Chapter 8
Miscellaneous Titrations
ARGENTOMETRY
Introduction and Principle
This titrimetric method is mainly based on the titrant used in the titration. The silver nitrate is used as the titrant which precipitates the subsequent halide. This method is similar to that of the precipitation titrations. It is mainly used for the determination of the halides and halide compounds.
Theory
The sample is mixed with the silver nitrate and forms the precipitate. In argentometric titrations, the sodium chloride is used as the primary standard for the standardisation of the silver nitrate.
Standard solution of the silver nitrate and the primary standard is prepared. Carry out the standardisation of the silver nitrate and calculate the normality of the prepared silver nitrate solution. Then the sample solution is prepared and the sample solution is titrated with the standard silver nitrate solution until the end point is obtained.
The concentration of the sample solution is determined by the equation or by the titration curve.
where N1 is the normality of the sample; V1 is the volume of the sample solution; N2 is the normality of the prepared silver nitrate solution; V2 is the volume of the titrant consumed up to the end point
Four Parameters Should be Considered in the Argentometry
- Precipitate must be insoluble.
- Precipitate formation should be fast and rapid.
- Co-precipitation must be avoided.
- End point should be clearly visible.
End Point Detection
The end point in the argentometric titrations are detected by the following methods.
- Chemical method: By using the chemical reagent, the colour change is detected at the end point. These indicators are similar as that of the precipitation titrations.
Examples: Chromate indicator: This method is named as Mohr's method. Fluorescein indicator: This method is named as Fajan's method. Ferric solution: This method is named as Volhard's method. - Potentiometric method: Potential difference between the silver electrode and a reference electrode is measured when immersed in the titration mixture.
- Amperometric method: In this method, current generated between the pairs of silver microelectrodes immersed in the titration mixture. Current plotted as the function of reagent volume.
Preparation of the Standard Silver Nitrate Solution
Accurately weighed silver nitrate is dissolved in the distilled water and made up to the required volume with the distilled water.
Standardisation of the Silver Nitrate Solution
The previously dried sodium chloride at 120 °C for 1 h 30 min is accurately weighed and is dissolved in the water. To this solution, equal amount of the acetic acid and ethyl alcohol is added. To this, little quantity of eosin is added as the indicator and then the standard silver nitrate solution is titrated until pink colour is appeared.
ADVANTAGES
- Simple
- Less time consumption
- Specific
DISADVANTAGES
- Interferences are more.
- Care should be taken while preparation of silver nitrate solution.
APPLICATIONS
- Used in the determination of the silver ores and alloys.
- Used in the determination of halogens.
- Used in the determination of the mercaptans.
- Used in the determination of the fatty acids.
- Used in the determination of the zinc compounds.
Method: Initially the zinc compound is precipitated with the precipitating agent. Then the precipitate formed is dissolved in the mineral acid and is titrated with the excess of silver nitrate solution. Next the excess of silver nitrate is titrated with the ammonium thiocyanate using ferric ammonium sulphate as the indicator.
ZnHg(CNS)4 + AgNO3 Zn(NO3)2 + Hg(CNS)2 + 2AgCNS + AgNO3 (Excess)
Zn(NO3)2 + Hg(CNS)2 + 2AgCNS + AgNO3 (Excess) - Used in the sulphonamides determination.
Method: An accurately weighed 200 mg of the sample is dissolved in small quantity of NaOH. Then thymolphthalein solution is added to adjust the sample solution colour to blue. Then 50 ml of distilled water is added. Then the blue colour is discharged with little quantity of sulphuric acid. To this, the silver nitrate is added until the black colour precipitate is formed. Collect the precipitate and acidify it with the nitric acid. Then the excess silver is titrated with the ammonium thiocyanate by using ferric alum as the indicator.
This method is used in the determination of the sulphadiazine, sulphamerazine and sulphamethazine.
IODOMETRY AND IODIMETRY
Introduction
These methods are widely used in the analysis of the compounds based on the reactions of the iodine. Iodine reacts directly and fastly for the determination of the compounds. The direct method is referred as iodimetry and the indirect method is referred as the iodometry.
Principle and Theory
The main principle involved in the iodometry and the iodimetry titration is the oxidation-reduction reaction.
In iodimetry, a reducing agent is titrated directly with the iodine to produce I−.
Example: Estimation of the vitamin C.
In this method the reducing agent is titrated with the iodine solution.
Iodine oxidises the sample. The end point is detected by the starch as indicator.
In iodometry, an oxidizing sample is added to excess of I− to produce iodine which is back titrated with the standard solution of the sodium thiosulphate. In this method the iodine is produced when an oxidizing substance is added to the excess I−.
Then, the iodine produced is back titrated with the help of an appropriate titrant like sodium thiosulphate.
Thiosulphate is a strong reducing agent which is mainly used in the determination of oxidizing agents.
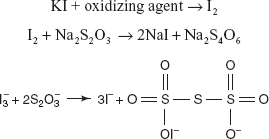
Starch is used as the indicator for both the reactions which produce intense blue colour for the iodimetry and the disappearance of the intense blue colour is the end point for the iodometry.
Example: Determination of chlorites.
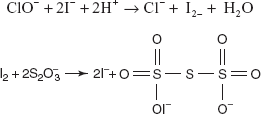
Preparation of 0.1N Iodine Solution
Weigh 3.2 g of iodine crystals in a watch glass and mix with 7.5 g of potassium iodide and distilled water. Stir the solution continuously to dissolve the iodine crystals and fill the required volume with the distilled water.
Standardisation of 0.1N Iodine Solution
Weigh 500 mg of arsenic trioxide in a beaker and the 2 ml of sodium hydroxide solution is added. Warm the solution to dissolve the crystals. Then fill the required volume with distilled water. Pipette out the above solution and acidify with the help of diluted HCl and little quantity of sodium carbonate is added to remove the excess of acid. The resulting solution is then titrated with the 0.1N iodine solution until a pale straw colour is obtained.
The demerits of the starch indicator are as follows:
- Insolube in cold water.
- Unstable in the water.
- It produces water insoluble complex with iodine.
Factors Affecting the Iodometry and Iodometric Titrations
- Hydrogen ion concentration: The hydrogen ion concentration increases the oxidizing power of the oxidizing agents. Hence the hydrogen ion concentration is directly proportional to the oxidizing power of the oxidants. So it increases the iodine activity.
- pH of the sample solution: The pH of the solution is inversely proportional to the oxidizing power of the oxidant. The increase in the pH decreases the iodine solution activity that is oxidizing power.
Errors that are Observed in the Iodometry and Iodometric Titrations
- Loss of iodine due to its high volatility.
- Readily oxidised when exposed to the air.
APPLICATIONS
- Used in the determination of the copper.
Method: A known quantity of copper is dissolved in the nitric acid. The excess of nitric acid is removed by the addition of sulphuric acid. Then pH of the solution is adjusted with the ammonium hydroxide. To this, 10% potassium iodide solution is added and the liberated iodine is titrated with the sodium thiosulphate using starch iodide as the indicator. The end point is detected by the formation of the white precipitate.
2Cu+2 + 4KI Cu2 Cl2 + 4K+ + I2 ↓
Cu2 Cl2 + 4K+ + I2 ↓ - Used in the determination of the alkaloids.
Method: Alkaloids are determined by the iodometric method. The alkaloid is dissolved in the alcohol and in the excess sulphuric acid. Then potassium iodide-potassium iodate solution is added and the liberated iodine is titrated with the 0.1N sodium thiosulphate using starch as the indicator. The reaction is as follows:
KIO3 + 5KI + 3H2SO4 3K2SO4 + 3H2O + 3I2
3K2SO4 + 3H2O + 3I2 - Used in the determination of the chlorates.
- Used in the determination of the adrenaline.
Method: Extract the drug with carbon tetra chloride and add starch solution and iodine solution. Then sodium thiosulphate is added and finally sodium bicarbonate is titrated.
- Used in the determination of analgesics and antipyretics.
Method: Sample is dissolved in acetic acid and water. Warm this solution not exceeding above 70 °C. To this, 0.25N iodine solution is added with continuous shaking followed by addition of concentrated HCl. The flask is stoppered and shake until the precipitate obtained. Then dilute with water and allow standing for 30 min. Next filter the precipitate through glass-sintered filter. Take 50 ml filtrate which is titrated with 0.1N sodium thiosulphate using starch as the indicator. This method is used for the determinations of acetophenatidin, cinchophen and antipyrine.
- Used in the determination of the ascorbic acid content of pure solution or purity can be determined by titration with 0.1N iodine solution.
- Used in the analysis of hydrogen peroxide.
- Used in the determination of the dissolved oxygen.
- Used in the determination of the arsenic.
- Used in the determination of the sulphurous acid.
REVIEW QUESTIONS
- What is the principle involved in the Argentometry?
- What are the conditions required for the Argentometry?
- What are examples drugs assayed by the Argentometry?
- What are the advantages of Argentometry?
- What are the factors that affecting the Argentometry?
- What are the different methods used for the end point detection for the Argentometry?
- What is the principle difference between the iodometry and iodimetry?
- What is the theory involved in the iodometry and iodimetry?
- What are the errors observed in the iodometry and iodimetry?
- What are the advantages and disadvantages of iodometry and iodimetry?
