Chapter 3
Analysis of Carboxylic Acids
INTRODUCTION
The most of the pharmaceutical agents contain the carboxylic acids. The carboxylic acid containing drugs are Salicylic acid derivatives used as antipyretics, Nicotinic acid, para-amino benzaldehyde, etc.
Based on the importance of these drugs, the analytical methods are proposed. They are as follows:
- IR method: This method is mainly based on the absorption of the IR radiation by the sample which is because of the stretching vibrations of the C=O bond which is measured at the 5.65–5.80 μm.
- UV method: This method is mainly based on the determination of the UV radiation absorption by the sample which under goes transition. This is carried by dissolving the sample in the appropriate solvent and is measured at absorption maxima. The carboxylic acids show the absorption maxima at 250–280 nm. The generally used solvent in this method is ethanol.
- Reaction methods: These methods are mainly based on the measurement of the samples reacted with any reagents and the absorption maxima is detected by the different methods such as visible, UV and other methods.
- Ferric hydroxamate method: The method involves the reaction of the carboxylic acid sample with the hydroxylamine to produce the hydroxymic acid. The hydroxymic formed is reacted with the ferric ion to form the coloured product which is measured at 520–550 nm.
RCOOH + NH2OH
 RCONHOH + H2O3RCONHOH + 3H2O + Fe+3
RCONHOH + H2O3RCONHOH + 3H2O + Fe+3 Fe(RCONHO)3 + 3H2O + 3H+
Fe(RCONHO)3 + 3H2O + 3H+The procedure involved is as follows: The sample is dissolved in the anhydrous alcohol and 3 ml of the reagent solution such as alkaline hydroxylamine hydrochloride solution is added. Then this solution is refluxed for 5 min. Then ferric hydroxide solution is added. Then the resulting solution absorption maxima at 500–520 nm is measured using the reagent blank solution.
- Gas chromatographic method: The carboxylic acids are determined by the GC method by heating the sample in the presence of the quinoline and cupric carbonate. Then the carbon dioxide produced is carried through the chromatographic column and is determined by its peak height which gives the amount of the carboxylic acid in the sample.
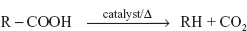
The procedure involves the packing of the column with the 25% SF 96 silicone oil on 30/60 mesh chromosorb at 51 °C. This is connected to the thermal conductivity detector. The sample is mixed with the cupric carbonate and 0.2 ml of the quinoline. Then this solution is heated to 225 °C for 45 min. Then cool the reaction mixture to 100 °C and the carbon dioxide formed is carried with the carrier gas and the peak height is determined.
- Electro analytical methods:
- Coulometric titration: The basic principle involved in this method is the carboxylic acids are determined by an electrolytic reaction by generating the titrant for a period of time. The procedure involved in this method is by dissolving the sample in the aqueous potassium bromide and dip the indicator electrode and the reference electrode in this solution. Titrate the sample at ca. 20 mA current and record the time at the end point.
Then the milliequivalents of the carboxylic acid is given by the following equation:
= Current (i) × time (t) 96,500 - Conductometric titration: In this method, the conductivity of the sample is determined when it is placed in the electrolytic solution. The method involves the sample is dissolved in the 0.2M ammonia and then transfer the sample into the conductivity cell. Then add ammonia to make up the volume to 125 ml followed by stirring. The resulting solution is titrated with the lithium hydroxide solution. Then the end point is located by the plotting between the conductance and the volume of the titrant. Then calculate the amount of the carboxylic acid present in the sample.
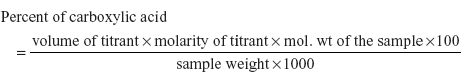
- Coulometric titration: The basic principle involved in this method is the carboxylic acids are determined by an electrolytic reaction by generating the titrant for a period of time. The procedure involved in this method is by dissolving the sample in the aqueous potassium bromide and dip the indicator electrode and the reference electrode in this solution. Titrate the sample at ca. 20 mA current and record the time at the end point.
- NMR method: The carboxylic acids are determined by the NMR method using 60 MHz proton NMR. This method is first proposed by Morris and Susi. Later Sawyer and Brannan proposed the other method that is the samples are dissolved in the D2O and are determined by using tetra methyl ammonium chloride as an internal standard.
- Radiochemical methods: Quantitative derivatisation of the sample is more important in the determination of the carboxylic acids by this method. The derivatisation involves treatment of the carboxylic acid with the following reagents:
- Diazomethane-C14 in ether-methanol.
- Tritiate water in ether and non-radioactive diazomethane.
- A solution of the sodium hydroxide and sodium methoxide-H3 in methanol-H3.
- Methanol C14 in the presence of boron trifluoride.
Then the excess reagent is removed.
RCOOH + C14CH2N2 R-COOC14CH3+N2
R-COOC14CH3+N2Then the sample attached to the radioactive compound is determined.
REVIEW QUESTIONS
- Explain the principles involved in the spectrophotometric analysis of the carboxylic acid group.
- What is the principle involved in the ferric hydroxamate method?
- Give the procedure for the analysis of the carboxylic acid group by the gas chromatographic method.
- Explain the principle involved in the coulometric analysis of the carboxylic acid group.
- What is the principle involved in the analysis of carboxylic acid group by the conductometric method?
- Give the procedure for the analysis of the carboxylic acid group by the radio chemical methods.
