Chapter 20
Metabolic Engineering of Microorganisms for the Production of Lactate-Containing Polyesters
Yokimiko David, Sang Yup Lee and Si Jae Park
Polylactic acid (PLA) is considered as a biomass-driven alternative to petroleum-based plastic and has biocompatible properties such as biodegradability and low toxicity to humans and environment [1, 2]. The current commercial process of PLA synthesis is not simple, in which ring-opening polymerization (ROP) of lactide, a cyclic dimer of lactate, is employed because direct polycondensation of lactic acid cannot provide PLA with high molecular weight over 200 000 Da [2, 3]. (l)-Lactic acid, the monomer source of PLA synthesis, is produced by the microbial fermentation, in which microorganisms such as lactic acid bacteria produce (l)-lactate as a major product with the trace amount of (d)-lactate from sugars at almost neutral pH [3]. And then lactic acid that is purified to a polymer grade is used for the synthesis of lactide. Even though it is now possible to provide PLA with advanced material properties by improved polymer processing technology, it is not still effective to alter material properties of PLA by copolymer synthesis since lactonized monomers for copolymerization are costly and are not easily available. Also, the possible toxic effects of heavy metal catalysts used for PLA synthesis remaining in trace after polymerization cannot be ignored for medical and food applications. Thus, a biological process such as lipase-mediated PLA synthesis has been suggested as an alternative, safe, and environmentally friendly process [4]. Recently, we have developed a complete biosynthetic process in which microorganisms synthesize PLA and PLA copolymers from glucose and accumulate them as distinct granules in their cytoplasm, by employing advanced PHA biosynthetic pathways [5–12]. Metabolic pathways for the production of PLA and PLA copolymers developed in recombinant Escherichia coli are shown in Figure 20.1. PHAs are natural polyesters consisting of various 3,4,5,6-hydroxycarboxylic acids, which are synthesized by PHA synthase (PhaC) that polymerizes various hydroxyacyl-CoAs (HA-CoAs) generated as metabolites of diverse metabolic pathways and are accumulated as distinct granules in the cell [15]. Even though lactate has been suggested as a possible monomer unit of natural PHA since it contains both hydroxyl and carboxyl groups, there have been no reports about the identification of lactate as monomer unit of natural PHAs screened up to date. A minimal requirement for the synthesis of PHA containing specific monomers is that cellular metabolic pathway should supply specific HA-CoAs to PHA synthase that uses these HA-CoAs as substrates for polymer synthesis [15]. In natural microorganisms, PHA is accumulated as a source of carbon and energy under unfavorable growth conditions; 3-HA-CoAs, the most abundant and easily available metabolites, have been used as substrates for PHA synthesis [15].
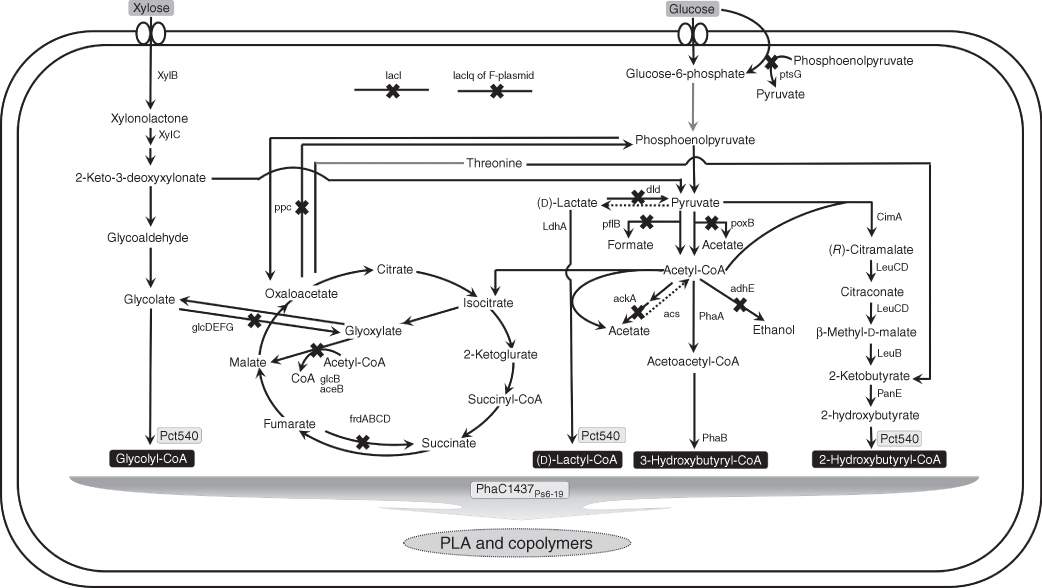
Figure 20.1 Metabolic engineering of E. coli for the production of PLA and copolymers. Cross marks show knocked out genes to eliminate competing pathways. Dotted arrows indicate overexpression by the use of strong promoters. Gray arrows represent multiple steps. (Ppc: phosphoenolpyruvate carboxylase or frdABCD: fumarate reductase, ptsG: IIBCGlc, glucose transporter, poxB: pyruvate oxidase, dld: d-lactate dehydrogenase, aceB: malate synthase A, glcB: malate synthase G, glcDEFG: glycolate oxidase, ilvA: threonine dehydratase, ldhA: (d)-lactate dehydrogenase, pflB: pyruvate formate lyase, ackA: acetate kinase, Acs: acetyl-CoA synthase, PhaA: β-ketothiolase, PhaB: acetoacetyl reductase, adhE: acetaldehyde/alcohol dehydrogenase, CimA: citramalate synthase, LeuB: 3-isopropylmalate dehydrogenase, LeuCD: Isopropylmalate (IPM) isomerase, PanE: 2-hydroxyacid dehydrogenase, Pct540: evolved propionyl-CoA transferase, PhaC1437: evolved PHA synthase, XylB: xylose dehydrogenase, XylC: xylonolactonase, lacI: transcriptional repressor) [10–14].
To construct a biosynthetic pathway for the production of PLA employing PHA biosynthetic pathway, it was preferentially necessary to develop the metabolic pathway for the generation of lactyl-CoA using Clostridium propionicum propionyl-CoA transferase (PctCp), which is suggested to make (d)-lactyl-CoA by transferring CoA from propionyl-CoA or acetyl-CoA to (d)-lactate [16]. It has also been reported that several hydroxy acids including lactate, 3-hydroxypropionate (3HP), 3-hydroxybutyrate (3HB), and 4-hydroxybutyrate (4HB) could be activated to their CoA derivatives by propionyl-CoA transferase using acetyl-CoA as a CoA donor. The possible candidates as PLA polymerases were selected from representative class I, II, III, and IV PHA synthases and examined for their activities to accept lactyl-CoA as a substrate in recombinant E. coli [5, 8]. Class I Ralstonia eutropha H16 PhaC, class II Pseudomonas sp. MBEL 6-19 PhaC1 (PhaC1Ps6-19), class III Allochromatium vinosum DSM 180 PhaEC, and class IV Bacillus cereus ATCC 14579 PhaRC were examined for the production of poly(3-hydroxybutyrate-co-lactate) [P(3HB-co-LA)] in recombinant E. coli expressing PctCp, which can supply (d)-lactyl-CoA and (d)-3HB-CoA [8]. P(3HB-co-LA) was used as a model PLA copolymer since lactate incorporation into polyester might be enhanced by copolymerization of lactyl-CoA with 3HB-CoA, the most favorable substrate of PHA synthase. It was found out that a very low amount of lactate was incorporated into P(3HB-co-LA) by recombinant E. coli expressing PctCp, and all the PHA synthases except PhaC1Ps6-19 from glucose and 3HB added to the culture medium [8]. However, only P(3HB) homopolymer without incorporation of lactate monomer was more often produced due to very low activity of natural PHA synthase toward lactyl-CoA. Thus, we decided to engineer PHA synthase to increase activity toward lactyl-CoA for the efficient production of PLA and PLA copolymers.
Among the examined PHA synthases, PhaC1Ps6-19 possesses exceptionally broad substrate specificities toward 3HA-CoAs of 4–12 carbons. Therefore, PhaC1Ps6-19 was chosen for protein engineering to make it accept lactyl-CoA as substrate even though this synthase could not support the production of P(3HB-co-LA). It was reasoned that engineering of PHA synthase having a broad range of substrate specificity might be useful to broaden the spectrum of monomer components for PLA copolymers.
Site-directed mutagenesis of PhaC1Ps6-19 was firstly carried out to enhance its activity toward SCL-monomers such as 3HB monomer since PhaC1Ps6-19 cannot efficiently accept SCL-HA-CoAs as substrates. The mutation sites for amino acid change were selected based on previous reports about substrate specificities of Pseudomonas sp. 61-3 PHA synthase 1 (PhaC1Ps61-3) that has amino acid homology of 84% to PhaC1Ps6-19 [17–20]. The effect of amino acid changes at E130, S325, S477, and Q481 of PhaC1Ps6-19 on the P(3HB) synthesis was examined in recombinant E. coli. When 3HB-CoA was supplied by the expression of R. eutropha β-ketothiolase (PhaA) and acetoacetyl-CoA reductase (PhaB), PhaC1Ps6-19 having mutations in these four amino acid residues supported the production of P(3HB) from glucose in recombinant E. coli, which means that PhaC1Ps6-19 was successfully engineered to accept SCL-monomers [6, 10].
To carry out the directed evolution of PhaC1Ps6-19 to screen PhaC1Ps6-19 mutants accepting lactyl-CoA as substrate, artificial operon consisting of the phaC1Ps6-19 mutant gene and the pctCp gene was constructed. At first, functional expression of PhaC1300Ps6-19 (PhaC1Ps6-19 E130D, S325T, and Q481M) and PctCp in artificial operon was firstly examined in recombinant E. coli in lysogeny broth (LB) medium containing 20 g/l of glucose and 2 g/l of 3HB using P(3HB) as model polymer. We were surprised to find out that P(97mol.%3HB-co-3mol.%LA) was synthesized instead of P(3HB) homopolymer during the analysis of polymer produced by recombinant E. coli expressing PhaC1300Ps6-19 and PctCp [10]. It was confirmed by repeated experiments that a small amount of lactate was reproducibly incorporated into P(3HB-co-LA), the lactate fraction of which varied depending on the culture condition [10].
This is why we did not further employ the PHA synthases from R. eutropha, A. vinosum, and B. cereus, even though these PHA synthases allowed the production of PHA containing a small amount of lactate monomer [5, 8]. We succeeded in screening superior Pseudomonas sp. MBEL 6-19 PHA synthase mutants efficiently accepting lactyl-CoA as substrate compared with PHA synthases of other strains. Actually, activity for the production of lactate-containing PHA by PhaC1Ps6-19 was serendipitously found during the site-directed mutagenesis of PhaC1Ps6-19 to enhance the substrate specificity of PhaC1Ps6-19 toward 3HB-CoA. To our knowledge, high throughput screening methods are not currently available to sort bacterial cells depending upon monomer composition of polymers accumulated in the cells; thus we decided to examine PhaC1Ps6-19 variants in detail, which might also be used to polymerize (d)-lactyl-CoA with different efficiencies. Also, it was found to be difficult to sort bacterial cells accumulating PLA homopolymer in the cells because typical methods to detect PHA-accumulating cells such as Nile red staining could not be employed to screen bacteria accumulating PLA homopolymer in our unpublished experiments. Further engineering of PhaC1Ps6-19 (Figure 20.2) by combinatorial mutagenesis on E130, S325, S477, and Q481 generated more PhaC1Ps6-19 mutants that resulted in the production of P(3HB-co-LA) having different lactate fraction. Among those, quadruple mutant PhaC1Ps6-19 having E130D, S325T, S477G, and Q481K (PhaC1400Ps6-19) increased lactate fraction in P(3HB-co-LA) up to 49 mol.% [10]. Importance of four amino acid residues (E130, S325, S477, and Q481) in conferring activity toward lactyl-CoA was further extended to almost type II PHA synthases that generally accept only medium-chain-length HA-CoAs having C6–C14 carbon numbers as substrates [21]. Representative type II PHA synthases from P. chlororaphis, Pseudomonas sp. 61-3, P. putida KT2440, P. resinovorans, and P. aeruginosa PAO1 were examined for the production of P(3HB-co-LA) by employing site-directed mutagenesis on these sites [21]. All PHA synthases with these mutations could accept (d)-lactyl-CoA to produce P(3HB-co-LA), but the wild-type PHA synthases could not support the production of the P(3HB-co-LA).

Figure 20.2 The key enzymes for biosynthesis of lactate-containing polymers. Particular residues of the Clostridium propionicum propionyl-CoA transferase (PctCp) and Pseudomonas sp. 6-19 PHA synthase I (PhaC1Ps6-19) are engineered to exhibit higher activities in vivo. The mutated sequences are shown in comparison with the wild-type residues in the original polypeptides. Pct532 and Pct540 are the mutants derived from the C. propionicum Pct by error-prone PCR. Pct532 has an amino acid mutation (A243T) in addition to the silent nucleotide mutation (A1200G). Pct540 has an amino acid mutation (V193A) and four silent nucleotide mutations (T78C, T669C, A1125G, and T1158C). PhaC1202, PhaC1310, PhaC1400, and PhaC1437 are the mutants derived from the Pseudomonas sp. 6-19 PHA synthase I (PhaC1Ps6-19). PhaC1202, PhaC1 mutant having double mutations (E130D and Q481K); PhaC1310, PhaC1 mutant having triple mutations (E130D, S447F, and Q481K); PhaC1400, PhaC1 mutant having quadruple mutations (E130D, S325T, S477R, and Q481M); PhaC1437, PhaC1 mutant having quadruple mutations (E130D, S325T, S477G, and Q481K) [10].
Along with the generation of PhaC1Ps6-19 mutants by site-directed mutagenesis of the amino acids at E130, S325, S477, and Q481 and by random mutagenesis using error-prone PCR, random mutagenesis of C. propionicum propionyl-CoA transferase was also carried out to enhance its activity to provide more (d)-lactyl-CoA with PhaC1Ps6-19 mutants in E. coli without cell growth inhibition. Since lactyl-CoA is synthesized by using acetyl-CoA as a CoA donor, the most important central metabolite for cell growth, high level expression of the C. propionicum propionyl-CoA transferase gene is suggested to inhibit cell growth. Two beneficial PctCp mutants (Figure 20.2), Pct532Cp (A243T, and one silent nucleotide mutation of A1200G) and Pct540Cp (V193A, and four silent nucleotide mutations of T78C, T669C, A1125G, and T1158C) were screened based on the increase of polymer content and lactate monomer fraction in P(3HB-co-LA)[9, 10].
It is important to supply enough substrates for PHA synthase to achieve efficient production of PHAs in microorganisms. Therefore, metabolic engineering of E. coli XL1-Blue was carried out to provide PhaC1Ps6-19 mutants with more lactyl-CoA to increase lactate fraction of P(3HB-co-LA), which ultimately makes the production of PLA homopolymer in microorganism from renewable resources.
Metabolic pathways competing for pyruvate and acetyl-CoA were removed since they are the direct precursors for lactate and CoA donor for the synthesis of lactyl-CoA. Also, biosynthesis pathway of lactate was amplified in E. coli to make more lactyl-CoA available for PHA synthase. P(3HB-co-LA) with high lactate fraction could be synthesized in recombinant E. coli JLX10 strain (ΔackA PldhA::Ptrc Δppc ΔadhE Pacs::Ptrc) and E. coli JLXF5 strain (ΔlacI ΔpflB ΔfrdABCD ΔadhE PldhA::Ptrc Pacs::Ptrc), both of which have enhanced lactyl-CoA synthesis pathway. Also, PLA hompolymer was synthesized in these strains [11, 12].
These results suggest that enhanced production of PLA homopolymer and P(3HB-co-LA) copolymers can be achieved by engineering metabolic capacities of E. coli strain to provide more precursors for polymer synthesis. It was found that the mechanical and thermal properties of the lactate-containing polyesters are highly dependent on their monomer compositions [10, 13, 22]. As the lactate monomer fraction in polyester increases, the molecular weight, crystallinity, and melt viscosity decrease. Glass transition temperature is also proportional to the LA fraction. On the other hand, no significant difference on the melting temperature is observed in polyesters consisting of different monomers with different compositions (10], Table 20.1). Thus, it is important to design monomers to be incorporated into PLA backbone to obtain the desired material properties of synthesized polyesters.
Table 20.1 Thermal and mechanical properties of PLA and PLA copolymers.a
| Polymer | Thermal properties | Mechanical properties | ||||
| Tg (°C) | Tm (°C) | ΔHm (cal/g) | Tensile strength (MPa) | Young's modulus (MPa) | Elongation at break (%) | |
| P(3HB) | −7 | 159, 176 | 1.6, 9.9 | 19 | 1079 | 9 |
| P(3HB-co-47 mol.% LA) | −8 | 140, 157 | 0.4, 1.9 | 7 | 153 | 84 |
| PLA | 60 | 153 | 2.2 | 52 | 1020 | 2 |
a Data were taken from [13, 22].
Besides P(3HB-co-LA) polymer, various copolymers consisting of lactate and various hydroxycarboxylic acids such as 3HP, 4HB, 3-hydroxyvalerate (3HV), and MCL-3-hydroxyalkanoate have been synthesized in recombinant E. coli expressing PhaC1Ps6-19 mutants along with engineered metabolic pathways to supply these monomers [5–12]. Also, PLA copolymers containing other 2-hydroxyacid monomer such as 2-hydroxybutyrate (2HB) could be synthesized in recombinant E. coli expressing PhaC1Ps6-19 mutant [14]. Moreover, recently, poly(lactate-co-glycolate) (PLGA), one of commercially available Food and Drug Administration (FDA)-approved polyester, could successfully be synthesized by recombinant E. coli expressing PhaC1Ps6-19 mutants [23]. Similarly, R. eutropha, a native producer of PHA, could produce 2-HA-containing PHA by functional expression of Pct540Cp and PhaC1Ps6-19 mutants [24–26]. Using the metabolic engineering strategies described in this chapter, in vivo synthesis of more diverse nonnatural polyesters having novel material properties can be produced from biomass-derived renewable resources by metabolically engineered bacteria, wherein these resources can be pretreated to provide cheap and suitable substrates for microbial fermentations [27].
Acknowledgments
This work was supported by the Technology Development Program to Solve Climate Changes (Systems Metabolic Engineering for Biorefineries) (NRF-2015M1A2A2035810 and NRF-2012M1A2A2026556) from the Ministry of Science, ICT, and Future Planning (MSIP) through the National Research Foundation (NRF) of Korea and the Mid-career Researcher Program from MSIP through the NRF of Korea (NRF-2016R1A2B4008707).
References
- 1. Södergård, A. and Stolt, M. (2002) Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci., 27 (6), 1123–1163.
- 2. Maharana, T., Mohanty, B., and Negi, Y.S. (2009) Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci., 34 (1), 99–124.
- 3. Vink, E.T., Rábago, K., Glassner, D.A., Springs, B. et al. (2004) The sustainability of NatureWorks™ polylactide polymers and Ingeo™ polylactide fibers: an update of the future. Macromol. Biosci., 4 (6), 551–564.
- 4. Lassalle, V.L. and Ferreira, M.L. (2008) Lipase-catalyzed synthesis of polylactic acid: an overview of the experimental aspects. J. Chem. Technol. Biotechnol., 83 (11), 1493–1502.
- 5. Park, S.J., Lee, S.Y., Jung, Y.K., and Cho, J. (2006) Cells or plants having an producing ability of polylactate or its copolymers and method for preparing polylactate or its copolymers using the same. PCT WO, 2,006,126,796, filed May 17, 2006 and issued Nov. 30, 2006.
- 6. Yang, T.H., Kang, H.O., Park, S.J., Lee, S.H., et al. (2013) Mutants of PHA synthase from Pseudomonas sp. 6-19 and method for preparing lactate homopolymer or copolymer using the same. US Patent 8,541,652, filed Nov. 21, 2007 and issued Sep. 24, 2013.
- 7. Park, S.J., Yang, T.H., Kang, H.O., Lee, S.H., et al. (2014) Copolymer containing 3-hydroxyalkanoate unit and lactate unit, and its manufacturing method. US Patent 8,765,402, filed Nov. 21, 2007 and issued Jul. 1, 2014.
- 8. Park, S.J., Yang, T.H., Kang, H.O., Lee, S.H., et al. (2013) Copolymer comprising 4-hydroxybutyrate unit and lactate unit and its manufacturing method. US Patent 8,383,379, filed Nov. 21, 2007 and issued Feb. 26, 2013.
- 9. Park, S.J., Yang, T.H., Kang, H.O., Lee, S.H., et al. (2014) Mutant of propionyl-CoA transferase from Clostridium propionicum and preparing method for PLA or PLA copolymer using the same. US Patent 8,883,476, filed Jul. 26, 2013 and issued May 11, 2014.
- 10. Yang, T.H., Kim, T.W., Kang, H.O., Lee, S.H. et al. (2010) Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase. Biotechnol. Bioeng., 105 (1), 150–160.
- 11. Jung, Y.K., Kim, T.Y., Park, S.J., and Lee, S.Y. (2010) Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol. Bioeng., 105 (1), 161–171.
- 12. Jung, Y.K. and Lee, S.Y. (2011) Efficient production of polylactic acid and its copolymers by metabolically engineered Escherichia coli. J. Biotechnol., 151 (1), 94–101.
- 13. Yamada, M., Matsumoto, K., Uramoto, S., Motohashi, R. et al. (2011) Lactate fraction dependent mechanical properties of semitransparent poly(lactate-co-3-hydroxybutyrate)s produced by control of lactyl-CoA monomer fluxes in recombinant Escherichia coli. J. Biotechnol., 154 (4), 255–260.
- 14. Chae, C.G., Kim, Y.J., Lee, S.J., Oh, Y.H. et al. (2016) Biosynthesis of poly(2-hydroxybutyrate-co-lactate) in metabolically engineered Escherichia coli. Biotechnol. Bioprocess Eng., 21 (1), 169–174.
- 15. Lee, S.Y. (1996) Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng., 49 (1), 1–14.
- 16. Selmer, T., Willanzheimer, A., and Hetzel, M. (2002) Propionate CoA-transferase from Clostridium propionicum. Eur. J. Biochem., 269 (1), 372–380.
- 17. Matsumoto, K.I., Aoki, E., Takase, K., Doi, Y. et al. (2006) In vivo and in vitro characterization of Ser477X mutations in polyhydroxyalkanoate (PHA) synthase 1 from Pseudomonas sp. 61-3: effects of beneficial mutations on enzymatic activity, substrate specificity, and molecular weight of PHA. Biomacromolecules, 7 (8), 2436–2442.
- 18. Matsumoto, K.I., Takase, K., Aoki, E., Doi, Y., and Taguchi, S. (2005) Synergistic effects of Glu130Asp substitution in the type II polyhydroxyalkanoate (PHA) synthase: enhancement of PHA production and alteration of polymer molecular weight. Biomacromolecules, 6 (1), 99–104.
- 19. Takase, K., Taguchi, S., and Doi, Y. (2003) Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the type II polyhydroxyalkanoate synthase gene. J. Biochem., 133 (1), 139–145.
- 20. Takase, K., Matsumoto, K.I., Taguchi, S., and Doi, Y. (2004) Alteration of substrate chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by in vitro evolution: in vivo and in vitro enzyme assays. Biomacromolecules, 5 (2), 480–485.
- 21. Yang, T.H., Jung, Y.K., Kang, H.O., Kim, T.W. et al. (2011) Tailor-made type II Pseudomonas PHA synthases and their use for the biosynthesis of polylactic acid and its copolymer in recombinant Escherichia coli. Appl. Microbiol. Biotechnol., 90 (2), 603–614.
- 22. Zaman, H.U., Song, J.C., Park, L.S., Kang, I.K., and Park, S.J. (2012) Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol., 93 (1), 273–283.
- 23. Choi, S.Y., Park, S.J., Kim, W.J., Yang, J.E. et al. (2016) One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat. Biotechnol., 34, 435–440.
- 24. Park, S.J., Jang, Y.A., Lee, H., Park, A.R. et al. (2013) Metabolic engineering of Ralstonia eutropha for the biosynthesis of 2-hydroxyacid-containing polyhydroxyalkanoates. Metab. Eng., 20, 20–28.
- 25. Yang, T.H., Park, S.J., Lee, E.J., Kang, H.O., et al. (2013). Recombinant Ralstonia eutropha capable of producing polyactic and acid or polylatic acid polymer, and method for producing polyactic acid or polylatic acid copolymer using the same. US Patent 20,140,242,650, filed May 18, 2013 and issued Aug. 28, 2014.
- 26. Park, S.J., Jang, Y.A., Noh, W., Oh, Y.H. et al. (2015) Metabolic engineering of Ralstonia eutropha for the production of polyhydroxyalkanoates from sucrose. Biotechnol. Bioeng., 112 (3), 638–643.
- 27. Oh, Y.H., Eom, I.Y., Joo, J.C., Yu, J.H. et al. (2015) Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean J. Chem. Eng., 32 (10), 1945–1959.
