Chapter 8
Supercritical Fluid Chromatography
INTRODUCTION
Chromatography is an essential group of techniques for the separation of the compounds of mixtures by their continuous distribution between two phases i.e. stationary phase and mobile phase and the system is associated with the following:
- A solid stationary phase and a liquid (or) gaseous mobile phase are named as adsorption chromatography.
Example: Gas chromatography (GC).
- A liquid stationary phase and a liquid (or) gaseous mobile phase are named as partition chromatography.
Example: Paper chromatography.
Advances in technology have resulted in wide range of techniques varying in complexity, separation ability, sensitivity of adsorption and partition chromatography provides an excellent separation and allows the accurate assay of very low concentrations of a wide variety of substances in complex mixtures.
Supercritical fluid chromatography (SFC) is a hybrid technique of gas and liquid chromatography because when mobile phase is gas and stationary phase is liquid this technique is called as liquid chromatography. When mobile phase is liquid and stationary phase is gas, then the technique is called the GC. So SFC combines the best features of both liquid chromatography and GC. SFC is an important technique because it permits separation and determination of group of compounds that are not conveniently handled either by GC (or) liquid chromatography.
The phenomenon and behaviour of supercritical fluid (SCF) have been the subject of research right from 1800s. Hanny and Hogarth in 1879 first demonstrated solubility in SCF but first suggestion of SFC was put forward in 1958 by Lovelock. In 1962, Klesper Corvin and Turner used SFC for separation of porphyrins. Giddings in 1966 and Sie Rijender in 1967 were responsible for further developments of SFC. Jentoft and Gouw in 1972 successfully carried out analysis of petroleum-derived mixture by SFC. Novotny and Lee et al. demonstrated the first experiments on capillary SFC in 1982. The first commercial packed column of SFC was made available in 1981 and the first commercial capillary column SFC instrument was introduced in 1985.
THEORY
SFC is defined as when the sample is carried through a separation column by a SCF where the mixture is divided into unique bands based on the amount of interaction between the individual analyte and the stationary phase in the column. As these bands leave the column, their identities and quantities are determined by a detector.
Here SCF is defined as from a phase diagram for a pure substance in which the regions corresponding to solid, liquid and gaseous states are clear.

Phase transitions in SFC
Here we have to know about critical temperature, i.e., temperature at which a liquid no longer exists as liquid, and critical pressure, i.e., pressure at which a gas no longer exists as gas.
From the above figure, this SCF is defined as a fluid obtained by heating above its critical temperature and compressing above its critical pressure. The liquid is converted to super critical fluid by increasing the temperature with constant pressure and the gas is converted to supercritical fluid by increasing pressure with constant temperature.
A substance such as CO2 can exist in solid, liquid and gaseous phases under various combinations of temperature and pressure. For every substance, there is a temperature above which it can no longer exist as a liquid, no matter how much pressure is applied. Likewise, there is a pressure above which the substance can no longer exist as a gas no matter how high the temperature is raised. These points are called critical temperature and critical pressure, respectively, and are the defining boundaries on a phase diagram for a pure substance. At this point, the liquid and vapour have the same density and the fluid cannot be liquefied by increasing the pressure. Above this point, where no phase change occurs, the substance acts as a SCF. So SCF can be described as a fluid obtained by heating above the critical temperature and compressing above the critical pressure. There is a continuous transition from liquid to SCF by increasing temperature at constant pressure or from gas to SCF by increasing pressure at constant temperature. The term compressed liquid is used frequently to describe a SCF, a near critical fluid, an expanded liquid or a highly compressed gas.
Examples of SCFs are the following: Supercritical ethane, nitrous oxide, ammonia, N-pentane, N-butane, carbon tetra fluoride, carbon dioxide.
IMPORTANT PROPERTIES OF SCFs
- SCFs have high densities so they have greater affinity to dissolve large, non-volatile molecules. Solvation strength of SCF is directly related to the fluid density. Thus solubility of solid can be manipulated by making slight changes in temperatures and pressures. Certain important processes are based on the high solubility of organic species in SC–CO2, for example, it has been employed for extracting caffeine from coffee beans to get decaffeinated coffee and for extracting nicotine from cigarette tobacco.
Example: Supercritical CO2 readily dissolves n-alkenes containing 5-30 carbon atoms.
- Another important property of SCFs is that dissolved analysis can be easily recovered by simply allowing the solutions to equilibration to atmosphere at low temperatures. Finally, SCFs have the advantage of higher diffusion constants and lower viscosities relative to liquid solvents. The low viscosity means that pressure drop across the column for a given flow rate is greatly reduced. The greater diffusibility means longer column length can be used. Higher diffusion coefficient means higher analysis speed that increases in the order high-performance liquid chromatography (HPLC), SFC and GC. These advantages are important in both chromatography and extractions with SCFs.
Example: An analyte dissolved in the supercritical CO2 can be recovered simply by reducing the pressure and allowing to evaporate.
The following are some of the advantages and disadvantages of SCFs compared to conventional liquid solvents for separations:
ADVANTAGES
- Dissolving power of the SCF is controlled by pressure and/or temperature.
- SCF is easily recoverable from the extract due to its volatility.
- Non-toxic solvents leave no harmful residue.
- High boiling components are extracted at relatively low temperatures.
- Separations not possible by more traditional processes can sometimes be effected.
- Thermally labile compounds can be extracted with minimal damage as low temperatures can be employed by the extraction.
DISADVANTAGES
- Elevated pressure required.
- Compression of solvent requires elaborate recycling measures to reduce energy costs.
- High capital investment for equipment.
Solvents of SCF Extraction
The choice of the SFE solvent is similar to the regular extraction. Principle considerations are the followings:
- Good solving property
- Inert to the product
- Easy separation from the product
- Cheap
- Low PC because of economic reasons
The following table shows critical properties of some commonly used SCFs
| Fluid | Critical temperature (in Kelvins) | Critical pressure (in lbs) |
|---|---|---|
| CO2 | 304.1 | 73.8 |
| Ethane | 305.4 | 48.8 |
| Toluene | 591.8 | 40.0 |
| Water | 647.3 | 221.2 |
INSTRUMENTATION
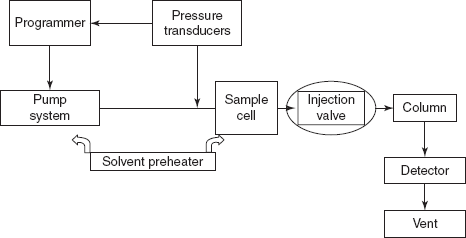
Flow chart of the SFC
The instrumentation of SFC is similar in most regards to instrumentation for HPLC because the temperature and pressure required for creating SCF from several liquids (or) gases lie within operation limits of HPLC equipment. There are mainly two differences between them. They are as follows:
- A thermostatic over
- A restrictor (to maintain pressure)
The instrumentation SFC contains the following components:
- Pumps: High-pressure pumps are used to obtain passing of mobile phase through the column high pressure at about 1000-3000 psi. For packed columns, they use reciprocating pumps and for capillary columns, syringe pumps are used.
- Restrictor: Restrictor is used to maintain desired pressure in the column by a pressure adjustable diaphragm.
- Microprocessors: Equipped with one (or) more microprocessors to control variables such as pumping pressure, oven temperature and detector performance.
- Columns: Two types of columns are mainly used in SFC:
- Capillary columns packed with octadecyl silyl (C18).
- Packed columns packed with alumina, silica, polystyrene.
- Injector: For packed columns, conventional HPLC injection system is used and the most commonly used is rheodyne injector. This has a fixed volume loop such as 20-50 ml (or) more. Injector has two modes, i.e. load position, when the sample is loaded in the loop, and inject mode, when the sample is injected.
For capillary columns, pneumatically driven valves are used.
- Thermostatic oven: A thermostat is required for precise temperature to control the temperature of mobile phase conventional GC (or) liquid chromatography ovens are used. Temperature maintenance is highly essential for, efficient separation. Two types of operations are available for the maintenance of temperature. They are as follows:
- Isothermal programming in which the same temperature is maintained throughout the process of separation.
- Linear programming in which the oven is heated linearly over a period of time. This type of programming is required where a sample has a mixture of low boiling and high boiling point compounds.
Example: -160 °C initially to 210 °C at the end of separation with an increase in temperature at the rate of 5 °C/min.
- Detectors: It is compatible with both GC and HPLC detectors. The most commonly used detectors are the following:
- Katharometer (or) thermal conductivity detector
- Flame ionisation detector
- Argon ionisation detector
- Electron capture detector
- UV detector
- Refractive index detector
- Flour metric detector
- Conductivity detector
- Amperometric detector
- Photodiode array detector
Above detectors are compatible with SFC instrument.
In method development of SFC, the following parameters have been considered:
Stationary phase: Octadecylsilyl (C18), alumina, silica, and polystyrene.
Mobile phase: Supercritical CO2, water, ethane, butane, and carbon tetra fluoride.
Modifiers: These modifiers play an important role in SFC to modify the stability and reactivity of SCFs. They can also enhance selectivity of separation and improve separation efficiency by blocking some of the highly active sites on the stationary phase. Small amount (3.5%) of methanol to CO2 increases the solubility of cholesterol. If an analyte is only soluble in an aqueous solution, it is probably a poor candidate for SFC. Apart from methanol other solvents such as acetonitrile, ethanol and 1-propanol are also used as modifiers. For highly retained non-polar solutes, modifiers increase the column efficiency. For polar solutes, they improve both retention and efficiency.
Example: Alcohols, cyclic ethers, methanol.
CATEGORISATION OF SFC

ADVANTAGES OF SCF OVER HPLC AND GC
- Faster separation than HPLC.
- Lower zone broadening than GC.
- High resolution than HPLC and GC.
- High molecular weight compounds can be handled conveniently than GC.
- Non-volatile compounds, thermally unstable compounds and polymers can be handled.
- The uses of organic chemicals are minimised with the use of SCF.
LIMITATIONS
- High capital equipment investment.
- Number of controllable factors is more hence more time is required for method development.
PRECAUTIONS
Only precaution should be taken in the development of SCF chromatogram is pressure programming. This is analogous to temperature programming in GC and gradient elution in HPLC. It is also called as density programming.
When pressure increased density also increases which results in increase in solvating ability and decrease in retention time (Rt) that indicates less time consuming.
There are two types of density programming:
- Linear density program: Pressure increased at fixed rate.
- Asymptotic density program: Increased pressure is linearly reduced as it approaches maximum.
COMPARISON OF SFC WITH HPLC AND GC
SFC combines some of the characteristics of gas and liquid chromatography, as several physical properties of SCF are intermediate between gases and liquids. Like GC, SFC is inherently faster than LC because the lower viscosity makes use of higher flow rates. Diffusion rates in SCFs are intermediate between gases and liquids.
As a consequence, band broadening is greater in SCFs but less than in gases. Thus, the intermediate diffusivities and viscosities of SCFs result in faster separation than is achieved in LC, accompanied by lower zone broadening than is encountered in GC.
The mobile phases play different role in GC, LC and SCF. In GC, the mobile phase causes the zone movement. In LC, the mobile phase transports the solute molecule and also interacts with them thus influencing the selectivity. When a molecule dissolves in supercritical medium, the process resembles volatilisation but at much lower temperature than that of GC. Thus, at a given temperature, the vapour pressure for a large molecule in SCF may be 1010 greater than in the absence of that fluid. As a consequence, high molecular weight compounds, thermally unstable species, polymers and large biological molecules can be eluted from a column at a reasonably low temperature.
The biggest advantage that SFC holds over GC is the ability to separate thermally labile compounds. This is appreciated in the pharmaceutical fields since roughly 20% of all drugs candidates fall in this category. Unlike GC, by changing the mobile phase, the selectivity can be varied in SFC.
Due to thermally unstable or non-volatile nature of many nitrogen and/or sulphur containing compounds, they cannot be analysed by GC. Even if HPLC is applicable to analyse these compounds, it generates a large number of organic solvents, which need to be ultimately disposed. The disposal cost of organic solvents typically ranges from $5 to $10 per gallon and is constantly rising due to the strict environmental regulations. With the desire for environmentally conscious technology, the use of organic chemicals as used in HPLC could be reduced with the use of SFC. Because SFC generally uses carbon dioxide, collected as a by-product of other chemical reactions or is collected directly from the atmosphere, it contributes no new chemicals to the environment.
Like GC, SFC is inherently faster than HPLC, because of its lower viscosity and higher diffusion rates. It is well documented that SFC provides a combination of 3-5 times increase in the speed of analysis and a decrease in the analysis cost through saving in organic solvent.
Unlike GC or HPLC where the mobile phase dominates the type of detector to be used, SFC utilises mobile phase, which can be either liquid like or gas like. Therefore, both GC and HPLC detectors are applicable to SFC. This multidetector compatibility makes SFC a technique of unparallel success in the analysis of thermally liable species and/or relatively high molecular weight compounds.
SFC has several main advantages over conventional chromatographic techniques (GC and HPLC). The biggest advantage that SFC has over HPLC lies within the differences in the mobile phases. SCFs are less viscous, possess a higher diffusivity than liquids under HPLC conditions and allow lower pressure drops along an analytical column. This provides not only the ability to increase column lengths but also allows for faster flow rates. These factors in turn affect capacity ratios, selectivities and theoretical plate heights. It has been reported that 200,000 theoretical plates have been achieved by using 11 analytical (4.6 mm id) columns in series. Additionally, SFC can be set up for sub-ambient temperatures, which has been a key in many chiral separations.
APPLICATIONS OF SFC
- Used in forensic science for separation and identification of opiate drugs, excipients, stimulant drugs, barbiturates, explosives, etc.
Example: Codeine, antioxidants, barbituric acid etc.
- Used in the micro-particle formulation technique.
Example: Liposome’s, nanoparticle, solvent-free solid dispersion dosage forms formulation.
- Used in the pharmaceutical final product sterilisation by using supercritical particle technology.
Example: Parentrals determination (or) stability increasing.
- Used in natural products analysis.
Example: Chlorophyll, carotenoids, tocopherols, vitamins, etc.
- Used in the separation of chiral compounds.
Example: Albendazole sulphoxide enantiomers. Cis and trans β-carotene enantiomers.
- Used in the separation of organometallic compounds.
Example: Lead, mercury and tin.
- Used in the identification of milk fat triglycerides.
Example: Lactose
- Used in the analysis of β-agonists.
Example: COX-II inhibitors.
- Used in the analysis of pesticide residues in canned foods, fruits in which parathyroid’s, herbicides, fungicides have been tested.
Example: DDT determination.
- Used in the separation of oligomers.
Example: Triton X 100 (non-ionic surfactant) separation.
- Used in the separation of polymers.
Example: Dimethyl poly siloxane oligomers.
- Used in the separation of anti-depressants, phenothiazine antipsychotic, β-blockers, etc.
Example: Phenothiazine separation.
- Used in the determination of mefloquine in blood.
REVIEW QUESTIONS
- What is the principle and theory involved in the supercritical fluid chromatography?
- What are the properties of the supercritical fluids?
- Explain the instrumentation of the SFC.
- Add a note on modifiers used in the SFC.
- What are the advantages and limitations of SFC?
- What are the applications of SFC?
- Compare the SFC with traditional chromatographic techniques.
