Chapter 3
Analysis of Anti-Pyretics and Analgesics
INTRODUCTION
These agents are mainly used to decrease the elevated body temperature and in the case of pain. These agents analyses are more important for their wide usage and high toxicity. Classification of these agents is as follows:
- Salicylic acid derivatives.
Example: Acetyl salicylic acid.
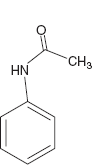
- Aniline derivatives.
Example: Acetanilide.
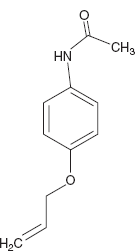
- p-Aminophenol derivatives.
Example: Acetophenacitidin.
- Quinoline derivatives.
Example: Cinchophen.
- Pyrazolone derivatives.
Example: Antipyrine and aminopyrine.
ANALYSIS METHODS
- Gravimetric method
- Titrimetric methods:
- Bromometric
- Iodometry
- Diazotisation titration
- Non-aqueous titration
- Complexometry
- Acidimetry
- Alkalimetry
- Ethoxy content method
- Colorimetric methods:
- Hydroxylamine method
- 2-Naphthol method
- Chromic acid method
- Nitric acid method
- Silico tungstic acid method
- PDAB method
- Diazotised nitroaniline method
- UV method
- IR method
- Polarographic method
- Chromatographic method
- Gravimetric method: Sample is extracted with petroleum benzein to remove the foreign matter. Then the extract is discarded and again extracted with chloroform. The resulting solution is evaporated to dryness and the residue obtained is dried at 80 °C for 2 h. Then cooled to room temperature and weighed. This method is mainly used for acetanilide and acetophenatidin determination.
The residue is dried at 60 °C for 2 h used for neocinchophen determination.
Other procedures are the following:
- Sample is reacted with bromide–bromate solution or potassium iodide–iodine solution in the presence of acid which forms the precipitate which is extracted with ether–chloroform mixture. Then dry the solvent and weigh the residue. This procedure is mainly used for cinchophen determination.
- Sample is reacted with iodine in the presence of sodium bicarbonate. Then extracted with chloroform and excess of iodine precipitated with silver nitrate. The precipitate is dried at 80 °C for 2 h and the weight of the residue is taken. This method is mainly used for the determination of antipyrine.
- Sample is reacted with excess of HCl. Evaporate to dryness and weighed. This method is mainly used for the determination of aminopyrine.
- Titrimetric methods:
- Bromometry:
Indirect method: Sample is hydrolysed with sulphuric acid and extracted with chloroform. To this, add bromine-bromate solution and keep a side for 10 min in dark. Then add potassium iodide solution and keep a side for 30 min in dark. Then the excess of iodine is back titrated with sodium thiosulphate using starch as an indicator. This method is used for acetanilide and aminopyrine determination.
Direct method: Sample is dissolved in 6N HCl and heated to 60 °C. Then titrated with 0.05N potassium bromated solution using methyl red as an indicator.
- Iodometry: Sample is dissolved in acetic acid and water. Warm this solution not exceeding 70 °C. To this, add 0.25N iodine solution with continuous shaking followed by addition of concentrated HCl. Stoppered the flask and shake until the precipitate obtains. Then dilute with water and allow standing for 30 min and filtered through glass-sintered filter. Take 50 ml of filtrate which is titrated with 0.1N sodium thiosulphate using starch as an indicator. This method is used for acetophenatidin, cinchophen and antipyrine determinations.
- Diazotisation titrimetry: Sample is refluxed for 1 h with 30 ml of 10% sulphuric acid and the solution is collected in the titration flask. Then add 10 ml of concentrated hydrochloric acid. Then keep the solution in the cold water bath to bring the temperature to 15 °C. Then titrate the solution with the 0.1N sodium nitrite using the starch iodide as an indicator. This method is used for the determination of the acetophenatidin.
- Ethoxy method: The ethoxy compound of the drug is reacted with the hydro iodic acid which forms the ethyl iodide and is extracted with the bromine–potassium acetate and acetic acid solution. The excess of bromine is removed by the addition of formic acid and the excess of iodine is back titrated with the sodium thiosulphate.
ROC2H5 + HI
 C2H5I + ROHC2H5I + Br2
C2H5I + ROHC2H5I + Br2 C2H5Br + IBrHIO3 + 5KI + 5H+
C2H5Br + IBrHIO3 + 5KI + 5H+ 3I2 + 3H2O + 5K+
3I2 + 3H2O + 5K+This method is used for the determination of the acetophenatidin.
- Non-aqueous method: The antipyretics and analgesics are bases which can be titrated by the non-aqueous titrants after hydrolysis of drugs. After hydrolysis, the drug produces the free amine which is titrated with the 0.02N perchloric acid in the dioxane solvent. The end point is determined potentiometrically.
- Acidimetry: The sample is extracted with 50 ml of alcohol and is treated with 40 ml 0.1N potassium hydroxide in alcohol followed by condensation for 1 h, which cools the solution and is titrated with the 0.1N hydrochloric acid using phenolphthalein as an indicator.
- Alkalimetry: The sample is extracted with 10 ml of the carbon tetra chloride, the solution is filtered and the filtrate is discarded. The residue obtained is dissolved in the neutralised alcohol followed by heating to 70 °C. Then the solution is filtered and the residue is washed with the several portions of neutralised alcohol and is titrated with the 0.1N sodium hydroxide using phenolphthalein as an indicator.
- Complexometry: The sample is dissolved in the water and is mixed with the precipitating reagent such as cadmium chloride and ammonium thio cyanate and keep the solution a side for the formation of the precipitate. The precipitate is filtered with the cadmium free ammonium thio cyanate water and dissolved in the appropriate quantity of concentrated ammonium hydroxide. Then add indicator solution such as erichrome black T and the buffer solution. Then the resulting solution is titrated with the 0.05M disodium ethylene diamine dihydro chloride solution as a titrant. The end point is colour change from red to steel blue.
- Bromometry:
- Colorimetric methods: These methods mainly based on the absorption of the radiation within the visible range. There are several methods based on the colouring reagents. They are as follows:
- Hydroxylamine method: The hydroxylamine reacts with the amide drugs which form the hydroxymic acid:
RCONX2 + NH2OH
 RCONHOH + NHX3
RCONHOH + NHX3
The formed hydroxymic acid produces the coloured product when reacted with the ferric ions.
The method involves the aqueous solution of the sample is mixed with the little quantity of the hydroxylamine reagent and allowed to stand for 3 h at 60 °C. Then the solution is immediately cooled to room temperature and concentrated HCl and ferric chloride are added. The absorbance is measured at 515 nm. This method is mainly used for the determination of acetophenatidin.
- 2-Naphtholmethod: This method is mainly used for the determination of the hydrolysed samples. The method involves the sample solution is mixed with the diazotising reagents such as HCl and NaNO2 and then the excess of HNO2 is removed by the addition of sulphanilic acid. The resulting solution is coupled with the 2-naphthol reagent which forms the orange-red colour solution measured at 420 nm.
- Chromic acid method: This method is mainly based on the reaction of the chromic acid with the sample in the presence of ammonium citrate. The method involves the sample is dissolved in the chloroform. To the aliquot of this solution, ammonium citrate solution and chromium trioxide solution are added. Then exactly after 7 min measure the absorbance at 543 nm. This method is used for the determination of the aminopyrine and p-acetyl amino phenol.
- Nitric acid method: This method is first proposed by Miller when reaction of the acetophen-atidin with the nitric acid which produces the yellow to orange red colour. The coloured solution is measured at 465 nm. This method is used for the determination of the aminopyrine and antipyrine.
- Silicotungstic acid method: The sample is mixed with the excess of silico tungestic acid and the excess of silicotungstic acid is coloured with the colouring reagent such as titanium trichloride. The resulting solution is measured at 650 nm. This method is used for the determination of the cinchophen.
- PDAB method: The sample is initially reacted with the nitrite in acidic conditions is the principle of this method. The method procedure involves the sample solution is mixed with the equal volume of the reagent solution that is with p-dimethyl amino benzaldehyde. Then the salmon pink coloured solution is measured after 1 h at 513 nm.
- Diazotisation method: The basic principle in this method is the diazotisation of the sample with HCl and the sodium nitrite and the diazotised compound is reacted with the p-nitro aniline which produces the yellow colour measured at 570 nm.
- Hydroxylamine method: The hydroxylamine reacts with the amide drugs which form the hydroxymic acid:
- UV method: This method is based on the absorption of UV radiation in different solvents. The sample is mixed with the solvent and measured at appropriate wavelength.
Example: Drug is mixed with the ethanol and is measured at 270 nm.
Aspirin is dissolved in the chloroform and the absorption is measured at 277 nm.
- IR method: The method involves the sample is extracted with the chloroform and the absorbance is measured. The following are the results obtained for different drugs:
Drugs Absorption maxima (μ) Aspirin 9.27 Acetophenatidin 8.99 Caffeine 10.26 - Polarographic method: The compounds which are reducible at the dropping mercury electrode are commonly determined by this method. First the compounds to be determined are diazotised with the diazotising reagents and are titrated with the sodium hydroxide to neutralise the excess nitrous acid produced by the reaction. Then run the polarogram. The reduction reaction is given as the following:
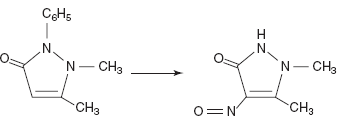
The procedure involves the sample is mixed with the sulphuric acid and sodium nitrite solution and allow to stand for 12 min at 23 °C. Then the excess nitrous acid is neutralised with the sodium hydroxide and 1% gelatin solution is added. Then the resulting solution is polarographed.
- Chromatographic method: This method is based on the principle of partition chromatography. The method involves the sample is dissolved in the chloroform. Then the column is filled with the pH 6–7 buffer. Then the sample solution is diluted with the di-isopropyl ether and the aliquot is pipette on to the column. Then the elution is carried out with the 75:25 of diisopropyl ether and chloroform, respectively. After elution, the eluates are collected.
- IP methods:
- For analgin: 400 mg of sample is dissolved in the mixture of 40 ml ethanol and 10 ml of 0.01M HCl. Then titrate the resulting solution with iodine until a yellow colour is stable for 30 s:
1 ml of 0.05M I2 ≡ 0.01667 g of analgin
- For aspirin: 1.5 g of sample is mixed with 15 ml of ethanol and 50 ml 0.5M NaOH. Then boil the solution for 10 min. Titrate the solution with 0.5M HCl using phenol red as an indicator:
1 ml of 0.5M NaOH ≡ 0.04504 g of aspirin
- For acetaminophen: 0.5 g of sample dissolved in 10 ml of water and 50 ml of 1 M H2SO4 followed by boiling by reflux condensation for 1 h. Then cool the solution and dilute with the water to 100 ml. To 20 ml aliquot, add 40 ml o water, 40 g of ice and 15 ml of 2M HCl. To this, add 0.1 ml ferroin solution as an indicator. Titrate the solution with 0.1M ceric ammonium sulphate:
1 ml of 0.1M ceric ammonium sulphate ≡ 0.00756 g of paracetamol
- For piroxicam: This can be assayed by the HPLC method. The column is packed with stationary phase LC 1. Then the mobile phase used is methanol and citrate buffer in the ratio of 45:55. The elutes are detected at 254 nm.
- For analgin: 400 mg of sample is dissolved in the mixture of 40 ml ethanol and 10 ml of 0.01M HCl. Then titrate the resulting solution with iodine until a yellow colour is stable for 30 s:
REVIEW QUESTIONS
- Explain the procedure involved in the gravimetric analysis of the antipyretics and analgesics.
- What are the titrimetric methods available for the analysis of the antipyretics and analgesics?
- Explain the colorimetric methods for the analysis of the antipyretics and analgesics.
- What is the principle involved in the analysis of the analgesics and antipyretics by the polarographic method?
- What is the principle involved in the IR analysis of the analgesics and antipyretics?
- What are the IP methods for the analysis of the antipyretics and the analgesics?