Chapter 9
Affinity Chromatography
INTRODUCTION
Affinity chromatography is highly specialised form of adsorption chromatography in which a specific ligand is immobilised chemically into an insoluble matrix to adsorb reversibly a single molecular species from a mixture of solutes.
This technique is originally developed for the purification of enzymes and also extended to nucleotides. This involves the immobilisation of appropriate ligands in such a way that the enzyme is still capable of re-engaging to that immobilised form of the ligand.
CLASSIFICATION OF AFFINITY CHROMATOGRAPHY
This is mainly classified into two classes:
- Bioselective: The affinity is based on biologically relevant binding groups involving specific ligands, lectins, nucleotide cofactors, immunobiotin-labelled proteins, rarely employed cofactors, receptor proteins and immunosorbents.
- Chemiselective: The affinity is based on chemically defined interactions including hydrophobic chromatography, ion exchange chromatography, conventional chromatography, etc.
GENERAL PROCEDURE FOR THE DEVELOPMENT OF AFFINITY CHROMATOGRAPHY
This involves several steps. They are as follows:
- A necessary chromatography packing (or) bioselective adsorbent is synthesised and the bioligand is immobilised.
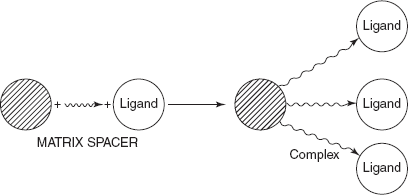
Attachment of the ligand to the matrix through spacer
- A crude extract is prepared and freed from endogenous substrate which is achieved by dialysis (or) enzyme precipitation.
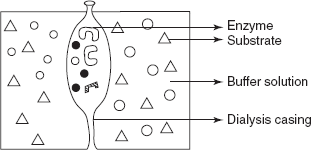
Dialysis
- The substrate free extract is applied to the chromatographic packing of immobilised bioligand.
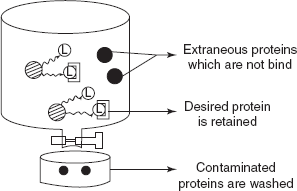
Extraction of the desired proteins
- The contaminated proteins which have no affinity for the column are removed by washing.
- The protein to be purified remains bounded to the column owing to its affinity for the immobilised ligand. The desired protein is eluted possibly with other soluble ligand.
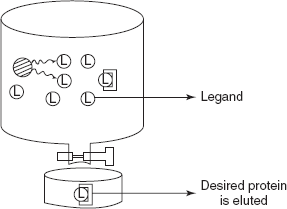
Separation of the mixture into the individual components
ADVANTAGES OF AFFINITY CHROMATOGRAPHY
- Selective/specific adsorption increases the purity by 100 folds.
- The number of purification steps is reduced.
- The species is concentrated.
- The species is stabilised on binding.
- This process is very rapid.
- This method is purely bowed on biological specificity not on physicochemical properties.
- Suitable for large-scale purification.
FACTORS AFFECTING AFFINITY CHROMATOGRAPHIC SEPARATION
This depends on the following factors:
- The ligand.
- The preparation of chromatographic packing.
- An inert matrix.
- The methods of attacking the ligand to matrix.
- Eluting the molecule from the bioselective adsorbent.
Selection of matrix: Selection of an appropriate matrix depends on the type of separation desired. It shows the considerable effect on the stability of complex formed between ligand and affine molecule. Support matrix should have the following properties:
- Adequate particle size and shape: Increased particle size reduces flow resistance and they become clogged. Irregularly shaped particles leads to unequal path lengths for substance to be separated and subsequently broadening of bond. Spherical shape is best sited.
- It should be stable and resistant against micro-organisms.
- It should be inert to prevent the affine from interacting non-specifically with the matrix itself.
- It should possess functional groups to which ligand can be attacked easily by vanity of chemical reactions.
- It should allow the free diffusing of the biomolecules to the binding sites.
- It should be economical.
TYPE OF MATRICES
The following are the most commonly used matrices in affinity chromatography:
- Agarose:
- It is a linear polysaccharide containing alternating residences of D-galactose and 3,6-anhydro-l-galactose.
- Ionic residences such as carboxylate and sulphate present in agarose can be removed by hydrolysis (or) reduction.
- Agarose can be prepared by mixing a hot aqueous solution of agar (2–6%) with an organic solution such as mineral oil and detergent. The aqueous solution is mixed rapidly with the organic phase to form droplets which forms agarose beads upon cooling.
- Agarose is frequently used as the cyanogenbromide derivative under alkaline conditions. The activated agarose reacts with proteins and other molecules containing amino groups.
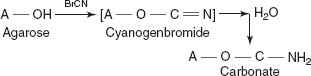
- The serious problem encountered with agarose is the formation of an unstable isourea from the reaction of cyanogen bromide activated agarose with primary amines.
- Agarose has the following disadvantages:
- It lacks thermal stability.
- It cannot be dried (or) frozen readily.
- It shrinks and swells upon changes in the ionic strength.
- Derivatised agarose after possess ion exchange properties. In addition to bioselective adsorption, agarose is derivatised by coupling which is achieved by means of bifunctional reagents such as bis-oxiranes.
Example: (1,4-Bis-2,3-poxy propoxy) butane (or) diphenyl sulfone.
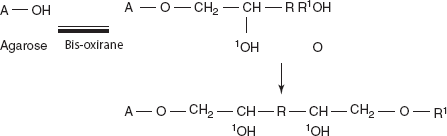
where R is the ligand attached to the agarose; R’ is the functional group.
- Other activation methods of agarose are with carbonyl-di-imidazole (CDI) (or) tresyl chloride.
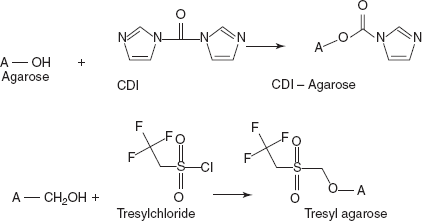
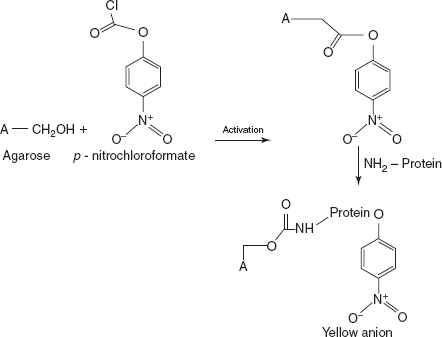
- Coupling:
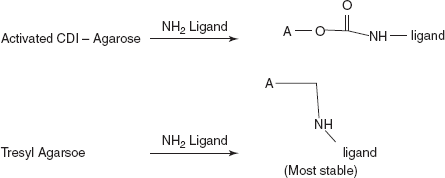
A new group of activation reagents have been recently developed in Israel. These reagents resemble phosgene and produce immobilised enzymes with the bond identical to those produced by CDI.
- Cellulose:
- The most widely used DNA affinity chromatography procedure depends on the physical trapping of DNA fibres in a mesh of cellulose fibres.
- The DNA cellulose is prepared by drying DNA and cellulose together in an appropriate buffer followed by washing the untrapped DNA. The DNA slowly elutes. Coherent cellulose (or) related polysaccharides are used as a bioselective adsorbent. DNA cellulose is used for the purification of DNA binding proteins.
- Its principle advantage is its low cost.
- Polyacrylamide: This serves as a support for polyacrylamide gel electrophoresis and a medium for gel permeation chromatography. These beads are hydrophilic, chemically stable and having an uniform pore diameter. Due to its easy derivatisation, it is mostly used as excelled affinity chromatographic support. Hydroxyl methacrylates are available numerically as spheron beds. These supports exhibit increased porosity with exclusion limits of up to a molecular weight of 108.
Controlled Pore Glass
- It is developed when borosilicate glass heated is up to 800 °C and separated into borate and silicate phases. The borate phase is removed by our acid-etching process leaving a porous of 96%. Silica network alkali leaching facilitates the migration of boron to the surface.
- Numerous boron Lewis acid sites adsorb nucleophiles such as proteins to yield positively charged centres which lead to non-specific adsorption while can be minimised by coating the glass with glyidyl oxypropyl timothy silate
Ligands
- The ligand to be immobilised can be an inhibitor, w-enzyme, substrate analogue (or) any other bimolecular with an affinity for a specific site such as active site, allosteric site, membrane binding site, etc., on the protein to be purified. Such biomolecules are usually small although macromolecules have been successfully employed. The ligand must have sufficient affinity for the enzyme being purified to retain on the ligand matrix completely.
- A ligand in affinity chromatography should possess the following properties:
- It should specifically form a reversible complex with the substance to be purified.
- The compound should possess a chemically modifiable group through C so that the covalent linkage to the matrix can occur.
- It should form a stable complex on binding to the substance to be purified.
- It should be able to withstand harsh conditions during the chromatographic process.
- It should be easy to dissociate the complex by a simple change in the medium without adversely affecting the compound to be purified.
- It should be consistently available and economical.
- Ligands are generally classified as non-specific (or) group specific. They are further divided into lower and higher molecular weight ligands.
- Ligands with monospecificity have a high affectivity for the purification of a particular substance. It has a disadvantage that is a special ligand-matrix combination is required for every substance to be purified and also monoclinic ligands bind more strongly and require harsh elements than group specific ligands.
Example: Steroid hormones, vitamins and certain enzyme inhibitors.
A ligand with group specificity called general ligands, which has one of the great values, allows a variety of substances to be purified with single affine matrix.
Example: Coenzymes such as NAD, NADP, FMN and FAD used in dehydrogenases enzymes separation:
- The above two are the biospecific ligands
- They are also non-biospecific ligands. They are as follows:
- Dyes as ligands which are used in dye-ligand chromatography:
Examples:
- Cibacron blue F 3G-A is very suitable group-specific ligand for the enzymes requiring adenylyl containing co-factors.
- Dextral-blue sepharose is used for purification and isolation of albumin.
- Metal chelates as ligands (metal chelate AC).
Examples:
- Zn+2 is carbonyl methyl amino sepharose 6B used to purify human fibroblast interferon.
- Fe+3 bis-carboxy methyl amino sephadex G25 used to purify tyrosine-containing peptides.
- Charge transfer chromatography: Change transfer materials such as acriflavine, acridine yellow, pentachlorophenol and malachite green are used in AC.
Example: Acriflavine enables to separate nucleotides, oligo nucleotides, single chain and double chain nucleic acids.
- Hydrophobic ligands.
Example: Octyl-sepharose-CL 4b is used to purify staphylococci lipase and phenyl-sepharose-CL 4b is used to purify heparin.
- Organo mercurial and disulphide-containing ligands.
Examples:
- 2-Phridyldisulfde-hydroxyl pyopyl ether agarose:
- To separate copper thiamine from human foetal liver.
- To separate disulphide anion reductive from beef liver.
- Agarose glutathione-2-pyridyl-disulphide:
Examples: Globulin phospholipids from human serum. Chains of haemoglobin from bovine haemoglobin. - 2-Phridyldisulfde-hydroxyl pyopyl ether agarose:
- Dyes as ligands which are used in dye-ligand chromatography:
Spacers
- Although a ligand may be directly attached to an activated matrix, an intervening organic group usually serves as a method of attachment (or) as spaces (or) to separate the ligand form the matrix.
- A spacer is important. For example, when a series of eight agarose-insulin complexes are used for the purification of insulin receptor protein, the derivative with the longest spacer arm is most effective.
- The spacer arm positions the ligand at a certain distance from the matrix in order to reduce stearic interference from the matrix.
There are three principle possibilities for introducing spacers. They are as follows:
- One end of the spacer is first bond to the matrix and then the biological ligand is bound to the other end of the spaces using various methods for matrix fixations.
- The spacer can be first bound to the ligand as a subsistent and then the other end of the spaces can be fixed to the matrix.
- Commonly used spacer arms are aliphatic, linear hydrocarbons C two functional groups one at each end of the chains.
Example: Of spacers: hetero ethylene domains and β-amino hexanes.
Elution
- In the elution step, it is attempted to weaken the interactions between the ligand-matrix and ligand analytic molecule to achieve the desired from the column.
- Different elution methods are as follows:
- Specific methods: Affinity elution with substrates, cofactors, inhibitors and other specific soluble compounds.
- Non-specific methods: Changing of pit, ionic strength, temperature, polarity (or) by using reagents such as deforming elements, urea, guanidine, detergents, etc.
- Special methods: Electrophoresis, cleavage of matrix-ligand bond, buffer effects, various elution variants such stepwise (or) gradient elution.
- In many cases, a change in pH leads to description, however, it is limited because of chemical stability of the substance, the matrix and the covalent linkage between matrix and ligand.
Example: In the case of proteases and inhibitors, adsorption occurs at pH 8.1 and elution at pH 3.1.
- Increase in the ionic strength either stepwise (or) in a gradient fashion is one of the gentle method of non-specific elution. NaCl/KCl mostly in concentration between 0.1 and 0.2M is used.
- In the case of high chaotropic salts, SCN–/CCl3 CN-can be used particularly in immune affinity chromatography for plasma protein isolation.
- In some cases, decrease of polarity by addition of ethanol/dioxan with human lymphoid interferon up to 50% ethylene glycol is used for desorption.
- A special method to elute biomacromolecules is electrophoresis. It is suited for desorption of antibiotics and hormone-binding proteins.
- Another special method is selective cleavage of bond between the matrix and the ligand. This method is mainly used for high affinity complex when normal elution leads to penetration.
Example: Oestradiol-binding proteins.
- The elution of the compound is monitored by several methods such as spectrophotometric methods, chemical detection methods and electrophoresis.
APPLICATIONS
- Purification of enzymes and proteins:
- Example: DNA-related enzymes can be purified such as homophile DNA-ligase which has been purified from thermos thermopiles using DNA cellulose.
- Example: Heparin agarose used to purify the heparin-binding domain of platelet thrombospondin.
- Determination of dissociation constant:
The dissociation constant of staphylococcal detained from tree and matrix bound thymidine-3 (p-aminophenyl phosphate)-5-phosphate, a competitive inhibitors of staphylococcal nuclease
- Kinetic studies of lactate dehydrogenase:
Lactate dehydrogenate was applied to bioselective matrix consisting of oxalate covalently coupled to agarose in the presence of nicotinamide adenine dinucleotide hydrogen (NADH).
- Therapeutic and clinical applications:
Example: The immunoadsorption chromatography has been used to treat hyper ester by passing the infected blood through an extra corporeal shunt consisting of a blood cell separator, and on the plasma side, an immunosorbent column containing antibodies to plasma low-density lipoprotein.
- Other application includes solid phase i.e., heterogeneous immune essay used in therapeutic drug monitoring, pregnancy testing and allergy testing.
- Used in lectins separation.
Example: RBC, tumour cells, embryonic cells, etc.
- Used in the study of antigen-antibody reactions.
- Used in synthesis of RNA on DNA template.
- Used in the identification of promoters of DNA for transcription.
- Used in the molecular biology.
Example: Histamine residues are separated from protein sequence.
- It is used in the determination of concentration of dilute protein solutions and storage of unstable proteins in the immobilised form.
REVIEW QUESTIONS
- What is the principle and theory involved in the affinity chromatography?
- What is procedure involved in the affinity chromatography?
- What are the different types of matrices used in the affinity chromatography?
- Define and give examples for the following terms: (a) Spacers (b) Ligands.
- What are the different elution methods used in the affinity chromatography?
- What are the applications of affinity chromatography?
