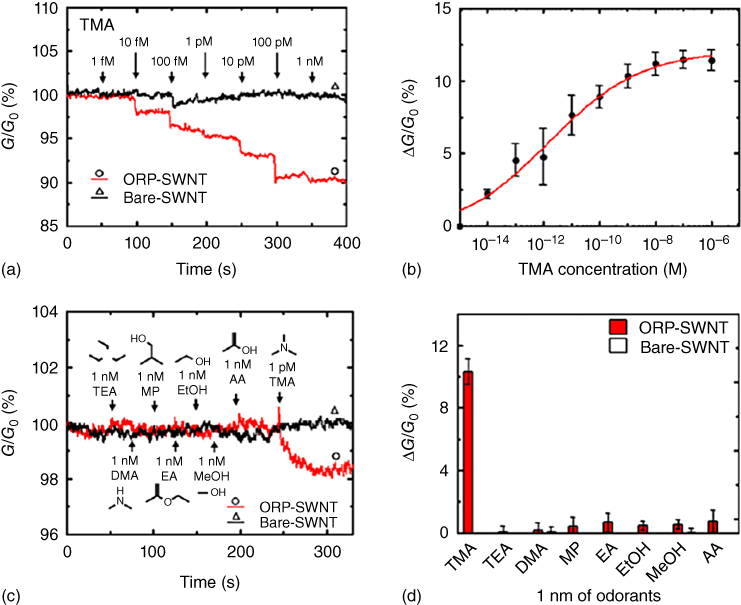
Figure 27.6 Sensitive and selective detection of TMA using PRBNs. (a) Real-time measurements of conductance changes generated by the introduction of TMA at concentrations ranging from 1 fM to 1 nM using bare (triangle) and ORP-coated (circle) SWNT-FETs. The decrease in conductance was visible after the introduction of 10 fM of TMA, and the responses increased, with an increase in TMA concentrations. (b) Dose-dependent response curve of PRBNs to TMA. Each point and error bar represents the mean and standard deviation (SD), respectively, calculated from five real-time measurements. (c) Real-time recognition of TMA from other small molecules using bare (triangle) and ORP-coated (circle) SWNT-FETs. The addition of 1 pM TMA solution caused a sharp decrease in the conductance of ORP-SWNT devices; however the addition of 1 nM of other molecules had no effect on the conductance. (d) Quantitative comparison of conductance changes, after the injection of TMA and other molecules at a concentration of 1 nM (n = 5). TMA, trimethylamine; TEA, triethylamine; DMA, dimethylamine; MP, 2-methyl-1-propanol; EA, ethyl acetate; EtOH, ethanol; MeOH, methanol; AA, acetic acid.
27.4 Secondary Transducer for Bioelectronic Nose
27.4.1 Quartz Crystal Microbalance
The QCM has been used as a secondary transducer for an odorant detection system. Since the QCM, as a piezoelectric crystal sensor, can detect the interaction between small molecules, many researchers used the QCM to detect the odorant by coating the QCM surface with various sensing elements, such as polymer, lipid, enzyme, peptide, and protein. QCM has several advantages, such as simplicity, high sensitivity, and convenience. The principle of QCM is that when specific gas molecules bind to the coating material of QCM, the total mass of the surface increases and this mass change reduces the resonant frequency of the crystal. Due to the ability of QCM to quantitatively detect gas molecules, many researchers have tried to construct an odor sensing system that uses a chemically modified QCM surface. For example, Wyszynski et al. used pegylated lipid, and Koshets et al. used calixarene films as a coating material of the QCM surface to detect various odorous molecules, such as alcohols, esters, acids, and aldehydes [38–40]. However, the chemically modified QCM had difficulty in discriminating structurally similar molecules.
The QCM sensor, functionalized with OR, is a useful odorant sensing system to detect and discriminate various odorants. Several researches show that by combining with an OR, the QCM can be a good odorant sensing device. Wu et al. coated a QCM with OR isolated from bullfrogs in order to construct an odorant sensor [17]. In this study, the QCM coated with bullfrog OR showed a significant change in frequency upon odorant simulation. In another study, the C. elegans OR ODR10 was heterologously expressed in Escherichia coli, and the purified protein was used as a sensing material of the QCM system. When the specific ligand diacetyl was injected in the range of 10−12–10−5 mM, the frequency changed in a ligand-specific and dose-dependent manner [11]. The OR that was expressed in mammalian cell was also used as a sensing material of the QCM system. The HEK-293 mammalian cell expressing the OR I7 was incubated on the surface of quartz crystal. When it was exposed to various odorants, the response was especially high for octanal, which is a known specific odorant of I7; and when it was exposed to various concentrations of octanal, the resonant frequency change increased in a dose-dependent manner [10]. In another study, OR-based synthetic polypeptide was deposited on the QCM to detect a low concentration of acetic acid. In this study, the QCM detected the acetic acid at 10 ppm [41]. These results clearly demonstrate that a piezoelectric system can be a useful odorant detection device, with high selectivity and sensitivity.
27.4.2 Surface Plasmon Resonance
The SPR technique is an optical method that uses a surface plasmon wave to monitor the change on the sensor surface. Surface plasmon is the fluctuation in electron density in a solid or liquid caused by incident light. SPR occurs in free electron-like metals, such as silver and gold. The electrons in a metal continuously move freely like a charged cloud. The resonance condition is established when light is focused onto the gold surface. Then, the energy is transferred from the light to the electrons in the surface. The critical angle where the reflectance falls to zero is called the resonance angle. The reflectance is altered by any surface changes or binding events near the surface of the SPR. Thus, the SPR system has been used as a biosensor to detect the interactions of various biomolecules in real time, without labeling [42, 43]. The use of SPR for biosensing was first demonstrated in 1983 [44]. Since then, SPR has been widely used in biological applications, such as ligand screening, cell biology, signal transduction, and DNA–DNA, DNA–protein, protein–protein, and protein–lipid interactions [45–47]. Recently, ligand screening or cellular signaling of GPCR using the SPR system has also been reported [48, 49].
This technique has also been used by Vidic et al. to detect the odorant–OR binding [50]. In this experiment, rat ORI7 was co-expressed with Gαolf in a yeast expression system, and nanosomes containing ORI7 were obtained by mechanical disruption. The nanosomes were immobilized on the surface of SPR, and the desorption of the Gαolf subunit from the lipidic bilayer, through the receptor activation upon the odorant stimulation, was monitored. Similarly, the nanosome containing human OR hOR3A1 was obtained from yeast and used as a sensing material of the SPR system [19]. Cell-based odorant detection using the SPR system was also carried out [20, 21]. The cells expressing ORI7 were cultured on the surface of SPR sensor chips, and the reflectance change was measured upon odorant stimulation. When various concentrations of odorant were injected, the higher concentration of odorant induced a greater change in reflectance. And when various odorants that have similar chemical structure were injected into the cells expressing ORI7, the cells responded specifically to their specific ligand octanal. In this study, it was shown that the SPR system can directly measure the odorant-induced cellular response. Besides, the SPR system was also used to study the functional role of the odorant-binding protein [51, 52].
27.4.3 Field Effect Transistor
A nanometer-scale sensor based on nanotube field-effect transistors (NT-FETs) has been developed in the field of chemical sensing. Recently, the SWNT and carboxylated polypyrrole nanotube (CPNT) have been combined with OR to detect the odorant with high sensitivity and selectivity. Kim et al. introduced a SWNT-FET combined with human OR protein as an odorant sensing system [3]. Human OR hOR2AG1, of which the specific odorant is amylbutyrate, was expressed in E. coli, and the partially purified hOR2AG1 was spread onto the SWNT-FET sensor. To detect the odorant binding to OR, the source–drain current was monitored, along with the addition of amylbutyrate. When the various concentrations of amylbutyrate were added, the source–drain current sharply decreased and gradually reached saturation value. This indicates that the binding of odorant to OR induced the change in current. The detection limit was 100 fM, which is significantly lower concentration than the concentration detected using other transducers. This olfactory biosensor also showed the specific detection of odorant when various odorants that have similar chemical structure were added to the sensor. In other experiments, nanovesicle containing OR was used as a sensing material in the CNT-FET sensor system. Jin et al. obtained nanovesicles from mammalian cell expressing hOR2AG1 [32]. The nanovesicles containing hOR2AG1 were immobilized on SWNT-FET, and the activity of the nanovesicles upon ligand stimulation was monitored. The activity of the nanovesicles was generated from the cellular signaling pathway, in which the calcium ions enter into cells upon ligand stimulation. Lim et al. used the nanovesicle-based SWNT-FET sensor as a diagnostic tool to detect the molecules produced from lung cancer patients [53]. A peptide-based SWNT-FET for odorant detection using OR-derived peptide was also reported [36]. Yoon et al. fabricated a FET sensor platform based on hOR2AG1-conjugated carboxylated polypyrrole nanotubes [4]. The hOR2AG1 was immobilized onto a CPNT, and the odorant–OR binding was detected by measuring the change in current. The interaction of odorant and OR can affect the charge carrier density of the conjugated CPNT. The detection limit of this sensor was tens of femtomoles. In 2012, Lee et al. used CPNT-FET to detect the odorant in gaseous phase [35]. Human OR hOR3A1, which was expressed in E. coli, was immobilized onto the CPNT-FET, and exposed to its specific ligand helional in gas phase, to mimic the natural olfactory mechanism. The minimum detectable level of this sensor was 0.02 ppt, which is much more highly sensitive than other gas-phase sensors. These results show that the nanotube based-FET system combined with OR or nanovesicle containing OR or peptide can be used as a highly sensitive odorant detection sensor and can also be applied to various areas that need to detect or diagnose meaningful odorant molecules.
27.5 Applications
There can be many applications of the bioelectronic nose. Applications include a variety of fields such as medicine, food, fragrance, and environmental monitoring (Table 27.1).
Table 27.1 Applications of the bioelectronic nose
| Industry | Application fields |
| Medicine | Detection of pathogen |
| Early diagnosis of severe diseases | |
| Patient condition control | |
| Food | Quality control (ingredient confirmation, off-flavor, and spoilage) |
| Fermentation process control | |
| Detection of bacterial contamination | |
| Fragrance and flavor | Consumer's expectation (cosmetics, perfumes) |
| Ripeness and freshness of fruits and vegetables | |
| Brand recognition (coffee, beer, etc.) | |
| Environmental monitoring | Air and water pollution monitoring (malodor, greenhouse gas emission) |
| Indoor air monitoring |
27.5.1 Medical Applications
Chemical compounds, especially volatile organic compounds from the human body, are important indicators of almost all diseases. These volatile organic compounds are emitted from various parts of the human body, such as the oral cavity, axillae, feet, scalp, skin, and breath; and they provide information about human health conditions, such as infection, diet, stress, immune status, and metabolic diseases [54]. It has been considered since ancient times that volatile organic compounds from the human breath are closely related to human diseases [55], and many researches have demonstrated a variety of key volatile compounds related to diseases. Blood, urine, and sweat are also the sources of odorous compounds that provide a lot of information about human health.
Lim et al. have developed a bioelectronic nose for the diagnosis of lung cancer [53]. Heptanal was a target odor molecule, because it is one of the major odor compounds from patients with lung cancer. The concentration of heptanal is much higher in the blood of patients than non-patients. Human OR, which specifically binds to heptanal, was identified and expressed in mammalian cells. Nanovesicles were derived from mammalian cells expressing the OR and immobilized on single-walled carbon nanotube field-effect transistors (SWNT-FETs) for the development of a bioelectronic nose. The constructed bioelectronic nose detected and discriminated heptanal at a concentration of 100 fM from human plasma, without any pretreatment (Figure 27.7).

Figure 27.7 Detection of heptanal from human blood plasma using a nanovesicle-based bioelectronic nose. The bioelectronic nose was fabricated using nanovesicles, with olfactory receptor (OR), Gαolf, and receptor-transporting protein 1S (RTP1S). (a) A real-time measurement for the influence of plasma on the conductance change in the bioelectronic nose. Human blood plasma samples were diluted to different ratios. The 1/10−7-diluted plasma did not show any effect on the conductance, which means that no pretreatment process is required, when 1/10−7-diluted plasma is used in the experiment. (b) The real-time detection of heptanal from 1/10−7-diluted plasma using a bioelectronic nose. A bioelectronic nose based on nanovesicles with OR, Gαolf, and RTP1S was able to detect 1 × 10−13 M heptanal (square), whereas a bioelectronic nose without ORs was not (triangle).
A bioelectronic nose using hormone receptors can also be used for medical application because numerous diseases can be induced by hormonal imbalance. Recent study has developed a bioelectronic nose that detects human parathyroid hormone (hPTH) [56]. hPTH receptors were overexpressed in E. coli and were combined with modified close-packed arrays of carboxylated polypyrrole nanoparticles (CPNTs). This hormone receptor-based bioelectronic nose detected hPTH with high sensitivity and selectivity. M1 muscarinic acetylcholine receptors (M1 mAChRs) were also used for detecting acetylcholine (ACh) [57]. ACh is one of the neurotransmitters in the human central nervous system, and dysregulation of ACh in the brain causes numerous neuropsychiatric disorders, such as Alzheimer's disease and Parkinson's disease. The bioelectronic nose device was developed with the combination of overexpressed M1 mAChRs and SWNT-FETs; and it detected ACh at a concentration of 100 pM and selectively differentiated ACh from other neurotransmitters. This bioelectronic nose system using various sensory receptors is expected to be used for the diagnosis of a variety of diseases by using various OR and hormone receptors.
27.5.2 Food Quality
The control and assessment of food quality is an important issue in the food industry, because food quality is closely related to the consumer's choice and the sales volume of the products. Generally, off-odor from spoiled food is generated by the oxidation of food or contamination of food by bacteria and fungi [58–60]. Intake of spoiled foods causes severe health problems. Thus, it is important to discriminate spoiled foods and to select normal and fresh foods.
It is known that the amount of linear aldehydes proportionally increases by the oxidation of unsaturated fatty acids, as foods spoil [61]. Recent study has demonstrated that the bioelectronic nose selectively and sensitively detects hexanal [6]. A bioelectronic nose was fabricated with canine OR as a primary transducer and SWNT-FETs as a secondary transducer and was able to detect hexanal at an extremely low concentration of 1 fM, as well as detected the hexanal in spoiled milk, without any pretreatment. Another approach has assessed seafood quality by measuring TMA from spoiled seafood by the combination of peptide receptor and SWNT-FETs [36]. This sensor was able to determine the degree of spoilage of seafood, without any pretreatment, by measuring the amount of TMA and also to discriminate spoiled seafood, such as oyster, shrimp, and lobster, from other spoiled foods, such as milk, tomato, broccoli, and beef (Figure 27.8). More recently, Ahn et al. have developed olfactory biosensor for detecting fungal contamination in grain [62]. Taken together, the bioelectronic nose is expected to be widely used to assess and control food quality at home as well as in industry.
27.5.3 Environmental Monitoring
There has been an increase in environmental pollutants, concomitant with the drastic development of industry, and these pollutants have threatened even our health and ecosystem, as well as the global environment. Environmental pollution is caused by the emission of carbon dioxide (greenhouse gases) and various toxic compounds from industrial processes into the atmosphere, soil, river, ocean, and so on. These pollutions need to be strictly monitored and controlled for the protection of the global environment and of our health from hazardous pollutants. An effective detection system is required to meet these needs.
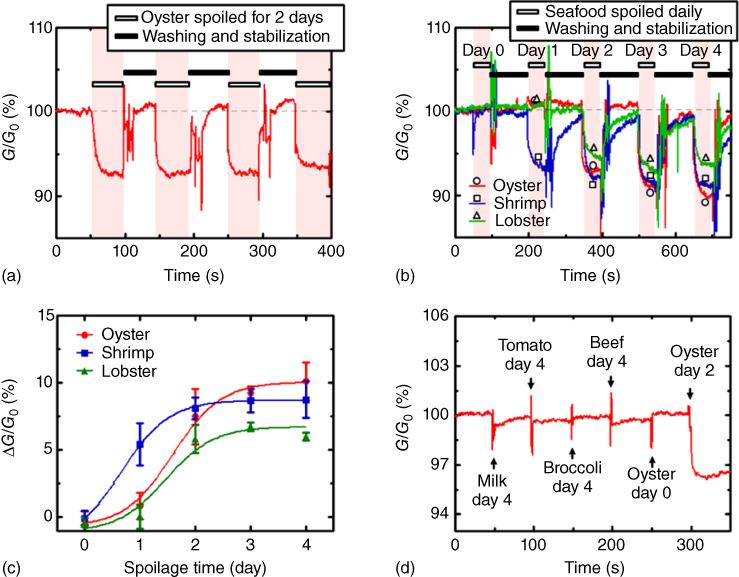
Figure 27.8 Detection of trimethylamine (TMA) from spoiled seafood using peptide receptor-based bioelectronic nose. (a) A real-time measurement data exhibiting the conductance change generated by repeated treatments with a spoiled oyster sample, which was produced by storing the oyster sample at 25 °C for 2 days. Consistent and prompt responses were generated. The gray dotted line represents the initial base line. (b) Real-time measurements of conductance changes generated by treatments with oyster (circle), shrimp (square), and lobster (triangle) samples, spoiled for different periods of time. Significant decreases in the conductance were observed for the 2-day spoiled oyster, 1-day spoiled shrimp, and 2-day spoiled lobster, and the responses increased with the spoilage time. (c) Response patterns versus the degree of spoilage of the three spoiled seafood samples (oyster, shrimp, and lobster). The generated responses tended to increase with the degree of spoilage. (d) Real-time recognition and distinction of spoiled oyster from other types of spoiled foods (milk, tomato, broccoli, and beef) and fresh oyster. The sample solutions of milk, tomato, broccoli, and beef spoiled for 4 days and the fresh oyster had no significant effect on the conductance. However, the injection of the oyster sample that had been spoiled for 2 days caused a sharp decrease in conductance.
The bioelectronic nose can be a suitable method for environmental monitoring. One major concern in monitoring environmental pollution is whether OR are still active, even in the dry condition, because air pollution is a severe environmental concern, and also, it is easier to detect the pollutants from the headspace of polluted soil and water samples, without any treatment. A few decades ago, one research group addressed this issue by using a synthetic peptide derived from an OR protein [63]. The sensor using a peptide effectively detected TMA and ammonia, which are well-known air pollutants. Lee et al. also showed that whole OR protein overexpressed in E. coli can detect odor molecules in the dry condition by combining carboxylated polypyrrole nanotubes (CPNTs) [35]. This device selectively detected gaseous odorant at a concentration as low as 0.02 ppt. As mentioned earlier, the OR is expected to have a proper function when incorporated in the lipid bilayer membrane. Recently, bioelectronic nose has been developed for real-time monitoring of water pollution using an SWNT-FET functionalized with olfactory nanovesicles [33]. All these characteristics of the bioelectronic nose show its potential to be used for environmental monitoring.
27.5.4 Other Applications
Potential applications of the bioelectronic nose are wide open in a variety of fields. The quality of our life can be improved through the development of fragrance and flavor industries, and the bioelectronic nose will be used to control the quality of their brands by constantly regulating fragrance and flavor. Process monitoring can be performed using the bioelectronic nose for the production of high-quality products. Another one is the security systems. Unwanted release of confidential documents can be detected by smell sensing. Regarding public safety, currently everybody is threatened with terrorism, for example, by explosives and toxicants. Peoples are concerned about the safety of public places, such as plazas, public buildings, subways, and airports, and drug smuggling is another severe issue threatening public health. Trained dogs are currently used for searching for explosives, toxicants, and drugs. However, an alternative method is needed to replace this method due to many limitations. The high cost of training and maintaining dogs is one limitation, and nose fatigue and the lifetime of dogs as a finder are other limitations. As described earlier, the bioelectronic nose has great potential for a myriad of applications.
27.6 Conclusion
The bioelectronic nose was designed using biological materials as a primary transducer and nonbiological materials as a secondary transducer. The primary transducer and secondary transducer play the role of a recognition element and a signal processing element, respectively.
Four types of primary transducers have been developed to enhance the selectivity of the bioelectronic nose. They are cell-, nanovesicle-, protein-, and peptide-based primary transducers. Cell- and nanovesicle-based primary transducers are excellent systems for mimicking the signaling pathway of the human olfactory system and discriminate odorant molecules with humanlike selectivity. However, in the case of cell-based systems, maintenance of the cell is not easy on the chip. In the case of the nanovesicle-based system, the small-sized vesicle is derived from live cells expressing OR and contains all components that are essential for olfactory signal transduction, such as G-proteins, cAMP, and ion-gated channels. The nanovesicle can be easily handled when it is applied to the secondary transducer, due to much smaller size, compared with the cells. However, the nanovesicle-based primary transducer is not reusable. The protein-based primary transducer uses OR proteins, which are produced from a heterologous expression system, such as an E. coli system. OR proteins are more stable, durable, and reusable than the nanovesicles under various measuring conditions. The OR proteins expressed in E. coli expression system may not have a normal tertiary structure. A refolding process is then required. As an alternative approach, peptides derived from OR proteins have been applied to the bioelectronic nose. The peptide-based primary transducer has the merit of high stability and is most suitable for commercialization.
Various kinds of devices have been used as a secondary transducer. The QCM can detect odor molecules with high selectivity and sensitivity by using various coating materials on a quartz crystal surface. However, it makes noise signals very sensitively responding to the environmental disturbance. The SPR can discriminate biomolecules without any labeling in real time, but the equipment for detection is expensive, and miniaturization for on-site measurement is not easy. The carbon nanotube and carboxylated polypyrrole nanotube using a FET extremely enhanced the sensitivity of the bioelectronic nose. The integration of the OR as a primary transducer with the FET sensor platform showed the detection of odor molecules with single-carbon-atomic resolution and femtomolar detection limits.
The bioelectronic nose using an OR was devised with a concept mimicking the human olfactory system, which is well known to recognize over ten thousands of odor molecules, through using only around 390 types of OR. So, the integration of all human OR on a single sensor platform gives the bioelectronic nose a powerful sensing ability, as well as realizing the human nose in vitro. The bioelectronic nose can be widely used for a huge variety of applications, for example, medical application for early diagnosis of severe diseases, food quality control, fragrance and flavor, environmental monitoring for the protection of the global environment and our health from toxic pollutants, and the maintenance of public safety, by blocking threats from terrorism, such as explosives and toxicants. Eventually, human welfare, as well as industrial development, is expected to be much improved through the development and application of the bioelectronic nose.
Acknowledgment
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT and Future Planning (No. 2015065103, 20110031870) and Ministry of Education (No. NRF-2013R1A1A2011174).
References
- 1. Lee, S.H. and Park, T.H. (2010) Recent advances in the development of bioelectronic nose. Biotechnol. Bioprocess Eng., 15 (1), 22–29.
- 2. Ko, H.J. (2015) Recent update of nanobiosensors using olfactory sensing elements and nanomaterials. Biosens. J., 4, 129. doi: 10.4172/2090-4967.1000129
- 3. Kim, T.H., Lee, S.H., Lee, J., Song, H.S. et al. (2009) Single-carbon-atomic-resolution detection of odorant molecules using a human olfactory receptor-based bioelectronic nose. Adv. Mater., 21 (1), 91–94.
- 4. Yoon, H., Lee, S.H., Kwon, O.S., Song, H.S. et al. (2009) Polypyrrole nanotubes conjugated with human olfactory receptors: high-performance transducers for FET-type bioelectronic noses. Angew. Chem. Int. Ed., 48 (15), 2755–2758.
- 5. Goldsmith, B.R., Mitala, J.J., Josue, J., Castro, A. et al. (2011) Biomimetic chemical sensors using nanoelectronic readout of olfactory receptor proteins. Am. Chem. Soc. Nano, 5 (7), 5408–5416.
- 6. Park, J., Lim, J.H., Jin, H.J., Namgung, S. et al. (2012) A bioelectronic sensor based on canine olfactory nanovesicle-carbon nanotube hybrid structures for the fast assessment of food quality. Analyst, 137 (14), 3249–3254.
- 7. Kwon, O.S., Song, H.S., Park, S.J., Lee, S.H. et al. (2015) An ultrasensitive, selective, multiplexed superbioelectronic nose that mimics the human sense of smell. Nano Lett., 15, 6559–6567.
- 8. Oh, E.H., Lee, S.H., Ko, H.J., Lim, J.H. et al. (2015) Coupling of olfactory receptor and ion channel for rapid and sensitive visualization of odorant response. Acta Biomater., 22, 1–7.
- 9. Lim, J.H., Oh, E.H., Park, J., Hong, S. et al. (2015) Ion-channel-coupled receptor-based platform for a real time measurement of G-protein-coupled receptor activities. Am. Chem. Soc. Nano, 9, 1699–1706.
- 10. Ko, H.J. and Park, T.H. (2005) Piezoelectric olfactory biosensor: ligand specificity and dose-dependence of an olfactory receptor expressed in a heterologous cell system. Biosens. Bioelectron., 20 (7), 1327–1332.
- 11. Sung, J.H., Ko, H.J., and Park, T.H. (2006) Piezoelectric biosensor using olfactory receptor protein expressed in Escherichia coli. Biosens. Bioelectron., 21 (10), 1981–1986.
- 12. Park, S.J., Kwon, O.S., Lee, S.H., Song, H.S. et al. (2012) Ultrasensitive flexible graphene based (FET)-type bioelectronic nose. Nano Lett., 12 (10), 5082–5090.
- 13. Song, H.S. and Park, T.H. (2011) Integration of biomolecules and nanomaterials: towards highly selective and sensitive biosensors. Biotechnol. J., 6 (11), 1310–1316.
- 14. Wang, J. (2005) Nanomaterial-based electrochemical biosensors. Analyst, 130 (4), 421–426.
- 15. Nambiar, S. and Yeow, J.T.W. (2010) Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron., 26 (5), 1825–1832.
- 16. Tombelli, S., Minnuni, M., and Mascini, M. (2005) Analytical applications of aptamers. Biosens. Bioelectron., 20 (12), 2424–2434.
- 17. Wu, T. (1999) A piezoelectric biosensor as an olfactory receptor for odour detection: electronic nose. Biosens. Bioelectron., 14 (1), 9–18.
- 18. Liedberg, B., Nylander, C., and Lunstrom, I. (1983) Surface plasmon resonance for gas detection and biosensing. Sens. Actuators, 4, 299–304.
- 19. Benilova, I., Chegel, V.I., Ushenin, Y.V., Vidic, J. et al. (2008) Stimulation of human olfactory receptor 17–40 with odorants probed by surface plasmon resonance. Eur. Biophys. J., 37 (6), 807–814.
- 20. Lee, S.H., Ko, H.J., and Park, T.H. (2009) Real-time monitoring of odorant-induced cellular reactions using surface plasmon resonance. Biosens. Bioelectron., 25 (1), 55–60.
- 21. Lee, J.Y., Ko, H.J., Lee, S.H., and Park, T.H. (2006) Cell-based measurement of odorant molecules using surface plasmon resonance. Enzyme Microb. Technol., 39 (3), 375–380.
- 22. Gao, X.P.A., Zheng, G., and Lieber, C.M. (2009) Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett., 10 (2), 547–552.
- 23. Malnic, B., Hirono, J., Sato, T., and Buck, L.B. (1999) Combinatorial receptor codes for odors. Cell, 96 (5), 713–723.
- 24. Buck, L. and Axel, R. (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65 (1), 175–187.
- 25. Niimuts, Y. and Nei, M. (2003) Evolution of olfactory receptor genes in the human genome. Proc. Natl. Acad. Sci. U. S. A., 100 (21), 12235–12240.
- 26. Glusman, G., Yanai, I., Rubin, I., and Lancet, D. (2001) The complete human olfactory subgenome. Genome Res., 11 (5), 685–702.
- 27. Zozulya, S., Echeverri, F., and Nguyen, T. (2001) The human olfactory receptor repertoire. Genome Biol., 2 (6), research0018.1–research0018.12.
- 28. Ben-Arie, N., Lancet, D., Tayler, C., Khen, M. et al. (1994) Olfactory receptor gene cluster on human chromosome 17: possible duplication of an ancestral receptor repertoire. Hum. Mol. Genet., 3 (2), 229–235.
- 29. Gilad, Y., Segre, D., Skorecki, K., Nachman, M.W. et al. (2000) Dichotomy of single-nucleotide polymorphism haplotypes in olfactory receptor genes and pseudogenes. Nat. Genet., 26 (2), 221–224.
- 30. Ko, H.J. and Park, T.H. (2006) Dual signal transduction mediated by a single type of olfactory receptor expressed in a heterologous system. Biol. Chem., 387 (1), 59–68.
- 31. Yang, H., Song, H.S., Ahn, S.R., and Park, T.H. (2015) Purification and functional reconstitution of human olfactory receptor expressed in Escherichia coli. Biotechnol. Bioprocess Eng., 20, 423–430.
- 32. Jin, H.J., Lee, S.H., Kim, T.H., Park, J. et al. (2012) Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens. Bioelectron., 35 (1), 335–341.
- 33. Son, M., Cho, D., Lim, J.H., Park, J. et al. (2015) Real-time monitoring of geosmin and 2-methylisoborneol, representative odor compounds in water pollution using bioelectronic nose with human-like performance. Biosens. Bioelectron., 74, 199–206.
- 34. Pick, H., Schmid, E.L., Tairi, A.-P., Ilegems, E. et al. (2005) Investigating cellular signaling reactions in single attoliter vesicles. J. Am. Chem. Soc., 127 (9), 2908–2912.
- 35. Lee, S.H., Kwon, O.S., Song, H.S., Park, S.J. et al. (2012) Mimicking the human smell sensing mechanism with an artificial nose platform. Biomaterials, 33 (6), 1722–1729.
- 36. Lim, J.H., Park, J., Ahn, J.H., Jin, H.J. et al. (2013) A peptide receptor-based bioelectronic nose for the real-time determination of seafood quality. Biosens. Bioelectron., 39 (1), 244–249.
- 37. Lee, S.H., Lim, J.H., Park, J., Hong, S. et al. (2015) Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron., 71, 179–185.
- 38. Wyszynski, B., Somboon, P., and Nakamoto, T. (2007) Pegylated lipids as coatings for QCM odor-sensors. Sens. Actuators B, 121 (2), 538–544.
- 39. Koshets, I.A., Kazantseva, Z.I., Shirshov, Y.M., Cherenok, S.A. et al. (2005) Calixarene films as sensitive coatings for QCM-based gas sensors. Sens. Actuators B, 106 (1), 177–181.
- 40. Oh, E.H., Song, H.S., and Park, T.H. (2011) Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb. Technol., 48 (6), 427–437.
- 41. Panigrahi, S., Sankaran, S., Mallik, S., Gaddam, B. et al. (2012) Olfactory receptor-based polypeptide sensor for acetic acid VOC detection. Mater. Sci. Eng. C, 32 (6), 1307–1313.
- 42. Harding, P.J., Hadingham, T.C., McDonnell, J.M., and Watts, A. (2006) Direct analysis of a GPCR-agonist interaction by surface plasmon resonance. Eur. Biophys. J., 35 (8), 709–712.
- 43. Szabo, A., Stolz, L., and Granzow, R. (1995) Surface plasmon resonance and its use in biomolecular interaction analysis (BIA). Curr. Opin. Struct. Biol., 5 (5), 699–705.
- 44. Liedberg, B., Nylander, C., and Lundström, I. (1995) Biosensing with surface plasmon resonance — how it all started. Biosens. Bioelectron., 10 (8), 1–6.
- 45. Alves, I., Park, C., and Hruby, V. (2005) Plasmon resonance methods in GPCR signaling and other membrane events. Curr. Protein Pept. Sci., 6 (4), 293–312.
- 46. Fägerstam, L.G., Frostell-Karlsson, Å., Karlsson, R., Persson, B. et al. (1992) Biospecific interaction analysis using surface plasmon resonance detection applied to kinetic, binding site and concentration analysis. J. Chromatogr. A, 597 (1), 397–410.
- 47. Homola, J., Yee, S.S., and Gauglitz, G. (1999) Surface plasmon resonance sensors: review. Sens. Actuators B, 54 (1), 3–15.
- 48. Navratilova, I., Besnard, J., and Hopkins, A.L. (2011) Screening for GPCR ligands using surface plasmon resonance. ACS Med. Chem. Lett., 2 (7), 549–554.
- 49. Chen, K., Obinata, H., and Izumi, T. (2010) Detection of G protein-coupled receptor-mediated cellular response involved in cytoskeletal rearrangement using surface plasmon resonance. Biosens. Bioelectron., 25 (7), 1675–1680.
- 50. Vidic, J.M., Grosclaude, J., Persuy, M.-A., Aioun, J. et al. (2006) Quantitative assessment of olfactory receptors activity in immobilized nanosomes: a novel concept for bioelectronic nose. Lab Chip, 6 (8), 1026–1032.
- 51. Vidic, J., Grosclaude, J., Monnerie, R., Persuy, M.-A. et al. (2008) On a chip demonstration of a functional role for odorant binding protein in the preservation of olfactory receptor activity at high odorant concentration. Lab Chip, 8 (5), 678–688.
- 52. Vidic, J., Pla-Roca, M., Grosclaude, J., Persuy, M.A. et al. (2007) Gold surface functionalization and patterning for specific immobilization of olfactory receptors carried by nanosomes. Anal. Chem., 79 (9), 3280–3290.
- 53. Lim, J.H., Park, J., Oh, E.H., Ko, H.J. et al. (2014) Nanovesicle-based bioelectronic nose for the diagnosis of lung cancer from human blood. Adv. Healthcare Mater., 3, 360–366.
- 54. Wysocki, C.J. and Preti, G. (2000) Human body odors and their perception. Jpn. J. Taste Smell Res., 7 (1), 19–42.
- 55. Ma, W., Liu, X., and Pawliszyn, J. (2006) Analysis of human breath with micro extraction techniques and continuous monitoring of carbon dioxide concentration. Anal. Bioanal. Chem., 385 (8), 1398–1408.
- 56. Kwon, O.S., Ahn, S.R., Park, S.J., Song, H.S. et al. (2012) Ultrasensitive and selective recognition of peptide hormone using close-packed arrays of hPTHR-conjugated polymer nanoparticles. Am. Chem. Soc. Nano, 6 (6), 5549–5558.
- 57. Kim, B., Song, H.S., Jin, H.J., Park, E.J. et al. (2013) Highly selective and sensitive detection of neurotransmitters using receptor-modified single-walled carbon nanotube sensors. Nanotechnology, 24 (28), 285501.
- 58. Kim, Y.D. and Morr, C.V. (1996) Dynamic headspace analysis of light activated flavor in milk. Int. Dairy J., 6 (2), 185–193.
- 59. Hu, M., McClements, D.J., and Decker, E.A. (2003) Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem., 51 (6), 1696–1700.
- 60. Gardner, H.W. (1989) Oxygen radical chemistry of polyunsaturated fatty acids. Free Radical Biol. Med., 7 (1), 65–86.
- 61. Shahidi, F. and Pegg, R.B. (1994) Hexanal as an indicator of meat flavor deterioration. J. Food Lipids, 1 (3), 177–186.
- 62. Ahn, J.H., Lim, J.H., Park, J., Oh, E.H. et al. (2015) Screening of target-specific olfactory receptor and development of olfactory biosensor for the assessment of fungal contamination in grain. Sens. Actuators B, 210, 9–16.
- 63. Wu, T.-Z. and Lo, Y.-R. (2000) Synthetic peptide mimicking of binding sites on olfactory receptor protein for use in ‘electronic nose’. J. Biotechnol., 80 (1), 63–73.
