Chapter 1
Radiometric Analysis
INTRODUCTION
George de Von Hevesy is the first person to use the radioactive compounds to determine the biological compounds. A radiotracer is defined as the chemical compound which contains the radioactive nuclei as an anion or a cation. The radioactivity is the emission of rays such as α, β and γ-rays. The examples of radionuclide are 3H and 14C which are frequently used for the analysis of the compounds:
The stability of the radioactive compounds is expressed by the half-life which is defined as the time taken for the decay of the half of the atoms. The radioactive decay law states the number of atoms remain after the decay:
where N0 is the starting number of particles; τ is the particles life time.
The choice of radiotracers based on the ability of the tracer readily mixes with the sample to be determined and the amount required for the determinations. The small amounts are appreciable for the determinations.
The factors affecting the radioactivity of the tracers are half-life of the isotope and the type of the radiation emitted.
The measurement of the radioactivity is by the following equation:
The above equation gives the percent efficiency of the radioactive tracer.
The following are the methods used for the measurement of the radioactivity:
- Ionisation method
- Liquid scintillation method
- Chemical method
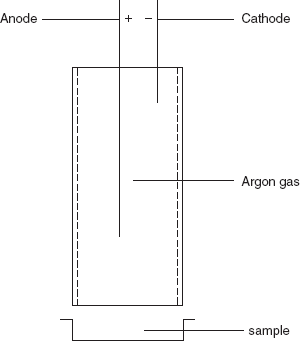
- Ionisation method: This method is carried by the Geiger–Muller tube. This is first invented by Hans Geiger in 1908 and it is developed by Walther Muller in 1928. Hence, it combining both together called as Geiger–Muller technique. This detects the low-level β and γ radiation. These rays are produced by the radioactive sources such as gas lantern mantle. A sensor is used a counter to monitor counts the radiation from the radiation source. It consists of a low-pressure inert gas filled in a tube. The inert gases such as helium, neon and argon are used. Then pair of electrodes are placed to pass the voltage but not the current. The mica end window enters the Geiger tube which is ionised and emits the radiation.
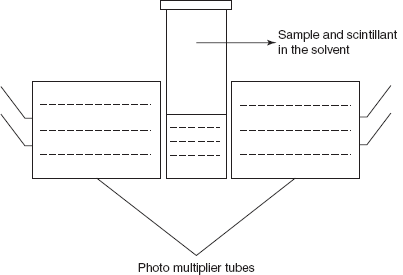
- Liquid scintillation method: Polach proposed the liquid scintillation method which is mainly used for the determination of the 14C. The liquid used in the scintillation method is the benzene or mixture of benzene and toluene. In this method, the sample is converted to carbon dioxide and is reacted with the lithium to produce lithium carbide and the fluorescence is determined by the photomultiplier tube.
2CO2 + 10Li
 Li2C2 + 4Li2O
Li2C2 + 4Li2OThis reaction is first proposed by Barker in 1953. Then Polach et al. in 1983 proposed the comparative study of the various scintillators and concluded that the powder butyl-PBD dissolved in the benzene shows the high stability. The scintillation is the process in which the energy of the fast moving electron is transferred with the help of the solvent produces the scintillant molecules. These molecules raise to the excited state and fluoresce radiation by emission which is detected by the photomultiplier tubes. The main drawbacks of this method are sample preparation should be in the clear solution form and variable quenching caused by the chemical quenching and colour quenching.
- Radiometric methods: These are the following types based on the principle involved in the methods:
- Isotope dilution analysis
- Radiometric titrations
- Radio chromatography
- Radio immuno assay
- Isotope dilution analysis: This method is introduced by Havesy and Hobbie in 1932. This is used for the determination of the inorganic compounds. The advantage is that the no separation is required for the determination. The principle involved is the that addition of the radiotracer measures the analyte mixture.
Specific activity of the tracer + sample
 specific activity of the isolated compound.
specific activity of the isolated compound.Specific activity is defined as the counts per minute.
The following ideal requirements are required for the radiotracer used in the isotopic dilution analysis:
- Radiotracer must be pure.
- Radiotracer should have the chemical identity.
- It should have uniform distribution of the radiotracer in the sample.
- It should have long shelf-life
The steps involved in the isotopic dilution procedure are the following:
– A small amount of the radiotracer is added to the solvent and followed by warming for isotope exchange.
– An organic solvent containing the suitable reagent is added.
– Solution is stirred well and the radiotracer is extracted and separates the aliquot of the organic phase.
– Then the organic phase is evaporated to dryness.
– Then the sample is counted by the Geiger–Muller counter.
– Then the planchet is weighed.
There are two types of isotopic dilution analysis methods. They are as follows:
- Direct isotopic dilution analysis: In this method, a known amount of the labelled isotope is added to the sample which contains the unlabelled compound:
S = A/m*
where S is the specific activity of the labelled compound; A is the counts/min; m* is the weight of the sample.
Then consider that m is the amount of unlabelled compound. Then the specific activity is given as the following:
Sa = A/m + m*Therefore, from the first equation
A = S × m*Then the second equation becomes as follows:
Sa = Sm/m + m*
- Inverse isotopic dilution analysis: In this method, the known quantity of the non-labelled compound is added to the sample containing an unknown amount of labelled compound.
m* = (A1/A2)m − m1
where A1 and A2 are specific activity of the sample solution and recovered sample, respectively; m* is the amount of the radioactive substance; m is the amount of the recovered sample; m1 is the amount of the inactive compound.
ADVANTAGES
- Highly sensitive when compared to other methods.
- Small amounts can be easily handled.
- Less expensive.
- Instrumentation is simple to handle.
- Specific for the biological and environmental studies.
- Reproducible values are obtained.
LIMITATIONS
- Destructive method hence the sample is not preserved.
- Only one sample can be handled at a time.
- Specific for compounds for which the suitable radiotracers are available.
APPLICATIONS
- Used for the elemental analysis in geological, biological, environmental, etc.
- Used in the determination of the industrial samples.
- Used in the determination of the vitamins, antibiotics, amino acids, hormones, etc.
- Used in the study of interaction of the elements in disease and regulation of the body.
- Used in the determination of the iodide in the mixture of halides.
- Used in the rapid analysis of Co in the different steels.
- Used in the determination of the total volume of the blood in the human body.
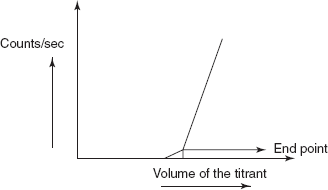
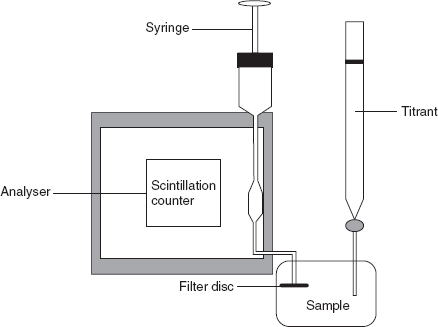
- Radiometric titrations: These titrations are based on the determination of the radioactivity by adding the radioactive indicator or titrant or sample. The end point is indicated by the sudden change in the radioactivity. The apparatus consists of the titration vessel containing the filter disc and is connected to the scintillation detector and lead shielding. A syringe is used to move the supernatant liquid into the scintillation counter unit.
Example: Determination of halides using 110Ag as a tracer.
110 AgNO3 + NaCl 110 AgCl precipitate + NaNO3
110 AgCl precipitate + NaNO3Initially, the sample solution containing the NaCl solution does not show the radioactivity. After the addition of the 110AgNO3, solution shows the slight increase in the radioactivity. The sudden change is observed after the precipitation of AgCl which can be taken as the end point. Then the plot is taken between the counts/s and volume of the titrant.
ADVANTAGE
- End point is not affected by the chemical reaction.
LIMITATION
The radiometric titration requires the phase separation.
- Radio chromatography: This method is first proposed by Glen T. Seaborg. Radio chromatography is the combination of the paper or thin layer chromatography with the ion exchange column or the gas chromatography by using the radioactive tracer and the spots are detected by the Geiger–Muller counter or scintillation counter.
The procedure involves the asbestos paper is spotted with the radiotracer and is placed in the glass chamber containing the dilute HCl and allow the asbestos paper for the development of the spots for 1 h. Then dry the paper in the drying chamber and cut the paper along the line and place in the counting chamber for 50 s. Then the plot is drawn between the activity of the strips and the strip number. From the peak obtained, calculate the Rf values. The following are the Rf values for some of the compounds:
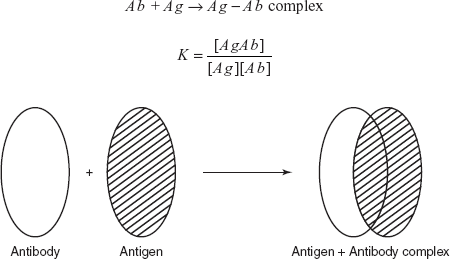
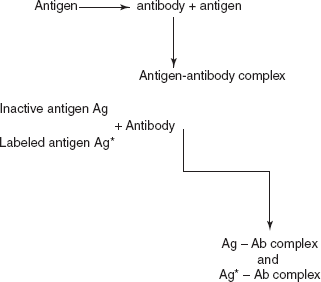
Compound Rf value Na+ 0.86 K+ 0.77 Rb+ 0.72 Cs+ 0.45 - Radio immuno assay (RIA): Radio immuno assay is first developed by Rosalyn Yallow and Soloman Berson in 1958 for the assay of biological compounds such as hormones, vitamins, drugs, etc. In 1947, Rosalyn awarded Nobel Prize for the determination of the insulin using the 131I as tracer. RIA involves the separation of the protein by antigen–antibody complex. This method is mainly used for the determination of the concentration of the antigen in the solution.
The principle involved in the radio immuno assay is the addition of the known quantity of the antigen which is labelled with the radioactive 131I. Then it is mixed with the sample and the reagent antibody. The labelled antigen is combined with the antibody to form an antigen–antibody complex.
The following are the requirements for RIA:
- Preparation and characterisation of antigen: Antigens are prepared by the synthesis or by isolation from the natural sources.
- Treatment of the antigen with the radioactive 131I: This can be done by the tagging procedure. Tagging of the antigen with the 131I.
- Preparation of specific antibody: Antibodies are prepared by injecting the antigen into the blood stream and which produces the specific antibody. These are extracted from the serum.
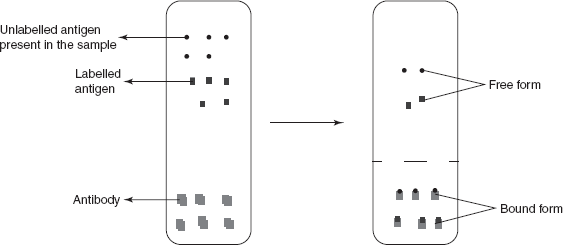
- Assay and separation of the complexes: This is for the separation of the unbound antigens. This step is carried out by the attachment of the antibodies to the micro titre well surface and the addition of the antigen binds to the active sites of the antibody. The unbound antigens are removed by the decanting followed by the washing of the well. The other method used for the separation is centrifugation. The solution is centrifuged at maximum rpm and the supernatant liquid is removed which contains the unbound antigen.
The amount of the labelled antigen bound to the antibody depends on the following:
- Amount of the antigen present (labelled and unlabelled)
- Amount of the antibody present
- The equilibrium constant
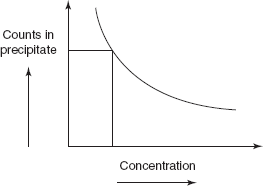
A calibration curve gives the concentration in the sample-
ADVANTAGES
- Highly specific.
- High sensitivity.
DISADVANTAGES
- Radiation hazards.
- Requires skilled person.
- High cost compared to other methods.
- Requires special license.
- Requires high care.
APPLICATIONS
- Used in the detection of narcotic drugs.
- Used in the blood bank screening for the hepatitis virus.
- Used in the cancer diagnosis.
- Used in the detection of hormonal levels.
- Used in the determination of neurotransmitters.
- Used in the determination of the drug poisoning.
- Used in the analysis of insulin.
- Used in the determination of steroids.
REVIEW QUESTIONS
- What are radiotracers and how they affected by the half-life?
- Explain the radioactivity measurement procedures by the Geiger–Muller counter.
- What is the principle involved in the liquid scintillation counter?
- What are the steps involved in the isotope dilution analysis?
- What is the difference between the direct and inverse isotopic dilution methods?
- What are the advantages of the isotopic dilution analysis?
- What are the applications of isotopic dilution analysis?
- What is the principle and procedure involved in the radiometric titrations?
- What is the procedure involved in the radio chromatography?
- What is the principle involved in the radio immuno assay?
- What are the requirements for the radio immuno assay?
- What are the applications of radio immuno assay methods?