Chapter 6
Mass Spectrometry
INTRODUCTION
Wein in 1898 proposed first crude mass spectra and identified the two neon isotopes by mass spectrometry by combining the electrostatic and magnetic fields. Aston developed mass spectra for more than 50 elements. Beynon in 1960 first wrote on the theory of mass spectrometry. Dampster proposed the instrumentation for the mass spectroscopy.
Mass spectrometry is defined as the measurement and interpretation of the positive ions based on their masses. It gives the information about the molecular structure of the organic and inorganic compounds.
PRINCIPLE
The basic principle involved in mass spectroscopy is when the compounds are bombarded with electrons, the compound may lose one electron and forms metastable ion:
Again increasing the energy leads to the formation of positive ions, which is separated and recorded by the mass spectrometer based on their mass-charge ratios.
Mass-charge ratio (m/e) is defined as the charge of the sample divided by the mass of the sample. This is useful for the measurement of the molecular structure based on the charges on the molecules.
THEORY
For an unit of charge (e) with mass (m), the acceleration after bombardment is v. The potential energy eV is equal to its kinetic energy:
where V is the acceleration voltage.
When this electron is placed in magnetic field the ion shows the force Hev which is equal to mv2/r. r is the radius of the semicircular electronic path.
Therefore, from the above equations
From this, we can observe that
- The radius r of an ion of given mass–charge ratio that can be changed by varying the values of H and V.
- Mass—charge ratio depends on the singly charged or doubly charged particles.
INSTRUMENTATION
The mass spectrometers should be able to perform the following functions:
- Ions are produced from the sample molecules when subjected to high energy beam of electrons.
- Ions are separated based on the mass–charge ratio when accelerated in the electric field.
- Ions are detected by the collector.
The following are the important components of mass spectrometer:
- The inlet system.
- The ion source.
- The electrostatic system.
- The separator.
- The collector.
- The vacuum system.

Flow chart for the mass spectrometer
- The inlet system: The sample introduced into the mass spectrometer should be at an atmospheric pressure. There are two main methods for the sample inlet:
- Direct introduction: This is commonly used in the matrix-assisted laser desorption/ionization (MALDI)-MS. The sample is initially placed in the probe and then introduced into the ionization source.
- Direct infusion: This is commonly used in the ESI-MS. A simple capillary is used to introduce the sample such as gas or solution form.
- Generally the sample introduced into the mass spectrometer should be in the form of vapour. To achieve this, the inlet system should be kept in the heating system.
- The ion source: The ionisation source is the mechanical device to convert the sample to ions. The common ionisation mechanisms used are as follows:
- Protonation: This is nothing but the addition of the proton to a molecule which increases net positive charge. The main advantage is it can be frequently used. The main disadvantage is that in some compounds they are not stable
Example: Carbohydrates.
M + H+ MH+
MH+
It is used in MALDI, electron spray ionisation (ESI) and atmosphere pressure chemical ionisation (APCI).
Example: Peptides are ionised by protonation.
- Deprotonation: This can be achieved by the removal of proton from a molecule which increases net negative charge. This is most useful for acidic compounds. The main disadvantage is that it is compound specific.
M – H+
 (M-H)+
(M-H)+
It is used in MALDI, ESI and APCI.
Example: Salicylic acid is ionised by deprotonation.
- Cationisation: This is the addition of positively charged ion to the neutral molecule with alkali or ammonium. This method is stable than protonation. Because of this, it is frequently used. But it is limited to some particular compounds.
M + cation
 Mcation+
Mcation+
It is used in the MALDI, ESI and APCI.
Example: D-galactose is ionised by the cationisation.
- Charge transfer: This is commonly known as desorption where the solution of the sample is converted to gas. It is mainly used for the charged complexes not for other compounds.
M+(solution)
 M+(gas)
M+(gas)
It is used in the MALDI and ESI.
Example: Tetraphenylphosphine is ionised by the desorption.
- Electron ejection: Electron ejection is achieved by the removal of the electron to produce positively charged molecule.

It is used in the electron ionisation.
Example: Anthracene is ionised by the electron ejection.
- Electron capture: Here, addition of the electron to the sample by absorption or by capture.
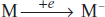
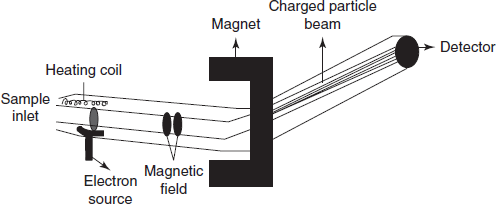
The sample is introduced into the ionisation chamber where the paths of electrons are placed. The molecules present in the sample are ionised by the ionisation source.

Schematic diagram for the ionization of the sample
- Protonation: This is nothing but the addition of the proton to a molecule which increases net positive charge. The main advantage is it can be frequently used. The main disadvantage is that in some compounds they are not stable
- The electrostatic system: The positive ions produced in the ionisation source are passed through the electric field which is placed between the accelerator plate and repeller plate which accelerates the ions of masses m1, m2 and m3 to their final velocities.
Energy eV = ½m1v12 = ½m2v22 = ½m3v32
The initial potential of the electronic field is set up to 4,000 V.
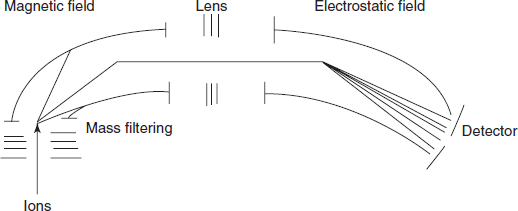
- The ion separator: This is commonly known as analyser which separates the ions according to their masses. An analyzer should have the following characteristics:
- It should have a higher resolution.
- High rate of transmission of ions.
The main types of analysers used in the mass spectroscopy are as follows:
- Single focusing magnetic deflection analyser
- Double focusing analyser
- Quadrapole analyser
- Time of fight analyser
- Single focusing magnetic deflection analyzer: It is most commonly used analyser. At a given voltage v, all the ions which are ionised produce the same energy.
eV = ½mv2
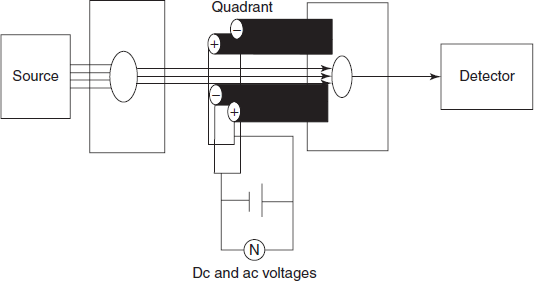
- Double focusing analysers: It is mainly used for the high resolution. In this, two ion beams are passed and detected by the separate collectors. The main advantages of this type of analyzer are high reproducibility and high sensitivity. The main disadvantage is its high cost and not suitable for pulsed ionisation methods.
Schematic diagram for the double beam analyser
ADVANTAGES
- Isobaric ions can be detected.
- High accuracy.
DISADVANTAGES
- Limited mass range.
- Very complex method.
- High cost.
- Quadrapole analyser: The ions are filtered by the quadrant of four parallel circular tungsten rods which focus ions by oscillating with radiofrequency.
ADVANTAGES
- Very simple instrument.
- Low cost.
- Highly robust technique.
DISADVANTAGES
- Limited mass range
- Limited resolving power
- Time of flight analyser: The ions are separated by changing their directions. Then the time of flight is given by
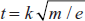
Schematic diagram for the time of flight analyser
ADVANTAGES
DISADVANTAGES
- High vacuum is required
- Recalibration is required after every use
- Ion collector or receiver: Ion beam is of 10 15-10 19 A. Most commonly used receivers are photographic plates, electron multipliers, electrometers and Faraday cylinders.
- Vacuum system: In this system, oil diffusion or mercury pumps are commonly used. High vacuum is maintained that is inlet at 0.015 torr, ion source at 10−5 torr and analyzer at 10−7.
TYPES OF MASS SPECTROMETRY
There are different types of mass spectrometry based on the combination of other analytical principle with that of the mass spectrometer.
Example: GC-MS, LC-MS, CIMS, FIMS and FABMS.
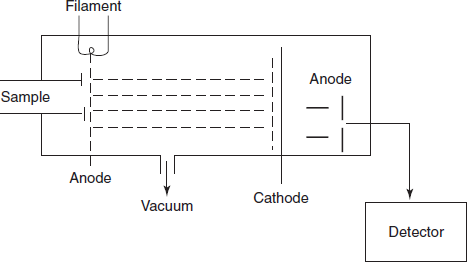
GC-MS: Gas liquid chromatography when combined with the mass spectrometry provides the high sensitivity of identification of compounds and structural elucidation. GC separates the volatile and semi-volatile compounds but it is not useful for the identification. This can be overcome by the MS. The only incompatibility is the pressure programming between the GC-MS. To overcome this, two types of separators are used. They are as follows:
- Jet separator: It is mainly used to introduce the more analyte into MS than carrier gas.
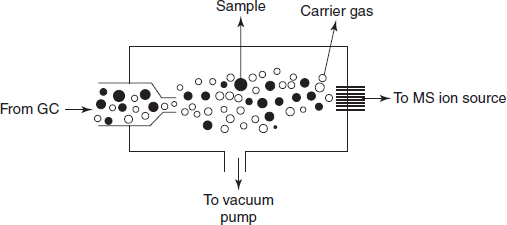
Schematic diagram for the zet separator
- Membrane separator: A membrane is placed between the spiral channels. At one end of the column, effluent is placed and on the other end MS is placed.
Modes of Operation of GC-MS:
There are three modes for the operation of GC-MS:
- Spectral mode.
- Total ion current.
- Selective ion monitoring.
Advantages
It is sensitive and used as a powerful tool for qualitative and quantitative determinations.
Disadvantages
It is time consuming.
LC-MS: LC-MS is more advanced than the GC-MS because no heating is required. This is conveniently used to analyse the non-volatile compounds and thermoliable compounds which cannot be handled by the GC-MS. This is mainly used for the molecular weight and structural determinations. The retention time is less when compared to the GC-MS. The main advantages of LC-MS: high sensitivity, selectivity and easy to use.
CIMS: This is mainly used for the physicochemical studies such as when ions collide with the molecules. These ions are present in the ion source. To attain this, reactant gas is used for this purpose.
Example: Methane, isobutene and ammonia.
Methane under goes the following reactions to obtain the reagent plasma. These reactions are collectively called as ion–molecule reactions.
CIMS forms the weak molecular ion (M+) and is taken as (M+1)+ which is commonly called as quasi-molecular ion.
FIMS: Field ionisation MS is used for the determination of the molecules lacking the parent ion. It consists of foil-type field ionisation source connected to mass analyser and the data are recorded in the recorder. Modification of the FIMS is known as field desorption MS (FDMS). In this method, the sample is allowed to evaporate by means of field ion emitter and introduced into the high electric field.
FABMS: Fast atom bombardment mass spectrometry involves the bombardment of the compound with the energy rich neutral particles.
Example: Xenon or Argon atoms with energies of 5,000–10,000 ev. This method is mainly used for the determination of large peptides, nucleotides and vitamin cyanocobalamin.
TYPES OF PEAKS IN MASS SPECTRA
The mass spectrum of the sample is the plot between intensity and m/e ratio on abscissa.
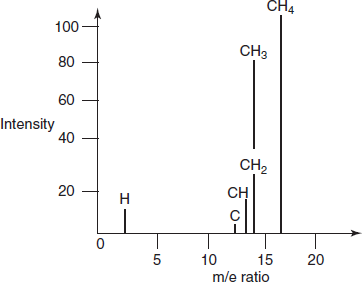
Peaks for the different compounds
There are different peaks observed in the MS. They are as follows:
- Molecular peak: This is also known as parent peak which is observed when the bombardment of the sample loses one electron and produces this peak.
This peak is the peak of highest mass number. The intensity of the peak depends on the stability of the ionized particle.
M + e− M+ + 2e−
M+ + 2e−
- Fragment peak: This peak is formed by the formation of fragment ions when the energy is given to the molecular ion. Many of the fragments in the MS are due to the fragments ions only.
M+
 M + 1 + M2
M + 1 + M2 - Rearrangement ion peak: This is due to the rearrangement of the fragment ions.
- Metastable ion peak: The ion resulting from the source and analyser is known metastable ion and the peak formed is known as metastable ion peak. These are broader with low intensity.
- Multicharged ion peaks: Some ions may exist with more than one charge.
Example: CO, N2, CO2, etc.
M + e− M++ + 3e−
M++ + 3e−
- Base peak: The largest peak in the mass spectrum is called base peak. It depends on the nature of the compound.
- Negative ion peak: In addition to the positive ions formed after energy increase, the negative ions also show the peaks. But these peaks are negligible in MS.
ADVANTAGES
- High sensitivity.
- Requires small sample size.
- Less time consuming.
- When it combines with other methods, it shows the high sensitivity and acceptability.
- Differentiates the isotopes.
DISADVANTAGES
- Only pure compounds are readily handled.
- Non-volatile compounds cannot be handled by the mass spectroscopy.
APPLICATIONS
- Used in the determination of isotopic compositions.
Example: Labelled isotopes are used in the quantification of proteins.
- Used in the trace gas analysis.
Example: Analysis of air.
- Used in the characterisation of polymers.
Example: Synthetic polymers.
- Used in the detection of the steroids
Example: Estrone and progesterone.
- Used in the determination of the anaesthetics.
Example: Lignocaine.
- Used in the determination of the dioxins.
Example: Digitoxin.
- Used in the determination of the gene damage.
Example: Gene theraphy
- Used in the detection of the oil deposits on rocks.
- Used in the determination of purity of the compounds.
- Used in the determination of molecular weights for new compounds.
- Used in the structural elucidation.
- Used in the determination of rate of reaction.
- Used the pharmacokinetic studies.
REVIEW QUESTIONS
- What are the different types of analysers?
- Name the receivers used in the mass spectrometry.
- Explain the principle involved in the time of fight mass spectrometer.
- What is metastable ion peak?
- What are the different ionization sources used in MS?
- Write about FABMS.
- Which method is highly sensitive: GC-MS or LC-MS?
- Explain the principle of quadrapole analyser.
- Write about the different methods used for the sample introduction.