Chapter 7
Production of Valuable Phenolic Compounds from Lignin by Biocatalysis: State-of-the-Art Perspective
>Somchart Maenpuen, Ruchanok Tinikul, Pirom Chenprakhon and Pimchai Chaiyen
7.1 Lignin and Its Composition
7.1.1 Composition of Lignin
Lignocellulose is the most abundant (bio)chemical found in nature. It is composed of ∼50% of cellulose, 20–35% of hemicellulose, and ∼15–30% of lignin. Lignin consists of amorphous rigid networks of phenylpropane units. The majority of lignin aromatic building blocks are syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H) moieties (Figure 7.1) that are condensation products of the corresponding monolignols – sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol (Figure 7.1). These aryl alcohols are tightly linked via covalent bonds [1–4] of carbon–carbon, ether, aryl ether, or biphenyl ether linkages [1, 3, 5–9]. The most predominant linkage found in lignin complexes of softwood and hardwood is a Cβ−O−C4 aryl ether linkage, which typically accounts for approximately 50% of the bonds in lignin (Figure 7.1). Other major linkages include Cβ−C5, Cβ−C1, Cβ−Cβ, C5−C5, and C4−O−C5 bonds [3, 10]. Due to the wide variety of linkages formed, the structural nature of lignin is highly amorphous and highly variable depending on the type of plant from which it was formed. Among the three major components of lignocellulose, lignin is the only feedstock that can provide aromatic monomers. Although lignin is abundant and found in quantities scalable for industrial process, its role as an industrial feedstock is currently underutilized. Therefore, better technology and processes to convert lignin into industrially relevant or high value-added compounds are needed.
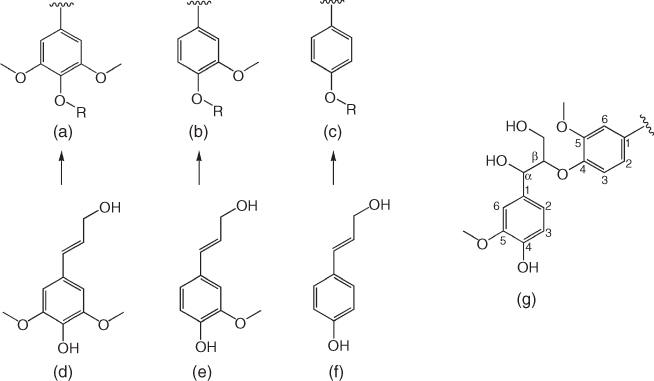
Figure 7.1 Chemical structures of syringyl (a), guaiacyl (b), and p-hydroxyphenyl (c) moieties that are derived from their corresponding monolignols – sinapyl alcohol (d), coniferyl alcohol (e), and p-coumaryl alcohol (f). Structure on the right shows the Cβ−O−C4−aryl ether linkages (g).
7.1.2 Process to Convert Lignin into Aromatic Monomers
7.1.2.1 Extraction of Lignin from Lignocellulose
In order to overcome the recalcitrant structure of lignocellulose and to obtain lignin from plant biomass, a number of pretreatment procedures have been established. These processes can be classified as either chemical or physicochemical pretreatments. For chemical pretreatments, plant biomass containing lignocellulose is treated with chemical reagents including acids (i.e., sulfuric acid), bases (i.e., sodium hydroxide), sulfites, organic solvents such as ethanol in the Alcell process, or ionic liquids such as imidazolium ion to generate lignins. The corresponding lignins generated from these processes are defined according to the pretreatment conditions: Klason lignin (acid treatment), Kraft lignin (base treatment), lignosulfonate lignin (sulfite treatment), organosolv lignin (organic solvent treatment), and ion liquid lignin [3, 7, 11, 12]. Among these processes, the pretreatments that use organic solvents and ionic liquids under mild conditions are the most environmentally friendly protocols. These processes also yield lignin with minimal changes in its native structure and with intact phenylpropane moieties. These two chemical pretreatment processes are regarded as promising methods for future use in lignin biorefinery [2, 3, 7, 12–14]. However, it should be noted that these processes are high cost because solvent and ionic liquid used cannot be recycled.
Lignin can also be extracted from lignocellulose using physicochemical pretreatments. In these processes, plant biomass is subjected to both physical force and treatment with chemical reagents. The ball milling or Björkman method uses a ball mill and a neutral solvent such as dioxane for extraction. The pyrolysis method uses high temperature and a gas feed. Steam explosion methods use high pressure and water, while ammonia fiber explosion (AFEX) uses high pressure and ammonia for extraction. A mixed process of mechanocatalysis uses ball milling and hydrochloric or sulfuric acids for extraction. Lignin produced from each of these processes is classified according to the extraction protocol, such as milled wood lignin (MWL), pyrolysis lignin, steam explosion lignin, AFEX lignin, and sulfur-free lignin (from mechanocatalysis) [3, 12, 15]. Among the processes mentioned, the ball milling or Björkman method is the most promising method that provides isolated lignin polymers with the most conserved native structure [3]. The process using ball milling in combination with chemical treatments such as acid, base, or neutral solvent is efficient for reducing particle size and crystallinity. It can also be scalable and adapted for wet and dried samples. The method resulted in good yield in lab scale but needs further development for real use in industries [16, 17].
7.1.2.2 Deconstruction of Lignin Using Physicochemical Processes
Due to the amorphous nature of lignin and its lack of a well-defined architecture, the mechanism of lignin deconstruction has remained unclear and elusive. As the most abundant linkage found in the lignin complexes of softwood and hardwood is a Cβ−O−C4 aryl ether linkage, processes to efficiently deconstruct the lignin polymer must be targeted to disrupt this bond. Recent reports based on computational calculations using density functional theory (DFT), molecular dynamics, and quantum mechanics indicate that the dissociation energy of this bond is relatively small (∼268 kJ/mol) [18, 19]. Various types of pretreatments have been developed to depolymerize lignin in order to obtain monolignols and other phenolic derivatives. Processes including chemical and physicochemical and biological and biochemical depolymerizations have been exploited for lignin deconstruction [3, 7, 20, 21]. Most of the chemical and physicochemical methods for lignin deconstruction result in phenylpropanoid derivatives that are mostly of the guaiacol type. Different deconstruction processes result in different products, as summarized in Figure 7.2. Although lignin deconstruction by chemical and physicochemical methods can be used in real industrial processes, these processes demand high energy consumption and are not as environmentally friendly as compared with the biological and biochemical depolymerization methods discussed later.
7.1.2.3 Deconstruction of Lignin Using Biological Processes
White-rot and brown-rot fungi isolated from decayed wood and bacteria isolated from the guts of wood-boring beetles and termites are among the major species reported to possess the inherent ability to degrade lignin to its monomeric units, which are high value-added phenolic derivatives [22–25]. A large number of bacteria were reported to be able to degrade Kraft lignin, a processed lignin derived from alkaline pretreatment of biomass and generally found in pulping waste. Typical valuable phenolic derivatives that are derived from degradation of Kraft lignin by bacteria include guaiacol, ferulic acid, gallic acid, cinnamic acid, vanillic acid, vanillin, benzaldehyde, and benzoic acid (see Table 7.1). These compounds can be further used as precursors for the generation of high value-added chemicals (discussed later). For lignin degradation in fungi (Phanerochaete chrysosporium, Botrytis cinerea, and Stropharia coronilla), fungal extracellular enzymes such as laccases, lignin peroxidases (LiP), and β-aryl ether-cleaving enzyme play important roles in the degradation of wood lignin via radical and non-radical mechanisms [22–24]. In addition to depolymerization by microbes, in vitro lignin deconstruction by enzymatic reactions has gained increasing interest because the process can be more tightly controlled in order to more readily obtain specific products as compared with in vivo enzymatic reactions.
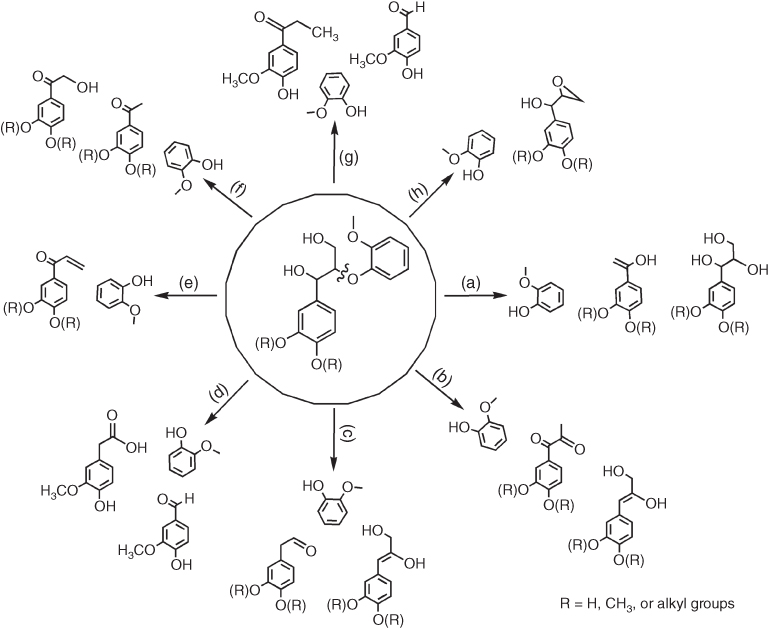
Figure 7.2 Overview summary of the phenylpropanoid derivatives resulting from different chemical processes (a, alkali; b, acid; c, ionic liquid; d, supercritical fluid; e, metal) and physicochemical processes (f, photochemistry; g, thermochemistry; h, mechanochemistry) used for the deconstruction of lignin model compounds containing Cβ−O−C4 aryl ether linkages.
Table 7.1 Examples of phenolic compounds derived from lignin deconstruction and their potential applications
| Product name | Chemical structure | Potential applications |
| Guaiacol |  |
A precursor for chemical synthesis of vanillin [26] |
| Vanillin |  |
A precursor for polyethylene terephthalate (PET) synthesis [10]A flavoring agent for food, beverage, perfumes, and pharmaceuticals [27]A potent suppressor for cancer cell migration and metastasis [28] |
| Vanillic acid | 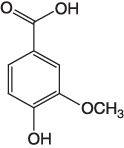 |
A precursor for synthesis of ester monomers and polyesters [10] |
| Vanillyl alcohol | 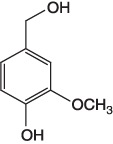 |
A precursor for synthesis of long-chain diene monomers [10] |
| p-Hydroxybenzoic acid |  |
A potent antiradical and anti-peroxide compound [29]A precursor for synthesis of potent antioxidants, that is, protocatechuic acid and gallic acid by enzymatic reaction [30] |
| Salicylic acid |  |
Anticancer agent [31, 32] |
| Gallic acid |  |
– Versatile biological activities including antioxidant, anticancer, anti-inflammatory, antimicrobial, anti-melanogenic, antiviral, antiallergic activities [33] – Ester derivatives are widely used in cosmetics and food [33]Antiproliferative and cytotoxic activities against cancer cells [34] |
| Cinnamic acid | 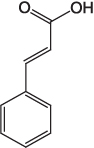 |
A precursor for polyester synthesis [10] |
| p-Coumaric acid |  |
Antioxidant [35]An anti-melanogenic agent [36]A precursor for synthesis of antiradical and antioxidant additives for prevention of oxidative damage of phenolic polymers [37] A precursor for polyester synthesis [10] |
| Ferulic acid |  |
A potent antioxidant [38–40] A precursor for synthesis of 4-vinylguaiacol, a precursor of styrene synthesis [41, 42] |
| Phenylacetic acid | 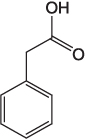 |
A precursor for penicillin synthesis [43] A flavoring and aroma agent, that is, honey-like odor [44] |
| 3,4-Dihydroxyphenylacetic acid |  |
Antioxidant [45] |
Laccase and peroxidase are among the top enzymes that are efficient in catalyzing lignin deconstruction. These enzymes are mostly extracellular enzymes that are secreted from fungi and bacteria. Laccase belongs to the polyphenol oxidase family. The enzyme contains a four-copper(II) cluster that can initiate radical formation of the lignin phenolic substrates by transferring one electron from the substrate to Cu2+, thus generating a substrate radical species and Cu+ [21, 46–49]. Laccases that are found in Streptomyces can degrade different types of lignins such as those from Miscanthus x giganteus lignocellulosic biomass, ethanosolv lignin, and Cβ−O−C4 lignin model compounds in both in vivo and in vitro assays [21, 50]. Class II peroxidases are H2O2-dependent enzymes with high redox potentials. Enzymes that can use lignin as a substrate include LiP, manganese peroxidase (MnP), and versatile peroxidase (VP). LiP (EC 1.11.1.14) contains a heme prosthetic group that promotes the formation of an aryl radical cation intermediate that can deconstruct the lignin model compound. MnP (EC 1.11.1.13) is a heme peroxidase that contains a Mn(II) as an additional cofactor to the heme group. VP (EC 1.11.1.16) is a heme peroxidase that combines together the catalytic features of LiP and MnP to give an enzyme with a broad range of substrate utilization capability [24, 46, 47]. Similar to the Cu(II)-dependent laccase reaction, the enzyme-bound transition metals (Fe(III) or Mn(II)) of LiP, MnP, and VP promote radical formation of the lignin substrate to initiate destabilization of the lignin structure [22, 47]. The LiP reaction has been studied using veratryl-glycerol-β-guaiacyl ether, a lignin model compound that is ubiquitously found (∼50–60%) in lignin complexes. This compound contains a common Cβ−O−C4 aryl ether linkage. Reaction with LiP demonstrated that a variety of cleavage products such as vanillin, guaiacol-type compounds, and various phenylpropanoid derivatives could be generated from this substrate (see Table 7.1) [22].
In addition to laccase and peroxidases, another enzyme that is important for lignin deconstruction is β-etherase. This enzyme is a non-radical ligninolytic enzyme that belongs to the glutathione-S-transferase superfamily. β-Etherase catalyzes the glutathione-dependent reductive β-aryl ether cleavage of lignin polymers. Use of a lignin model compound, veratryl-glycerol-β-guaiacyl ether containing a Cβ−O−C4 aryl ether linkage, as a substrate for the β-etherase reaction indicated that the cleavage products are guaiacol-type derivatives and a phenylpropanoid–glutathione adduct [24, 25, 47]. The glutathione adduct can be subsequently removed by a glutathione lyase to liberate a free phenylpropanoid derivative product that can be further oxidized and decarboxylated to generate high value-added products such as vanillic acid and vanillin [24, 25].
In summary, lignin deconstruction by biological processes can be catalyzed by bacteria, fungi, and enzymes such as laccase and LiP to result in a variety of aromatic monomers of guaiacol-type as well as phenylpropanoid derivatives (Figure 7.3) such as vanillin, vanillic acid, vanillyl alcohol, cinnamic acid, and p-coumaric acid. These compounds can be directly used as industrial feedstock to synthesize high value-added products [10]. Furthermore, some of the compounds such as gallic acid, p-coumaric acid, 3,4-dihydroxyphenylacetic acid, and ferulic acid showed biological activities that are useful for pharmaceutical applications. Although microbial degradation of lignin can be operated effectively under mild conditions [51], the process is still too slow to be applicable in industry.
7.2 Phenol Derivatives Derived from Lignin Deconstruction
Section 7.1 discussed the various processes of lignin deconstruction that can result in different types of phenol derivatives. Some of these compounds such as ferulic acid, gallic acid, vanillin, and vanillic acid can be used directly for their intended applications, while other compounds need further processing in order to convert them into high value-added compounds. Table 7.1 summarizes the lignin cleavage products and their potential usage in pharmaceutical and biotechnological applications.

Figure 7.3 Overview of the phenylpropanoid derivatives that can be derived from lignin deconstruction by biological processes. (a) Compounds that can be derived from reactions of bacteria and fungi with lignins containing Cβ−O−C4 aryl ether linkages. (b) Products from the cleavage of lignin model compounds with laccase, LiP, and β-etherase.
7.3 Biocatalysis to Increase the Value of Lignin-Derived Phenolic Compounds
Compounds listed in Table 7.1 can be further transformed by various enzymatic reactions to yield products of higher value. We classify the nature of such biotransformation processes into two categories: addition of an extra moiety (esterification and glycosylation) and modification of phenolic substituents (hydroxylation/monooxygenation, methylation, demethylation, and decarboxylation/carboxylation). Products derived from these biocatalytic reactions are useful in biotechnological, pharmaceutical, and industrial applications.
7.3.1 Addition of an Extra Moiety
7.3.1.1 Esterification
Esterification is one of the major means by which the biological activities of lignin-derived phenolic derivatives can be enhanced. Many phenolic derivatives that might be obtained from lignin (see Section 7.2) such as vanillyl alcohol, vanillic acid, p-hydroxyphenylacetate, 3,4-dihydroxyphenylacetate, ferulic acid, p-coumaric acid, cinnamic acid, and caffeic acid have biological activities such as antimicrobial, antioxidant, antiviral, anti-inflammatory, antitumor, antiallergenic, and anti-UV properties [52, 53]. They can potentially be used as bioactive ingredients in food, beverages, and cosmetics. Compounds with acid moieties are often plagued by poor cellular uptake due to their high polarity. Esterification of the acid is a simple method that can address this problem by increasing the lipophilicity of the compound such that their cell permeability can be enhanced.
Enzymatic esterification is an efficient, green, and clean method for the esterification of phenolic acids or phenolic derivatives. Chemical esterification using base or acid catalysts has the disadvantage of requiring high temperature and acid/base waste management. Furthermore, incomplete product conversion yield requires an additional purification process [54]. Lipases from various sources serve as biocatalysts to catalyze the esterification of phenolic acids. A series of alkyl coumarates including methyl coumarate, ethyl coumarate, propyl coumarate, and butyl coumarate were successfully synthesized using celite-bound lipase from Bacillus licheniformis SCD11501 as a biocatalyst [55]. The immobilized lipase B from Candida antarctica (Novozyme 435) is a good biocatalyst for the synthesis of caffeic acid phenethyl ester (CAPE), a valuable natural caffeic acid ester found in honeybee propolis [52, 56], with a bioconversion yield in the range of 91.7–100% [57, 58].
7.3.1.2 Glycosylation
Addition of a glycosyl moiety onto lignin-derived phenolic derivatives generally results in an increase in the biological and pharmacological activities of the compound because of the improved stability and water solubility imparted by the glycosyl group [59–62]. Glycosylation can also protect reactive groups from oxidation, enhancing their stability and bioavailability [63]. For example, glycosylation can increase the water solubility of hydroxycinnamate, allowing the glycosylated hydroxycinnamate to be used as food additive [64]. Glycosylation can also allow better uptake of polyphenols into enterocytes [60].
Glucosylation of caffeic acid can be catalyzed by α-amylase [59, 65, 66], by UDP-glucose glucosyltransferase [67], and by the whole cell of Eucalyptus perriniana [64]. The glucosylation of caffeic acid catalyzed by α-amylase can yield both caffeic acid 3-O- and 4-O-glucosides with 20% conversion (Figure 7.4). The glucosylated form of caffeic acid (caffeic acid 3-O-α-glucopyranoside and 4-O-α-glucopyranoside) has similar antioxidant and antimutagenic activities as caffeic acid, but the glucosylated form is less prone to browning reactions, suggesting that glucosylation can improve the functionality of the compounds in food and cosmetic applications [59]. Different biocatalysts usually result in different types of glucosylated products. UDP-glucose/glucosyltransferase from Arabidopsis specifically transfers UDP-glucose to the 3-OH position of caffeic acid to result in caffeic acid 3-O-glucoside [67]. The same enzyme can also use other hydroxycinnamate compounds including o-coumaric, m-coumaric, and isoferulic acid as substrates [67]. Whole cell of E. perriniana can produce glucosyl compounds of p-coumaric acid, caffeic acid, and ferulic acid, in which both the phenolic hydroxyl group and carboxyl group can be glucosylated [64].

Figure 7.4 Caffeic acid 3-O- and 4-O-α-glucopyranoside products of glucosylation catalyzed by α-amylase.
7.3.2 Modification of Aromatic Ring Substituent
Phenolic compounds derived from lignin deconstruction can serve as precursors for use in further reactions involving group modifications, including hydroxylation/monooxygenation, methylation, demethylation, and decarboxylation/carboxylation. These reactions add more value to phenolic acids by altering their functional properties such that their application as bio-based materials and/or their functional biological activities can be improved.
7.3.2.1 Hydroxylation/Monooxygenation
Hydroxylation or the addition of a hydroxyl group into the aromatic ring of phenolic acids generally increases the radical scavenging ability of the compounds, thus enhancing their applications in the pharmaceutical, food, and biotechnological industries. Various enzymes are employed in hydroxylation of phenolic acids such as cinnamic acid, ferulic acid, and p-coumaric acid (Figure 7.5).

Figure 7.5 Hydroxylation of phenolic acids such as cinnamic acid, p-coumaric acid, and ferulic acid by various hydroxylases to synthesize the corresponding products – p-coumaric acid (a), caffeic acid (b), and 5-hydroxyferulic acid (c), respectively. p-Coumaric acid can be completely converted to 3,4,5-trihydroxycinnamic acid (3,4,5-THCA) (d) via caffeic acid. This reaction is catalyzed by the Y398S variant of HPAH from Acinetobacter baumannii. (Dhammaraj et al. 2015 [30]. Reproduced with permission of American Chemical Society.)
A recent report has shown that the Y398S variant of flavin-dependent p-hydroxyphenylacetate 3-hydroxylase (HPAH) from Acinetobacter baumannii [30, 68, 69] can be used as a biocatalyst to synthesize 3,4,5-trihydroxycinnamic acid (3,4,5-THCA) from p-coumaric acid with 100% conversion (Figure 7.5) [30]. The wild-type enzyme can produce 3,4,5-THCA from p-coumaric acid, 3-(3,4,5-trihydroxyphenyl)propionic acid (3,4,5-THPPA) from 3-(4-hydroxyphenyl)propionic acid, 3,4,5-trihydroxyphenylacetic acid (THPA) from 4-hydroxyphenylacetic acid, and 3,4,5-trihydroxybenzoic acid (gallic acid) from 4-hydroxybenzoic acid (4-HBA) [30]. As the wild-type enzyme cannot efficiently use p-coumaric acid as a substrate, rational protein engineering was carried out in order to obtain the enzyme variant Y398S, which is more efficient than the wild-type enzyme in catalyzing the production of caffeic acid and 3,4,5-THCA from p-coumaric acid [30]. These trihydroxyphenolic acid products (THCA, THPA, and gallic acid) have higher antioxidant, anticancer, anti-inflammatory, and antimicrobial activities than the original phenolic acid substrates [33, 45, 70].
Ferulic acid can be hydroxylated by ferulic acid 5-hydroxylase (F5H), a P450-dependent monooxygenase, to synthesize 5-hydroxyferulic acid, a compound that can be used as a precursor to synthesize sinapic acid. The same enzyme can also use coniferyl alcohol and coniferyl aldehyde to form 5-hydroxyconiferyl alcohol and 5-hydroxyconiferyl aldehyde. Both compounds can be also used as precursors in sinapic acid synthesis (Figure 7.6) [71–74].

Figure 7.6 Hydroxylation of coniferyl alcohol derivatives (a) by ferulic acid 5-hydroxylase (F5H) to synthesize hydroxylated products (b) that can be used as precursors for sinapic acid synthesis (c).
7.3.2.2 Methylation
A phenolic group of caffeyl alcohol/aldehyde and caffeic acid can be methylated by S-adenosylmethionine (SAM)-dependent caffeate O-methyltransferase (COMT) to produce coniferyl alcohol/aldehyde and ferulic acid, respectively. The 5-hydroxyl moiety of 5-hydroxyconiferyl alcohol/aldehyde and 5-hydroxycaffeic acid can be further methylated by COMT to synthesize sinapyl alcohol/aldehyde and sinapic acid, respectively (Figure 7.7) [73–75].
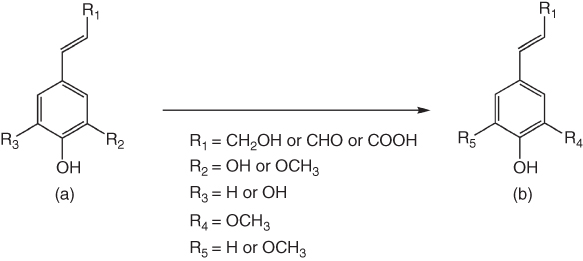
Figure 7.7 Methylation of caffeyl and 5-hydroxyconiferyl derivatives (a) by SAM-dependent O-methyltransferase to produce coniferyl and sinapyl derivatives (b).
7.3.2.3 Demethylation
The 3-methoxy group of vanillic acid, one of the major lignin-derived phenolic acids, can be demethylated by H4folate-dependent O-demethylase (LigM) in Sphingomonas paucimobilis SYK-6 [24, 76] or a nonheme iron-dependent demethylase in Pseudomonas sp. and Acinetobacter sp. to generate protocatechuic acid (PCA) or 3,4-dihydroxybenzoic acid (3,4-DHBA) (Figure 7.8) [24, 77, 78]. The conversion yield is rather high up to 80% [76]. PCA is a catecholic derivative that can be used as a precursor for the synthesis of gallic acid via C5-hydroxylation. Gallic acid has long been known to contain high antioxidative and therapeutic activities [33].
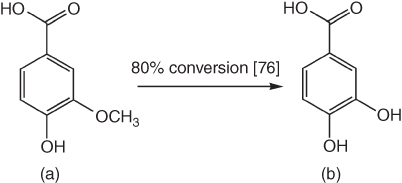
Figure 7.8 Demethylation of vanillic acid (a) to generate protocatechuic acid (PCA) (b).
7.3.2.4 Decarboxylation/Carboxylation
Ferulic acid, one of the major products of lignin depolymerization, can be decarboxylated via biotransformation to produce 4-vinylguaiacol (4-VG) (Figure 7.9), a styrene derivative that can be used in material industries or as a flavoring agent (clove-like spice) [79]. Streptomyces setonii ATCC 39116 can be used to convert ferulic acid into 4-VG with a yield of ∼0.8–0.9 g/l (∼89%) over 12–168 h [42]. 4-VG can also be generated from ferulic acid during fermentation of wheat beer by a decarboxylase (FDC1) in Saccharomyces cerevisiae [41]. Phenolic acid decarboxylase (PAD) in Bacillus subtilis and its homolog (UbiX) in Escherichia coli are enzymes that can convert ferulic acid, p-coumaric acid, and caffeic acid to produce styrene derivatives. PAD, UbiX, and FDC1 are homologous enzymes [80, 81]. Interestingly, PAD can also catalyze the reverse reaction to add CO2 regioselectively at the β-position of p-hydroxystyrene derivatives to produce ferulic acid derivatives with 40% yield [82] (Figure 7.9).

Figure 7.9 Reversible conversion of ferulic acid (a) to generate 4-vinylguaiacol (b) via phenolic acid decarboxylase (PAD)-catalyzed decarboxylation/carboxylation reaction.
7.4 Outlook and Future Perspectives
Although various technologies are available to convert lignin into phenolic compounds, the use of lignin in biorefinery is still limited and underutilized. New processes that can generate more homogeneous phenolic compounds from lignin deconstruction should be helpful in providing feedstock for efficient downstream processes. Research in biocatalysis or enzymes that can transform or convert lignin-derived phenolic compounds into high value substances will provide new avenues of lignin or lignocellulose utilization. Although the current price of petroleum does not allow bio-based industries to compete with fossil-based technology, research in the area of biomass utilization in conjunction with biocatalysis is still needed in order to reduce energy consumption, CO2 emission, and environmental damage.
Acknowledgments
The financial support by the Thailand Research Fund (grants RTA5980001, MRG5980001, TRG5880106, and TRG5780122), Vidyasirimedhi Institute of Science and Technology (VISTEC), Mahidol University, Burapha University is gratefully acknowledged.
References
- 1. Hu, W.J., Harding, S.A., Lung, J., Popko, J.L., Ralph, J., Stokke, D.D., Tsai, C.J., and Chiang, V.L. (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol., 17, 808–812.
- 2. Chatel, G. and Rogers, R.D. (2014) Review: oxidation of lignin using ionic liquids-an innovative strategy to produce renewable chemicals. ACS Sustainable Chem. Eng., 2, 322–339.
- 3. Li, C., Zhao, X., Wang, A., Huber, G.W., and Zhang, T. (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev., 115, 11559–11624.
- 4. Ten, E. and Vermerris, W. (2015) Recent developments in polymers derived from industrial lignin. J. Appl. Polym. Sci., 132, 42069–42081.
- 5. Ralph, J., Brunow, G., and Boerjan, W. (2007) Lignins, in Encyclopedia of Life Sciences, John Wiley & Sons, Ltd, pp. 1–10, doi:10.1002/9780470015902.a0020104.
- 6. Morreel, K., Kim, H., Lu, F., Dima, O., Akiyama, T., Vanholme, R., Vanholme, C., Goeminne, G., Inze, D., Messens, E., Ralph, J., and Boerjan, W. (2010) Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal. Chem., 82, 8095–8105.
- 7. Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., and Weckhuysen, B.M. (2010) The chemical valorization of lignin for the production of renewable chemicals. Chem. Rev., 110, 3552–3599.
- 8. Pandey, M.P. and Kim, C.S. (2011) Lignin depolymerization and conversion: a review of thermochemical methods. Chem. Eng. Technol., 34, 29–41.
- 9. Bai, Y.Y., Xio, L.P., Shi, Z.J., and Sun, R.C. (2013) Structure variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci., 14, 21394–21413.
- 10. Upton, B.M. and Kasko, A.M. (2016) Strategies for the conversion of lignin to high-value polymeric materials: review and perspectives. Chem. Rev., 116, 2275–2306.
- 11. Hendriks, A.T.W.M. and Zeeman, G. (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol., 100, 10–18.
- 12. Ma, R., Xu, Y., and Zhang, X. (2015) Catalytic oxidation of biorefinery lignin to value added chemicals to support sustainable biofuel production. ChemSusChem, 8, 24–51.
- 13. Zhao, X., Cheng, K., and Liu, D. (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol., 82, 815–827.
- 14. Ragauskas, A.J., Beckham, G.T., Biddy, M.J., Chandra, R., Chen, F., Davis, M.F., Davison, B.H., Dixon, R.A., Gilna, P., Keller, M., Langan, P., Naskar, A.K., Saddler, J.N., Tschaplinski, T.J., Tuskan, G.A., and Wyman, C.E. (2014) Lignin valorization: improving lignin processing in the biorefinery. Science, 344, 1246843–1246852.
- 15. Schüth, F., Rinaldi, R., Meine, N., Käldström, M., Hilgert, J., and Rechulski, K.M.D. (2014) Mechanocatalytic depolymerization of cellulose and raw biomass and downstream processing of the products. Catal. Today, 234, 24–30.
- 16. Kleine, T., Buendia, J., and Bolm, C. (2013) Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive a medium. Green Chem., 15, 160–166.
- 17. Kim, S.M., Dien, B.S., and Singh, V. (2016) Promise of combined hydrothermal/chemical and mechanical refining for pretreatment of woody and herbaceous biomass. Biotechnol. Biofuels, 9, 97–111.
- 18. Hu, J., Shen, D., Xiao, R., Wu, S., and Zhang, H. (2013) Free-radical analysis on thermochemical transformation of lignin to phenolic compounds. Energy Fuels, 27, 285–293.
- 19. Nguyen, J.D., Matsuura, B.S., and Stephenson, C.R.J. (2014) A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc., 136, 1218–1221.
- 20. Wang, H., Tucker, M., and Ji, Y. (2013) Recent development in chemical depolymerization of lignin: a review. J. Appl. Chem., 2013, 838645–838653.
- 21. Dutta, S. (2015) Lignin deconstruction: chemical and biological approaches, in Sustainable Catalytic Processes, 1st edn (eds B. Saha, M. Fan , and J. Wang), Elsivier B.V, Amsterdam, Netherlands, pp. 125–155.
- 22. ten Have, R. and Teunissen, P.J.M. (2001) Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem. Rev., 101, 3397–3413.
- 23. Sanchez, C. (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv., 27, 185–194.
- 24. Bugg, T.D.H., Ahmad, M., Hardiman, E.M., and Rahmanpour, R. (2011) Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep., 28, 1883–1896.
- 25. Bugg, T.D.H., Ahmad, M., Hardiman, E.M., and Singh, R. (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol., 22, 394–400.
- 26. Priefert, H., Rabenhorst, J., and Steinbüchel, A. (2001) Biotechnological production of vanillin. Appl. Microbiol. Biotechnol., 56, 296–314.
- 27. Walton, N.J., Mayer, M.J., and Narbad, A. (2003) Vanillin. Phytochemistry, 63, 505–515.
- 28. Lirdprapamongkol, K., Kramb, J.P., Suthiphongchai, T., Surarit, R., Srisomsap, C., Danhardt, G., and Svsti, J. (2009) Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agric. Food Chem., 57, 3055–3063.
- 29. Farhoosh, R., Johnny, S., Asnaashari, M., Molaahmadibahraseman, N., and Sharif, A. (2016) Structure–antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem., 194, 128–134.
- 30. Dhammaraj, T., Phintha, A., Pinthong, C., Medhanavyn, D., Tinikul, R., Chenprakhon, P., Sucharitakul, J., Vardhanabhuti, N., Jiarpinitnun, C., and Chaiyen, P. (2015) p-Hydroxyphenylacetate 3-hydroxylase as a biocatalyst for the synthesis of trihydroxyphenolic acids. ACS Catal., 5, 4492–4502.
- 31. Zitta, K., Meybohm, P., Bein, B., Huang, Y., Heinrich, C., Scholz, J., Steinfath, M., and Albrecht, M. (2012) Salicylic acid induces apoptosis in colon carcinoma cells grown in-vitro: influence of oxygen and salicylic acid concentration. Exp. Cell Res., 318, 828–834.
- 32. Scheit, K. and Bauer, G. (2015) Direct and indirect inactivation of tumor cell protective catalase by salicylic acid and anthocyanidins reactivates intercellular ROS signaling and allows for synergistic effects. Carcinogenesis, 36, 400–411.
- 33. Badhani, B., Sharma, N., and Kakar, R. (2015) Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv., 5, 27540–27557.
- 34. Gomes, C.A., da Cruz, T.G., Andrade, J.L., Milhazes, N., Borges, F., and Marques, M.P.M. (2003) Anticancer activity of phenolic acids of natural or synthetic origin: a structure–activity study. J. Med. Chem., 46, 5395–5401.
- 35. Teixeira, J., Gaspar, A., Garrido, E.M., Garrido, J., and Borges, F. (2013) Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed. Res. Int., 2013, 1–11.
- 36. An, S.M., Lee, S.I., Choi, S.W., Moon, S.W., and Boo, Y.C. (2008) p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by α-melanocyte stimulating hormone. Br. J. Dermatol., 159, 292–299.
- 37. Reano, A.F., Cherubin, J., Peru, A.M.M., Wang, Q., Clement, T., Domenek, S., and Allais, F. (2015) Structure–activity relationships and structural design optimization of a series of p-hydroxycinnamic acids-based bis- and trisphenols as novel sustainable antiradical/antioxidant additives. ACS Sustainable Chem. Eng., 3, 3486–3496.
- 38. Srinivasan, M., Sudheer, A.R., and Menon, V.P. (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr, 40, 92–100.
- 39. Graf, E. (1992) Antioxidant potential of ferulic acid. Free Radic. Biol. Med., 13, 435–448.
- 40. Kikuzaki, H., Hisamoto, M., Hirose, K., Akiyama, K., and Taniguchi, H. (2002) Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem., 50, 2161–2168.
- 41. Coghe, S., Benoot, K., Delvaux, F., Vanderhaegen, B., and Delvaux, F.R. (2004) Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem., 52, 602–608.
- 42. Max, B., Carballo, J., Cortes, S., and Dominguez, J.M. (2012) Decarboxylation of ferulic acid to 4-vinyl guaiacol by Streptomyces setonii. Appl. Biochem. Biotechnol., 166, 289–299.
- 43. Corse, J.W., Jones, R.G., Soper, Q.F., Whitehead, C.W., and Behrens, O.K. (1948) Biosynthesis of penicillins. V.1 substituted phenylacetic acid derivatives as penicillin precursors. J. Am. Chem. Soc., 70, 2837–2843.
- 44. Ruisinger, B. and Scieberle, P. (2012) Characterization of the key aroma compounds in rape honey by means of the molecular sensory science concept. J. Agric. Food Chem., 60, 4186–4194.
- 45. Siquet, C., Paiva-Martins, F., Lima, J.L.F.C., Reis, S., and Borges, F. (2006) Antioxidant profile of dihydroxy- and trihydroxyphenolic acids- a structure–activity relationship study. Free Radic. Res., 40, 433–442.
- 46. Alcalde, M. (2015) Engineering the ligninolytic enzyme consortium. Trends Biotechnol., 33, 155–162.
- 47. Pollegioni, L., Tonin, F., and Rosini, E. (2015) Lignin-degrading enzymes. FEBS J., 282, 1190–1213.
- 48. Riva, S. (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol., 24, 219–226.
- 49. Witayakran, S. and Ragauskas, A.J. (2009) Synthetic applications of laccase in green chemistry. Adv. Synth. Catal., 351, 1187–1209.
- 50. Majumdar, S., Lukk, T., Solbiati, J.O., Bauer, S., Nair, S.K., Cronan, J.E., and Gerlt, J.A. (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry, 53, 4047–4058.
- 51. Kumar, P., Barrett, D.M., Delwiche, M.J., and Stroeve, P. (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res., 2009 (48), 3713–3729.
- 52. Khan, N.R. and Rathod, V.K. (2015) Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Biochem., 50, 1793–1806.
- 53. Chigorimbo-Murefu, N.T.L., Riva, S., and Burton, S.G. (2009) Lipase-catalysed synthesis of esters of ferulic acid with natural compounds and evaluation of their antioxidant properties. J. Mol. Catal. B: Enzym., 56, 277–282.
- 54. Villeneuve, P. (2007) Lipase in lipophilization reaction. Biotechnol. Adv., 25, 515–536.
- 55. Sharma, S., Dogra, P., Chauhan, G.S., and Kanwar, S.S. (2014) Synthesis of alkyl coumarate esters by celite-bound lipase of Bacillus licheniformis SCD11501. J. Mol. Catal. B: Enzym., 101, 80–86.
- 56. Grunberger, D., Banerjee, R., Eisinger, K., Oltz, E.M., Efros, L., Caldwell, M., Estevez, V., and Nakanishi, K. (1988) Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia, 44, 230–232.
- 57. Widjaja, A., Yeh, T.H., and Ju, Y.H. (2008) Enzymatic synthesis of caffeic acid phenethyl ester. J. Chin. Inst. Chem. Eng., 39, 413–418.
- 58. Chen, H.C., Ju, H.Y., Twu, Y.K., Chen, J.H., Chang, C.M., Liu, Y.C., Chang, C., and Shieh, C.J. (2010) Optimized enzymatic synthesis of caffeic acid phenethyl ester by RSM. N. Biotechnol., 27, 89–93.
- 59. Nishimura, T., Kometani, T., Takii, H., Terada, Y., and Okada, S. (1995) Glucosylation of caffeic acid with Bacillus subtilis X-23 α-amylase and a description of the glucosides. J. Ferment. Bioeng., 80, 18–23.
- 60. Setchell, K.D.R., Brown, N.M., Zimmer-Nechemias, L., Brashear, W.T., Wolfe, B.E., Kirschner, A.S., and Heubi, J.E. (2002) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr., 76, 447–453.
- 61. Ratnam, D.V., Ankola, D.D., Bhardwaj, V., Sahana, D.K., and Kumar, M.N.V.R. (2006) Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J. Control. Release, 113, 189–207.
- 62. Zhang, P., Zhang, E., Xiao, M., Chen, C., and Xu, W. (2013) Enhanced chemical and biological activities of a newly biosynthesized eugenol glycoconjugate, eugenol-α-d-glucopyranoside. Appl. Microbiol. Biotechnol., 97, 1043–1050.
- 63. Torres, P., Poveda, A., Jimenez-Barbero, J., Parra, J.L., Comelles, F., Ballesteros, A.O., and Plou, F.J. (2011) Enzymatic synthesis of α-glucosides of resveratrol with surfactant activity. Adv. Synth. Catal., 353, 1077–1086.
- 64. Katsuragi, H., Shimoda, K., Yamamoto, R., Ishihara, K., and Hamada, H. (2011) Glycosylation of capsaicin derivatives and phenylpropanoid derivatives using cultured plant cells. Biochem. Insights, 4, 1–12.
- 65. Nishimura, T., Kometani, T., Takii, H., Terada, Y., and Okada, S. (1994) Purification and some properties of α-amylase from Bacillus subtilis X-23 that glucosylates phenolic compounds such as hydroquinone. J. Ferment. Bioeng., 78, 31–36.
- 66. Nishimura, T., Kometani, T., Takii, I.L., Terada, Y., and Okada, S. (1994) Acceptor specificity in the glucosylation reaction of Bacillus subtilis X-23 α-amylase towards various phenolic compounds and the structure of kojic acid glucoside. J. Ferment. Bioeng., 78, 37–41.
- 67. Lim, E.-K., Higgins, G.S., Li, Y., and Bowles, D.J. (2003) Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem. J., 373, 987–992.
- 68. Chaiyen, P., Suadee, C., and Wilairat, P. (2001) A novel two-protein component flavoprotein hydroxylase. Eur. J. Biochem., 268, 5550–5561.
- 69. Sucharitakul, J., Chaiyen, P., Entsch, B., and Ballou, D.P. (2006) Kinetic mechanisms of the oxygenase from a two-component enzyme, p-hydroxyphenylacetate 3-hydroxylase from Acinetobacter baumannii. J. Biol. Chem., 281, 17044–17053.
- 70. Esteves, M., Siquet, C., Gaspar, A., Rio, V., Sousa, J.B., Reis, S., Marques, M.P., and Borges, F. (2008) Antioxidant versus cytotoxic properties of hydroxycinnamic acid derivatives-a new paradigm in phenolic research. Arch. Pharm. (Weinheim), 34, 164–173.
- 71. Humphreys, J.M., Hemm, M.R., and Chapple, C. (1999) New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase-a multifunctional cytochrome P450-dependent monooxygenase. Proc. Natl. Acad. Sci. U. S. A., 96, 10045–10050.
- 72. Osakabe, K., Tsao, C.C., Li, L., Popko, J.L., Umezawa, T., Carraway, D.T., Smeltzer, R.H., Joshi, C.P., and Chiang, V.L. (1999) Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc. Natl. Acad. Sci. U. S. A., 96, 8955–8960.
- 73. Petersen, M., Hans, J., and Matern, U. (2010) Biosynthesis of phenylpropanoids and related compounds, in Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism, 2nd edn (ed. M. Wink), Wiley-Blackwell, Oxford, pp. 182–257.
- 74. Buchanan, B.B., Gruissem, W., and Jones, R.L. (2015) Biochemistry and Molecular Biology of Plants, 2nd edn, Wiley Blackwell, pp. 1178–1201.
- 75. Humphreys, J.M. and Chapple, C. (2002) Rewriting the lignin roadmap. Curr. Opin. Plant Biol., 5, 224–229.
- 76. Abe, T., Masai, E., Miyauchi, K., Katayama, Y., and Fukuda, M. (2005) A tetrahydrofolate- dependent O-demethylase, LigM, is crucial for catabolism of vanillate and syringate in Sphingomonas paucimobilis SYK-6. J. Bacteriol., 187, 2030–2037.
- 77. Brunel, F. and Davison, J. (1988) Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J. Bacteriol., 170, 4924–4930.
- 78. Segura, A., Bünz, P.V., D'Argenio, D.A., and Ornston, L.N. (1999) Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol., 181, 3494–3504.
- 79. Mathew, S. and Abraham, T.E. (2006) Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit. Rev. Microbiol., 32, 115–125.
- 80. Frank, A., Eborall, W., Hyde, R., Hart, S., Turkenburg, J.P., and Grogan, G. (2012) Mutational analysis of phenolic acid decarboxylase from Bacillus subtilis (BsPAD), which converts bio-derived phenolic acids to styrene derivatives. Catal. Sci. Technol., 2, 1568–1574.
- 81. Lin, F., Ferguson, K.L., Boyer, D.R., Lin, X.N., and Marsh, E.N.G. (2015) Isofunctional enzymes PAD1 and UbiX catalyze formation of a novel cofactor required by ferulic acid decarboxylase and 4-hydroxy-3-polyprenylbenzoic acid decarboxylase. ACS Chem. Biol., 10, 1137–1144.
- 82. Wuensch, C., Pavkov-Keller, T., Steinkellner, G., Gross, J., Fuchs, M., Hromic, A., Lyskowski, A., Fauland, K., Gruber, K., Glueck, S.M., and Faber, K. (2015) Regioselective enzymatic β-carboxylation of para-hydroxy-styrene derivatives catalyzed by phenolic acid decarboxylases. Adv. Synth. Catal., 357, 1909–1918.
