Chapter 8
Analysis of Sulphonamides
These compounds are mainly composed of amino group and the sulphonamide group. The general formula of sulphonamides is as follows:
These are commonly used as antibiotics. This group of compounds are mainly analysed based on the aromatic amino group or the acidic hydrogen in the molecule, that is, the aromatic amino group is diazotised or titrated in non-aqueous medium and the acidic hydrogen is titrated with base in non-aqueous medium.
These compounds are generally analysed by the following:
- Titrimetric methods
- Spectrometric methods
- Chromatographic methods
- Salt formation
- Electro-analytical methods
- Diazotisation titration: Diazotisation is the reaction where an aromatic amine reacts with nitrous acid to form a diazonium salt:
ArNH2+ HNO2+ HCl
 ArN=NCl + H2O
ArN=NCl + H2O
The method is as follows: sample is dissolved in an excess acid (HCl) and titrated with a standard solution of NaNO2. The end point is detected by the blue colour obtained upon streaking few drops of resulting solution on a starch iodide paper. The only precaution is the excess nitrous acid is removed by the addition of sulphamic acid:

where A are the millilitres of NaNO2; W is the weight of the sample; M is the molarity of NaNO2; EW is the equivalent weight factor.
All sulphonamides show positive reaction with diazotising reagents except sulphamethoxypyridazone and sulphisoxazole.
- Non-aqueous titration: The basic principle involved in non-aqueous titration is when titrating weak bases, a solvent is more acidic than when water is used.
In the case of weak acid, a more basic solvent is required.
Examples: Di methyl formamide, butylamine and ethylene diamine.
Various types of non-aqueous titrations have been applied to the sulphonamides. Because of the acidic properties of their –SO2NH– group, most sulphonamides can be titrated with basic titrant.
The method is as follows: the sample is dissolved in 50 ml of dimethyl formamide and five drops of thymol blue is added as the indicator. Resulting solution is titrated with sodium methoxide; the end point is detected as blue colour.

where A are the millilitres of sodium methoxide; W is the weight of the sample; N is the normality of methoxide; EW is the equivalent weight factor.
The main disadvantage of this method is that the titrant sodium methoxide is not stable with NaNO2.
- Bromometric titration: The sulphonamides react with bromine which will substitute on the benzene moiety and in some cases, on the heterocyclic moiety.
NH2C6H4SO2NHR + 2Br2
 NH2C6H2 Br2 SO2 NHR + 2HBr
NH2C6H2 Br2 SO2 NHR + 2HBr
Two general types of procedures are used. They are as follows:
- The sulphonamide may be directly titrated with bromated in the presence of bromide and the end point is detected potentiometrically or by means of indicator such as methyl red.
- In another method, a small excess of bromated is added and the solution is allowed to stand for a definite time before the excess is back titrated.
Procedures:
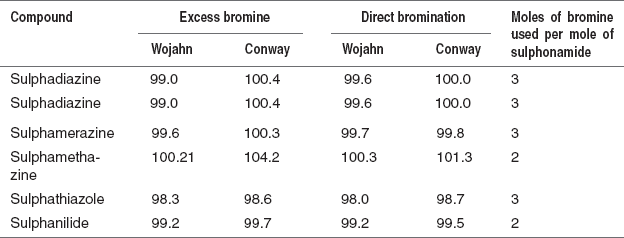
- Excess method of Wojahn: An accurately weighed sample of 0.2–0.3 g is dissolved in a minimum volume of 3% HCl. To this, 3 g of KBr and conc. HCl are added.
Dropwise 0.1N KBrO3 is added from burette until a yellow colour persists. To this, add 1 g of KI which is immediately titrated with 0.1N Na2S2O3 using starch as the indicator.
- Direct method of Wojahn: The sample is dissolved in a minimum volume of 3% HCl. To this, 3 g of KBr and conc. HCl are added which is titrated with 0.1N KBrO3 using methyl red as the indicator and the end point is detected as red colour.
- Excess method of Conway: The sample is dissolved in 20 ml of 2% NaOH and 0.1N KBrO3−KBr solution. To this, 80 ml of glacial acetic acid and 5 ml conc. HCl are added. Then flask is stoppered and shaken for 30 s and allowed to stand for 1.5 min. The excess of bromine is titrated with 0.1N arsenite until bromine colour is discharged.
Bromometric determinations
- Argentometric titration: A 0.2–0.3 g of sulphonamide is dissolved in 0.1N NaOH. The solution is adjusted to give a faint blue colour with thymolphthalien and adjusted to 50 ml with H2O. The blue colour is discharged with one or two drops of 0.1N H2SO4 and 25 ml of 0.1N AgNO3. The solution is allowed to stand in dark for 30 min. Then the precipitate is collected on double filter paper followed by washing thoroughly with H2O. The filterate is acidified with HNO3 and the excess silver is titrated with 0.1N ammonium thiocyanate with ferrion as the indicator.
- Calorimetry: The method consists of diazotising the sulphonilamide with NaNO2 in dilute acid decomposing the excess nitrite in sulphonic acid and coupling the diazo compound with N-(1-naphthyl)-ethylene diamine dihydrochloride (BM reagent) and the intensity of pink colour is measured within the range of 540–550 nm.
These specify the following:
- The coupling should occur in acid solution since acidification is required for diazotisation.
- The dye stuff obtained should be soluble in dilute acid.
- The compound used for coupling should be easy to purify.
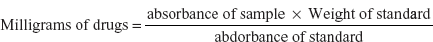
Other colorimetric methods are also available but these are not that much accurate than BM reagent method:
- Dimethyl amino benzaldehyde method: This method was proposed by Werner. Dimethyl benzaldehyde condensed through the amino group to produce a yellow colour.
- β-Naphthaquinone-4-sulphonate method: This method was proposed by Schmidt. Here the sample is added to one drop of HCl and 1 ml of 0.5% 1,2-naphthoquinone-4-sulphonic sulphonate reagent which is allowed to stand in dark for 1 h and the colour intensity is measured.
- Thiobarbituric acid method: Red colour is formed by condensation of thiobarbituric acid with pyrimidines substituted in the second position and the resulting colour solution is measured at 532 nm.
- UV-method: Sulphonilamide is diazotised with diazotising reagents and the excess nitrite is removed by urea and the solution absorbance is measured at 380 nm. This method is proposed by Halse and Wold.
- IR-Method: Dolensky determined the infrared spectra for a number of sulphonamides by suspending them in carbon di-sulphide or carbon tetra chloride with the use of aluminium stearate.
- Paper chromatography: The application of paper chromatography for the separation of mixture of sulphonamides is described by Shepherd by using descending, one-dimensional paper chromatography. It involves the flow of a mixture of solvents over a spot of material deposited on a strip of filter paper. As the solvent mixture flows over the spot, a liquid–liquid extraction system is established and the solutes present are carried along the paper strip at characteristic rates determined by their respective distribution coefficients. After a period of time, each component of the mixture was separated from the others in the form of spot. These spots can be identified by treating the chromatogram strip with diazotising agent followed by coupling agent.
The procedure is nothing but the little quantity of butanol–ammonia mixture is placed in the bottom of the chromatographic chamber to saturate the air space of the chamber with the solvent vapours through out the test period. Sampled end of the strip is placed in the butanol phase of the solvent mixture. Keep a side for 18 h for the development of the chromatogram. When the solvent front is descended to about 1 in. from the lower end of the strips, remove from the chamber and mark with a pencil. Dry the strip with the help of warm air and spray the paper strip with the help of diazotising reagents followed by coupling agents such as BM reagent for colour development. Violet–pink colour spots locate the sulphonamides.
- Electro-analytical methods: Sulphonamides can be easily determined by electro-analytical methods such as potentiometry, amperometry and polarography. They are as follows:
- Potentiometry: Agren investigated possibility of using a suitable redox indicator (di phenyl benzidine disulphonic acid) in the presence of oxidising agent (K2Cr2O7) which changes colour from green to violet colour at the end point with the potential change of +0.80 to +0.95 V.
- Amperometry: Scholten and Stone described an amperometric method of detecting the end point by using the electrical circuit of Wernimont and Hopkinson. A potential of 0.4 V is applied across two platinum electrodes. No current flows during the diazotisation reaction until excess of nitrite has been added. The end point is indicated by the first flow of current.
- Polarography: Korshunov and co-workers had shown that sulphonamides are reduced polarographically at dropping mercury electrode using 0.1N tetra methyl ammonium iodide with potential difference of −1.66 to −1.80 V.
- Salt formation method: This method involves the complex salt formation where sample is dissolved in 50 ml water, titrated with 0.1M methylene blue solution. The end point is the formation of light blue ring when one drop of solution is spotted on white filter paper.
1 ml of methylene blue ≡ 0.0294 g of sulphonilamide
REVIEW QUESTIONS
- What are the methods for the analysis of the sulphonamides by the titrimetric methods?
- What are the methods for the analysis of the sulphonamides by the spectrometric methods?
- Explain the principle involved in the analysis of sulphonamides by chromatography.
