Chapter 18
Synthetic Biology for Corynebacterium glutamicum: An Industrial Host for White Biotechnology
Han Min Woo
18.1 Introduction
Synthetic biology is a redefined biological discipline based on engineering concepts with computer-aided tools and has enabled construction of efficient biological systems under precise control by gene expression [1]. Metabolic engineering combined with synthetic biology has been boosted, constructing microbial cell factories that produce biochemicals for the replacement of petroleum-based chemicals from low-cost carbon feedstock without compromising the metabolic imbalances in a host. Synthetic biology-powered microbial cell factories require functional components including synthetic DNA parts, devices, chassis, and artificial network circuits for genetic regulation. These elements are assembled in a microbial cell factory in order to reprogram cellular properties (Figure 18.1).
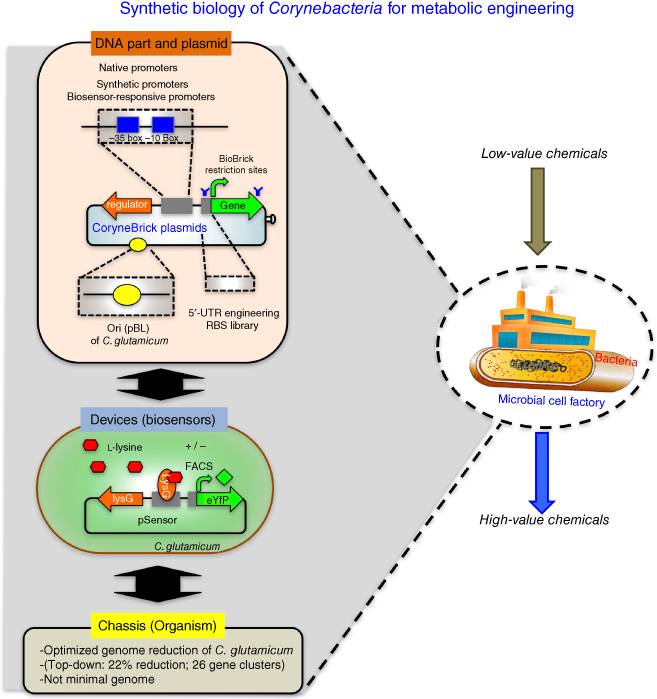
Figure 18.1 Synthetic biology platform for Corynebacterium glutamicum. For DNA parts, native promoters have been characterized and fully synthetic promoters for C. glutamicum have been developed. Small RNAs of C. glutamicum have been identified, and engineered antisense RNAs could be used to regulate the transcription of target genes. BioBrick restriction enzyme sites have been integrated in expression vectors of pTGR and CoryneBrick vectors (details in the text). Several replications of origins have been cloned to provide the different copies of the plasmid for different expression levels. For devices or biosensors, l-lysine-detecting metabolite sensor has been developed, based on the transcriptional regulator and its hybrid promoter. Native LysG of C. glutamicum activates the eYFP proteins whose signals were detected and sorted out by FACS. For chassis, optimum genome of C. glutamicum has been implemented to the wild-type by reducing 22% genome size (details in the text).
The engineering of Escherichia coli and Saccharomyces cerevisiae has been extensively applied with synthetic biology platform because they are relatively versatile hosts with numerous genetic tools available. As a result, genetically modified E. coli and S. cerevisiae have been shown capabilities of producing advanced biofuels, materials, and pharmaceuticals. Besides E. coli and S. cerevisiae, new opportunities in synthetic biology have been explored with Bacillus subtilis [2], Pichia pastoris [3], Streptomyces avermitilis [4], Pseudomonas putida KT2440 [5], and cyanobacteria [6].
Corynebacterium glutamicum is a Gram-positive, facultative anaerobic industrial host (GRAS: generally recognized as safe) that is used for large-scale industrial production of the flavor enhancer l-glutamate (2.93 million tons in 2012) and the food additive l-lysine (2.2 million tons in 2015). In addition, metabolic engineering has been applied to C. glutamicum for producing a variety of other commercially interesting chemicals and materials from renewable resources [7–10]. Recent reviews covering engineering of C. glutamicum have been focused on biological production of natural or nonnatural chemicals and materials [11–13] and its systems biology and physiology [14]. In this chapter, I describe on development of synthetic biology platform for C. glutamicum toward microbial cell factories for white biotechnology.
18.2 Synthetic Elements of Synthetic Biology for C. glutamicum
18.2.1 DNA Parts and Plasmids of Synthetic Biology for C. glutamicum
18.2.1.1 DNA Parts for C. glutamicum
DNA parts are usually considered DNA sequences with features for biological activities. DNA parts deposited at the main distributor Registry of Standard Biological Parts (RSBP; www.parts.igem.org) are classified as promoters, ribosome binding sites, protein coding sequences that encode particular proteins, and terminators for each element of transcription and translation of gene expression.
Promoter is one of the crucial DNA parts essential for gene transcription. The series of promoters in C. glutamicum have been studied with sigma subunits of RNA polymerase and gene expression profiles, using fluorescent proteins. First, endogenous promoters were selected, and its sequences were analyzed for identifying consensus sequence for RNA polymerase of C. glutamicum [15, 16]. As a result, DNA sequences of the promoter regions are well conserved at −10 regions (as tgngnTA(c/t)aaTgg), but the promoter sequences at −35 regions are less conserved. In addition, σ-factor-dependent promoters were dissected into seven groups with multiple promoters [15]. Strong constitutive promoters such as the gapA promoter (encoding glyceraldehyde 3-phosphate dehydrogenase), tuf promoter (encoding the translational elongation factor EF-Tu), and sodA (encoding manganese superoxide dismutase) were extensively used for gene expression in an expression plasmid or for replacement of the native promoters. These strategies have been applied to many biotechnological applications that were discussed in a recent review [17].
Recently, the library of synthetic promoters with leaderless transcripts has been constructed, and green fluorescent protein was used as reporter to screen the spectrum of synthetic promoters using fluorescent-activated cell sorting (FACS) [18]. The resulting synthetic promoters led to the constitutive gene expression of the target gene, and they were applied to high-level production of endoxylanase and antibody fragments. The synthetic promoter-based expression cassettes have been constructed for C. glutamicum to produce cadaverine [19]. Together with 2D proteomic analysis for extracellular proteome, the synthetic promoters were combined with signal peptide sequences for efficient platform of secretory production of recombinant proteins in C. glutamicum [20].
Secondly, engineering of synthetic pathways in C. glutamicum needs predictable promoters to adjust gene expression strengths for optimal gene expression of target genes. To develop new promoters, a synthetic promoter library for T7 RNA polymerase can be used as useful DNA parts for tunable gene expression in C. glutamicum since T7 RNA polymerase-dependent gene expression system for C. glutamicum has been developed [21]. Depending on the dosage of the inducer, that is, IPTG, expressions of the reporter gene of eYFP were tightly regulated and 450-fold changes of the specific fluorescence can be achieved with un-induced state. Other DNA parts such as 5′-UTRs, ribosome binding sites, and terminators could also be used to design tunable gene expression in C. glutamicum.
18.2.1.2 Synthetic Platform of Plasmids for C. glutamicum
In addition to constitutive gene expressions using either endogenous or synthetic promoters, controllable gene expression systems in synthetic biology platforms are also in high demand for optimizing target genes in synthetic pathways. Ninety-six different BioBrick (four unique restriction enzyme sites; EcoRI, BglII, BamHI, and XhoI) compatible plasmids for E. coli have been developed as standard DNA elements in a synthetic biology platform for gene expression [22]. These include a variety of origins of replication, controllable promoters, and ribosomal binding sites (RBS). These BglBrick vectors provide quantitative data according to induction systems, culture media, and incubation time.
Together with engineering endogenous plasmids in C. glutamicum [23] with well-known E. coli vectors, there are chances to choose mutated versions of conventional expression vectors of C. glutamicum for enhanced expression of desired genes [24]. A plasmid-based platform of synthetic biology (called pTGR) with corynebacterial DNA elements for C. glutamicum was developed and tested with the two reporter genes eGFP and mCherry [25]. With pTGR plasmids, gene of interests can be transcribed under either constitutive promoters or inducible tac promoter. These plasmids are predictive and easy for gene expression studies. To assemble DNA fragments, pTGR utilizes unique enzyme sites: XbaI and NheI for promoter, NheI and NdeI for RBS, NdeI and EcoRI/AvrII for the target gene, and AvrII and SpeI for transcriptional terminator (tt). Co-expression of multiple genes in pTGR can be assembled. Another type of synthetic platform for gene expression in C. glutamicum was also developed based on BglBrick vectors: CoryneBrick vectors [26]. They were applied for the reconstruction of xylose utilization by expressing multiple genes of xylose isomerase and xylulose kinase. In addition, a tetracycline-inducible gene expression vector, pCLTON1, showed a tightly controllable gene expression in C. glutamicum without leaky expression [27] and allowed gradual GFP expression based upon the level of anhydrotetracycline as inducer.
18.2.2 Devices and Genetic Biosensors of Synthetic Biology for C. glutamicum
Transcriptional regulator regulates the level of gene expression by altering transcription rates in a local genetic circuit or in a global network by different class of regulators. DNA elements widely used in a plasmid were derived from a bacterial, transcriptional, regulation system including LacI and TetR. Thus, engineering of sensor-regulator system to optimize cellular resources in a host would allow the cell to efficiently utilize carbon sources and produce target chemicals. For example, native transcriptional factor (TF) [28] or artificial genetic sensor combined with TFs [29] has been successfully implemented in E. coli for optimizing gene expressions
For development of synthetic biosensor in C. glutamicum, its native transcriptional regulators are the first choices to make up the regulatory system to control gene expressions of interest. Such database of the regulatory gene repertoire and the transcriptional regulatory network in C. glutamicum are informative for constructing the genetic sensors used in metabolic engineering and synthetic biology. This has been reviewed in detail [30]. When native transcriptional regulation is applied for the role of synthetic genetic sensors, the inherent regulatory mode of the transcriptional regulators must be carefully considered due to their complex network. For instance, global regulators (e.g., GlxR) control the expression of a large number of genes, either directly or indirectly, by hierarchical cross-regulation. A mutant of GlxR showed more than 100 changed gene expressions [31], and 439 genes were considered to be putative regulons after a bioinformatics search. In addition, the master regulators, RamA [32] and RamB [33], are involved in acetate metabolism, and SugR [34] controls central carbon metabolism. GntR1 and GntR2 regulators control the utilization of gluconate and regulate more than 50 genes including the ptsG and ptsS genes [35], while UriR and RbsR regulators control the utilization of uridine and ribose [36]. Local regulators FruR and LldR regulate the utilization of fructose and l-lactate [37], while PrpR and the two-component system CitAB control the utilization of propionate and citrate [38].
Due to the industrial importance of C. glutamicum as an amino acid host, genetic biosensors have been developed using native transcriptional regulators that recognize the amino acids [39–41]. These biosensor devices were combined with fluorescent protein to create a high-throughput screening and applied for laboratory adaptive evolution after random mutagenesis. Assisted with FACS, transcriptional regulator LysG that activates the expression of the l-lysine transporter LysE when the internal level of l-lysine is elevated has been used as a genetic biosensor in C. glutamicum to screen out the best l-lysine producer. Thus, a synthetic C. glutamicum LysG-biosensor has been engineered with eYFP fluorescence protein. Subsequently, it was applied to isolate l-lysine-producing mutants [39]. Similarly, the Lrp-based biosensor, in which the transcriptional regulator Lrp activates the expression of the brnFE operon encoding two-component permease in the presence of increased levels of branched-chain amino acids, also has been used for isolation of mutants to produce high amount of target branched-chain amino acids, including l-methionine and l-valine [40]. As a result, the top five mutants with mutations on pyruvate dehydrogenase complex produced up to 8 mM l-valine. In addition, in vivo biosensor-based enzyme screening method was applied to identify the mutations at key enzymes (LysC and HisG) in the arginine biosynthesis pathway [41]. Besides amino acid-related transcriptional regulators, those metabolite sensors, used as synthetic biology devices, will show potential for application of metabolic engineering of C. glutamicum.
18.2.3 Synthetic Biology of a Chassis for C. glutamicum
Chassis is a living platform to supply the components and has a genome that encodes biological function for cell growth and self-maintenance. B. subtilis and E. coli have been used for long time as a laboratory strain with extensive studies. B. subtilis and E. coli minimal genome strains have been developed since essential genes were exploited as building blocks. However, current engineering does not come up the levels that nature has evolved bacteria to develop novel features and advantages. Thus, different chassis have been developed with unique bacterial traits. For example, pseudomonas has been a model of environmental bacteria and biotechnological applications [42], and photosynthetic bacteria Synechococcus sp. PCC 6803 and Synechococcus elongatus PCC7942 have been used for a phototrophic bio-solar cell factory to convert CO2 to various chemicals [6, 43]. Recently, a methylotrophic bacterium, Methylobacterium extorquens AM1, has been extensively studied for conversion of C1 compounds to high value chemicals [44].
For C. glutamicum, large fragments of bacteriophage or phage-related elements have been deleted to prevent the genetically modified strains from genome rearrangements and genetic instabilities. A prophage-free variant of C. glutamicum ATCC 13032 showed a 6% reduction of genome [45]. Ethylene glycol has been produced in engineered C. glutamicum derived from a chassis of C. glutamicum MB001 strain [46]. Moreover, the top-down construction of a chassis of C. glutamicum has been applied, and 22% of genome-reduced C. glutamicum has been constructed and ready for biotechnological application [47].
18.3 Conclusion and Outlook
Recent studies described in this chapter have involved development of synthetic DNA parts, functional devices, and genome-reduced chassis for C. glutamicum. For construction of efficient microbial cell factory, new genetic tools and application of synthetic biology will appear soon for C. glutamicum. Especially, CRISPR-guided genome editing technology and its related gene regulation technology will boost engineering of microbial cell factories [48]. Combining with metabolic engineering, artificial genetic network and biosensors could be integrated in a chassis of C. glutamicum to dynamically regulate gene expressions in either native or heterologous pathway by optimizing metabolic balances and maximizing the production of the desired targets. Currently, the research community has attention on the use of C. glutamicum for biotechnological application and has moved toward development of synthetic biology for C. glutamicum.
References
- 1. Lee, S.Y. (2012) Metabolic engineering and synthetic biology in strain development. ACS Synth. Biol., 1, 491–492.
- 2. van Dijl, J.M. and Hecker, M. (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb. Cell Fact., 12, 3.
- 3. Vogl, T., Ruth, C., Pitzer, J., Kickenweiz, T., and Glieder, A. (2014) Synthetic core promoters for Pichia pastoris. ACS Synth. Biol., 3, 188–191.
- 4. Komatsu, M., Komatsu, K., Koiwai, H., Yamada, Y., Kozone, I., Izumikawa, M., Hashimoto, J., Takagi, M., Omura, S., Shin-ya, K. et al (2013) Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth. Biol., 2, 384–396.
- 5. Belda, E., van Heck, R.G., Lopez-Sanchez, M.J., Cruveiller, S., Barbe, V., Fraser, C., Klenk, H.P., Petersen, J., Morgat, A., Nikel, P.I. et al. (2016) The revisited genome of Pseudomonas putida KT2440 enlightens its value as a robust metabolic chassis. Environ. Microbiol. doi: 10.1111/1462-2920.13230
- 6. Atsumi, S., Higashide, W., and Liao, J.C. (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol., 27, 1177–1180.
- 7. Kim, E.-M., Um, Y., Bott, M., Woo, H.M., and Sauer, M. (2015) Engineering of Corynebacterium glutamicum for growth and succinate production from levoglucosan, a pyrolytic sugar substrate. FEMS Microbiol. Lett., 362, fnv161.
- 8. Lee, J., Saddler, J.N., Um, Y., and Woo, H.M. (2016) Adaptive evolution and metabolic engineering of a cellobiose- and xylose- negative Corynebacterium glutamicum that co-utilizes cellobiose and xylose. Microb. Cell Fact., 15, 20.
- 9. Choo, S., Um, Y., Han, S.O., and Woo, H.M. (2016) Engineering of Corynebacterium glutamicum to utilize methyl acetate, a potential feedstock derived by carbonylation of methanol with CO. J. Biotechnol., 224, 47–50.
- 10. Lee, J., Sim, S.J., Bott, M., Um, Y., Oh, M.K., and Woo, H.M. (2014) Succinate production from CO2-grown microalgal biomass as carbon source using engineered Corynebacterium glutamicum through consolidated bioprocessing. Sci. Rep., 4, 5819.
- 11. Becker, J. and Wittmann, C. (2012) Bio-based production of chemicals, materials and fuels – Corynebacterium glutamicum as versatile cell factory. Curr. Opin. Biotechnol., 23, 631–640.
- 12. Wieschalka, S., Blombach, B., Bott, M., and Eikmanns, B.J. (2013) Bio-based production of organic acids with Corynebacterium glutamicum. Microb. Biotechnol., 6, 87–102.
- 13. Wendisch, V.F., Bott, M., and Eikmanns, B.J. (2006) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr. Opin. Biotechnol. Microbiol., 9, 268–274.
- 14. Bott, M. and Niebisch, A. (2003) The respiratory chain of Corynebacterium glutamicum. J. Biotechnol., 104, 129–153.
- 15. Pátek, M. and Nesvera, J. (2011) Sigma factors and promoters in Corynebacterium glutamicum. J. Biotechnol., 154, 101–113.
- 16. Pátek, M., Nesvera, J., Guyonvarch, A., Reyes, O., and Leblon, G. (2003) Promoters of Corynebacterium glutamicum. J. Biotechnol., 104, 311–323.
- 17. Pátek, M., Holatko, J., Busche, T., Kalinowski, J., and Nesvera, J. (2013) Corynebacterium glutamicum promoters: a practical approach. Microb. Biotechnol., 6, 103–117.
- 18. Yim, S.S., An, S.J., Kang, M., Lee, J., and Jeong, K.J. (2013) Isolation of fully synthetic promoters for high-level gene expression in Corynebacterium glutamicum. Biotechnol. Bioeng., 110, 2959–2969.
- 19. Oh, Y.H., Choi, J.W., Kim, E.Y., Song, B.K., Jeong, K.J., Park, K., Kim, I.-K., Woo, H.M., Lee, S.H., and Park, S.J. (2015) Construction of synthetic promoter-based expression cassettes for the production of cadaverine in recombinant Corynebacterium glutamicum. Appl. Biochem. Biotechnol., 176, 2065–2075.
- 20. Yim, S.S., Choi, J.W., Lee, R.J., Lee, Y.J., Lee, S.H., Kim, S.Y., and Jeong, K.J. (2016) Development of a new platform for secretory production of recombinant proteins in Corynebacterium glutamicum. Biotechnol. Bioeng., 113, 163–172.
- 21. Kortmann, M., Kuhl, V., Klaffl, S., and Bott, M. (2015) A chromosomally encoded T7 RNA polymerase-dependent gene expression system for Corynebacterium glutamicum: construction and comparative evaluation at the single-cell level. Microb. Biotechnol., 8, 253–265.
- 22. Lee, T.S., Krupa, R.A., Zhang, F., Hajimorad, M., Holtz, W.J., Prasad, N., Lee, S.K., and Keasling, J.D. (2011) BglBrick vectors and datasheets: a synthetic biology platform for gene expression. J. Biol. Eng., 5, 12.
- 23. Tauch, A., Puhler, A., Kalinowski, J., and Thierbach, G. (2003) Plasmids in Corynebacterium glutamicum and their molecular classification by comparative genomics. J. Biotechnol., 104, 27–40.
- 24. Nesvera, J., Pátek, M., Hochmannova, J., Abrhamova, Z., Becvarova, V., Jelinkova, M., and Vohradsky, J. (1997) Plasmid pGA1 from Corynebacterium glutamicum codes for a gene product that positively influences plasmid copy number. J. Bacteriol., 179, 1525–1532.
- 25. Ravasi, P., Peiru, S., Gramajo, H., and Menzella, H.G. (2012) Design and testing of a synthetic biology framework for genetic engineering of Corynebacterium glutamicum. Microb. Cell Fact., 11, 147.
- 26. Kang, M.-K., Lee, J., Um, Y., Lee, T.S., Bott, M., Park, S.J., and Woo, H.M. (2014) Synthetic biology platform of CoryneBrick vectors for gene expression in Corynebacterium glutamicum and its application to xylose utilization. Appl. Microbiol. Biotechnol., 98, 5991–6002.
- 27. Lausberg, F., Chattopadhyay, A.R., Heyer, A., Eggeling, L., and Freudl, R. (2012) A tetracycline inducible expression vector for Corynebacterium glutamicum allowing tightly regulable gene expression. Plasmid, 68, 142–147.
- 28. Zhang, F. and Keasling, J. (2011) Biosensors and their applications in microbial metabolic engineering. Trends Microbiol., 19, 323–329.
- 29. Chou, H.H. and Keasling, J.D. (2013) Programming adaptive control to evolve increased metabolite production. Nat. Commun., 4, 2595.
- 30. Schroder, J. and Tauch, A. (2010) Transcriptional regulation of gene expression in Corynebacterium glutamicum: the role of global, master and local regulators in the modular and hierarchical gene regulatory network. FEMS Microbiol. Rev., 34, 685–737.
- 31. Moon, M.W., Park, S.Y., Choi, S.K., and Lee, J.K. (2007) The phosphotransferase system of Corynebacterium glutamicum: features of sugar transport and carbon regulation. J. Mol. Microbiol. Biotechnol., 12, 43–50.
- 32. Cramer, A., Gerstmeir, R., Schaffer, S., Bott, M., and Eikmanns, B.J. (2006) Identification of RamA, a novel LuxR-type transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol., 188, 2554–2567.
- 33. Cramer, A., Auchter, M., Frunzke, J., Bott, M., and Eikmanns, B.J. (2007) RamB, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum, is subject to regulation by RamA and RamB. J. Bacteriol., 189, 1145–1149.
- 34. Engels, V. and Wendisch, V.F. (2007) The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J. Bacteriol., 189, 2955–2966.
- 35. Frunzke, J., Engels, V., Hasenbein, S., Gätgens, C., and Bott, M. (2008) Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol. Microbiol., 67, 305–322.
- 36. Nentwich, S.S., Brinkrolf, K., Gaigalat, L., Huser, A.T., Rey, D.A., Mohrbach, T., Marin, K., Puhler, A., Tauch, A., and Kalinowski, J. (2009) Characterization of the LacI-type transcriptional repressor RbsR controlling ribose transport in Corynebacterium glutamicum ATCC 13032. Microbiology, 155, 150–164.
- 37. Gao, Y.G., Suzuki, H., Itou, H., Zhou, Y., Tanaka, Y., Wachi, M., Watanabe, N., Tanaka, I., and Yao, M. (2008) Structural and functional characterization of the LldR from Corynebacterium glutamicum: a transcriptional repressor involved in l-lactate and sugar utilization. Nucleic Acids Res., 36, 7110–7123.
- 38. Brocker, M., Schaffer, S., Mack, C., and Bott, M. (2009) Citrate utilization by Corynebacterium glutamicum is controlled by the CitAB two-component system through positive regulation of the citrate transport genes citH and tctCBA. J. Bacteriol., 191, 3869–3880.
- 39. Binder, S., Schendzielorz, G., Stäbler, N., Krumbach, K., Hoffmann, K., Bott, M., and Eggeling, L. (2012) A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol., 13, R40.
- 40. Mustafi, N., Grünberger, A., Kohlheyer, D., Bott, M., and Frunzke, J. (2012) The development and application of a single-cell biosensor for the detection of l-methionine and branched-chain amino acids. Metab. Eng., 14, 449–457.
- 41. Schendzielorz, G., Dippong, M., Grünberger, A., Kohlheyer, D., Yoshida, A., Binder, S., Nishiyama, C., Nishiyama, M., Bott, M., and Eggeling, L. (2014) Taking control over control: use of product sensing in single cells to remove flux control at key enzymes in biosynthesis pathways. ACS Synth. Biol., 3, 21–29.
- 42. Nikel, P.I., Martínez-García, E., and de Lorenzo, V. (2014) Biotechnological domestication of pseudomonads using synthetic biology. Nat. Rev. Microbiol., 12, 368–379.
- 43. Chwa, J.-W., Kim, W.J., Sim, S.J., Um, Y., and Woo, H.M. (2016) Engineering of a modular and synthetic phosphoketolase pathway for photosynthetic production of acetone from CO2 in Synechococcus elongatus PCC 7942 under light and aerobic condition. Plant Biotechnol. J. doi: 10.1111/pbi.12536
- 44. Ochsner, A.M., Sonntag, F., Buchhaupt, M., Schrader, J., and Vorholt, J.A. (2014) Methylobacterium extorquens: methylotrophy and biotechnological applications. Appl. Microbiol. Biotechnol., 99, 517–534.
- 45. Baumgart, M., Unthan, S., Ruckert, C., Sivalingam, J., Grunberger, A., Kalinowski, J., Bott, M., Noack, S., and Frunzke, J. (2013) Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl. Environ. Microbiol., 79, 6006–6015.
- 46. Chen, Z., Huang, J., Wu, Y., and Liu, D. (2016) Metabolic engineering of Corynebacterium glutamicum for the de novo production of ethylene glycol from glucose. Metab. Eng., 33, 12–18.
- 47. Unthan, S., Baumgart, M., Radek, A., Herbst, M., Siebert, D., Bruhl, N., Bartsch, A., Bott, M., Wiechert, W., Marin, K. et al (2015) Chassis organism from Corynebacterium glutamicum--a top-down approach to identify and delete irrelevant gene clusters. Biotechnol. J., 10, 290–301.
- 48. Cleto, S., Jensen, J.V., Wendisch, V.F., and Lu, T.K. (2016) Corynebacterium glutamicum Metabolic Engineering with CRISPR Interference (CRISPRi). ACS Synth. Biol., 5, 375–385.
