Chapter 5
Analysis of Local Anaesthetic Drugs
Local anaesthetics are the drugs used for the anaesthetic effect on the local area. The commonly employed drugs are as follows:
- Benzocaine
- Procaine
- Cocaine
- Orthocaine
The following are analytical methods used for the determination of the drugs. They are as follows:
- Spectrometric methods
- Visible method
- UV method
- Fluorimetry
- Titrimetric methods
- Residual titration
- Bromimetry
- Precipitate titration
- Titration with acid dyes
- Gravimetry
- IP methods
- Spectrometric methods:
- Visible method:
- Diazotization method: Sample is mixed with HCl and NaNO2 which act as diazotizing reagents. Then keep the solution a side for 3 min. Then add 95% alcohol and add coupling reagent such as 0.1% Bartton–Marshall reagent. Then within 15 min measure the absorbance at 545 nm. This method is used for the determination of benzocaine and procaine.
- Dye method: Sample is mixed with methyl orange which is a strong indicator. Then extract the coloured solution with the chloroform. Then measure the solution at 520 nm using reagent blank. This method is used for the determination of benzocaine and procaine.
- FC reagent method: The local anaesthetics contain the amine group which reacts with the Folin–Ciocalteau reagent in the presence of alkaline conditions. The method is as follows: sample is mixed with sodium hydroxide and Folin–Ciocalteau reagent. Then keep a side for 20 min for colour development and measure the absorbance of the blue coloured solution at 760 nm. This method is used for the determination of the procaine.
- UV method: This method is mainly based on the absorption of the UV radiation when the drug is dissolved in the appropriate solvent.
Drug Solvent Absorption maxima (nm) Benzocaine Alcohol 249 Cocaine HCl 275 Orthocaine Alcohol 265 Procaine Water 290 - Fluorometric method: This method is mainly based on the florescence producing drugs which is determined when dissolved in the common solvents in the presence of alkali. The method is as follows: the sample is dissolved in the water and measured at 345 nm.
- Visible method:
- Titrimetric methods:
- Residual titration: In this method, the sample is mixed with the excess of standard acid such as HCl or H2SO4. Then the uncombined acid is back titrated with the strong base such as NaOH or KOH. The end point is detected by the visible indicator such as phenolphthalein or by potentiometer.
- Bromimetry: The amines are dissolved in HCl and are reacted with the potassium bromide. Then the solution is titrated with the potassium bromate and the liberated bromine is reacted with the excess of iodine solution. Then the excess of iodine solution is back titrated with the sodium thio sulphate using starch iodide paste as the indicator.
- Precipitate titration: In this method, the sample is dissolved in water and the precipitating reagent such as sodium tetra phenyl boron is added and allowed to settle down the precipitate. Then the precipitate is washed with water. Then dissolve in the acetone and add HgCl2 and methyl red as the indicator. Then alkalize the solution with the standard NaOH and heat the solution and cool to room temperature and add potassium iodide solution. Then titrate with the HCl.
- Titration with the acid dyes: In this method, the sample is dissolved in the water and chloroform is added. Then titrate with the chloroformic solution of bromothymol blue indicator.
- Gravimetric method: In this method, the sample is dissolved in water and the precipitating reagent such as sodium tetra phenyl boron is added and allowed to settle down the precipitate. Then wash the precipitate with water. Then dry the precipitate in hot air oven to remove the moisture present in the precipitate. Then weigh the precipitate. Then the percent of drug is calculated by the following formula:
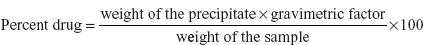
- IP methods:
- For benzocaine: Sample (400 mg) is dissolved in 25 ml of HCl and 50 ml of water. The solution is cooled to 10 °C. Then titrate the solution with the 1M sodium nitrite solution.
1 ml of 1M NaNO2 ≡ 0.01652 g of drug
- For bupiracaine: Sample (250 mg) is mixed with 5 ml of 0.01M HCl and 50 ml of ethanol and titrate the solution with 1M NaOH ethanolic solution.
1 ml of 1M NaOH ≡ 0.03249 g of drug
- For lignocaine: Sample (500 mg) is dissolved in 30 ml of acetic acid and 6 ml of mercuric acetate solution. Then add crystal violet as the indicator. Then titrate the resulting solution with 0.1M perchloric acid.
1 ml of 0.1M perchloric acid ≡ 0.027089 g of drug
- For benzocaine: Sample (400 mg) is dissolved in 25 ml of HCl and 50 ml of water. The solution is cooled to 10 °C. Then titrate the solution with the 1M sodium nitrite solution.
REVIEW QUESTIONS
- What are the methods used for the analysis of the local anaesthetics by the spectrometric methods?
- Explain the principle involved in the analysis of the local anaesthetics by the UV methods.
- What is the principle involved in the fluorometric analysis of the local anaesthetics?
- What are the IP methods for the analysis of the local anaesthetics?
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
