Chapter 7
Analysis of Adrenergic Drugs
INTRODUCTION
These drugs are used to treat life-threatening disorders such as bronchial asthma, shock, cardiac arrest and allergic reactions.
Example: Nor epinephrine
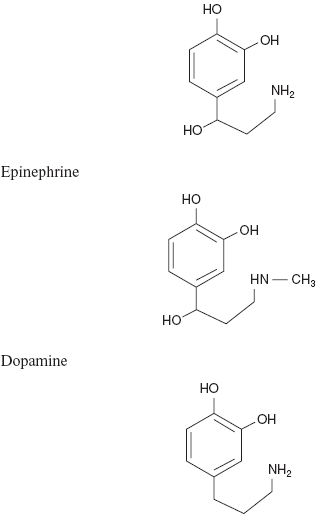
Based on their importance, these drugs are analysed mostly by the spectrometric methods and titrimetric methods.
- Colorimetric methods: These colorimetric methods are mainly based on the principle involved in the colour formation.
- Oxidative coupling reactions: This reaction involves the use of MBTH, N, N-dimethylamino-paraphenylenediamine (DMPD) and PAP in the presence of appropriate oxidant or Gibb's reagent under slightly acidic, basic or neutral conditions to form highly coloured products which are used for the analysis of adrenergic drugs.
- MBTH-Ce(IV) for salbutamol, isoxsuprine, nylidrin and pseudoephedrine: MBTH is generally known as 3-methyl-2,3-dihydro-1,3-benzothiazole which undergoes oxidative coupling with phenols, aromatic amines and heterocyclic bases.
Under reaction conditions MBTH looses 2e− and 1H+. On oxidation, it forms the electrophilic intermediate which is the active coupling species. The intermediate reacts with the adrenergic drug by electrophilic attack on most of the nucleophilic sites on the aromatic ring. When para position is substituted with electron-releasing group, then coupling proceeds through o-position.
Reaction:
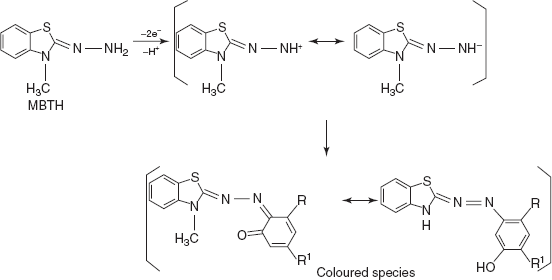
The coloured species is measured at 510 nm
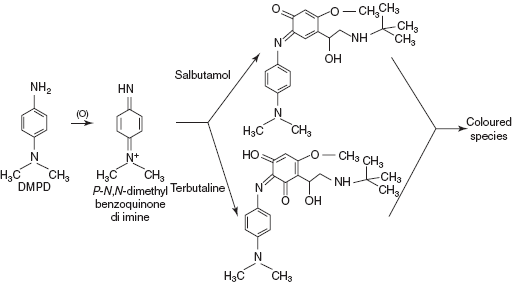
- DMPD-CAT method for salbutamol, isoxeprine, terbutaline and nylidrin: DMPD is chemically known as N, N-dimethyl benzene 1,4-diamine which undergoes oxidation with two electron's transfer to yield very unstable and highly reacting p-N, N-dimethyl benzo quinone di-imine. This is reacted with adrenergic drugs and forms the coloured species.
In this method, terbutaline shows absorption maxima at 500 nm; and salbutamol, isoxeprine and nylidrin show the absorption maxima at 620 nm.
Reaction:
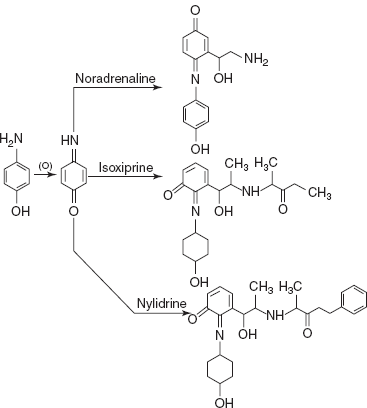
- PAP-O2 method for noradrenalin, isoxeprine and nylidrin: p-Amino phenol is a bi-functional substance containing the primary aromatic amine group and phenolic hydroxyl group in the presence of oxidising agent and followed by coupling of p-benzo quinone monoamine with the adrenergic drugs.
The coloured species formed is measured at different λmax based on the drug. For nor-adrenaline, the λmax is 600 nm and for isoxeprine and nylidrin, it is at 640 nm.
Reaction:
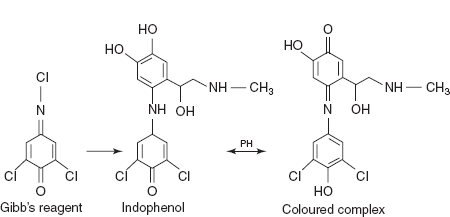
DCQC method for adrenaline, noradrenalin and oxymetazoline: DCQC is chemically known as the 2,6-dichloro quinone 4-chlorimide which is also known as Gibb's reagent attached to the para position of the phenol ring and forms indo-phenols which are brightly coloured and undergoes a drastic colour change with pH due to ionisation of phenolic proton. This coloured product is measured for different drugs.
Adrenaline shows the λmax at 510 nm, noradrenalin shows the λmax at 480 nm and oxy-metazoline shows the λmax at 660 nm.
Reaction:
- MBTH-Ce(IV) for salbutamol, isoxsuprine, nylidrin and pseudoephedrine: MBTH is generally known as 3-methyl-2,3-dihydro-1,3-benzothiazole which undergoes oxidative coupling with phenols, aromatic amines and heterocyclic bases.
- Diazo coupling reactions: The main principle involved in this reaction is diazonium salts react with certain aromatic compounds to form azo compounds by coupling. The aromatic ring is attacked by the diazonium ion. Substitution usually proceeds to para and then to ortho positions in alkaline conditions.
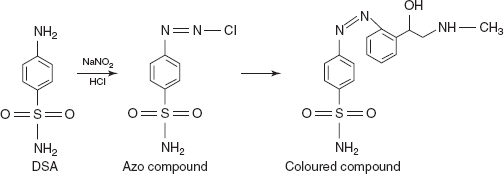
- DSA for adrenaline, noradrenalin, salbutamol, terbutaline, isoxeprine, nylidrin and phenyl ephedrine: The DSA is mixed with the diazotising reagents such as NaNO2 and HCl. Then it forms the azo compound which is coupled with the adrenergic drug to form a coloured complex.
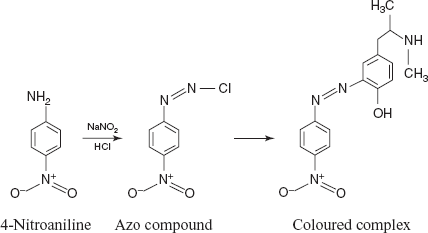
Drug name Λmax(nm) Adrenaline 410–420 Noradrenalin 410–420 Salbutamol 450 Isoxeprine 445 Nylidrin 445 Phenylephedrine 430 - DDP for adrenaline, noradrenalin, salbutamol, isoxeprine, nylidrin and phenyl ephedrine: The DDP is mixed with the diazotising reagents like NaNO2 and HCl. Then it forms the azo compound which is coupled with the adrenergic drug to form a coloured complex. Adrenaline and noradrenalin show the λmax at 465 nm, salbutamol shows the λmax at 465 nm, isoxeprine shows the λmax at 445 nm, nylidrin shows the λmax at 445 nm and phenylepherine shows the λmax at 410–420 nm.
Reaction:
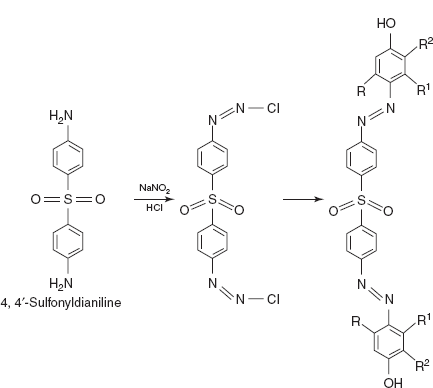
- DPNA for pseudoephedrine: The DPNA is mixed with the diazotising reagents such as NaNO2 and HCl. Then it forms the azo compound which is coupled with the adrenergic drug to form a coloured complex. The coloured complex is measured at 500 nm.
- DSA for adrenaline, noradrenalin, salbutamol, terbutaline, isoxeprine, nylidrin and phenyl ephedrine: The DSA is mixed with the diazotising reagents such as NaNO2 and HCl. Then it forms the azo compound which is coupled with the adrenergic drug to form a coloured complex.
- Condensation reactions: These reactions are mainly based on the aldehydes condensation under acidic conditions.
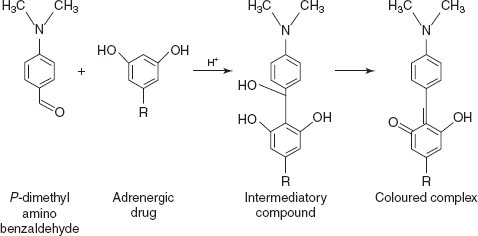
- MHB-H2So4 for terbutaline and orciprenaline: On condensation, methoxy benzaldehyde in acidic medium forms the intermediatory compound. On process, it shows the absorption maxima at 610 nm for terbutaline and orciprenaline.
Reaction:
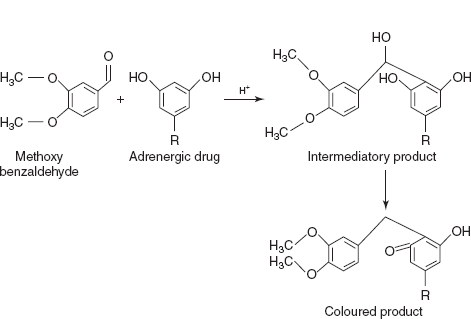
- PDAB–H2SO4 for terbutaline and orciprenaline: Same mechanism as the methyl benzaldehyde and shows the absorption maxima at 565 nm.
- PDAC–H2SO4 for terbutaline and orciprenaline: para-dimethyl amino cinnamaldehyde undergoes condensation in acidic medium and the coloured complex is measured at 575nm.
Reaction:
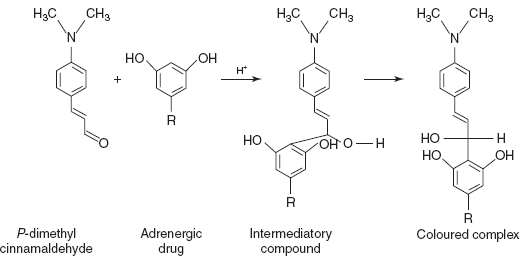
- MHB-H2So4 for terbutaline and orciprenaline: On condensation, methoxy benzaldehyde in acidic medium forms the intermediatory compound. On process, it shows the absorption maxima at 610 nm for terbutaline and orciprenaline.
- Redox reactions: Adrenergic drugs exhibit reducing properties in the presence of oxidants. The reduced form of oxidant is either self-coloured or form coloured complex with other reagent. Example: Fe(II) from Fe(III) with 2,4,6-triphenyl tetrazolium salt and
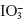 from
from 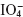 with metol sulphanilamide after masking excess of
with metol sulphanilamide after masking excess of 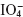 with Mo(VI).
with Mo(VI).
- Folin–Ciocalteu (FC) reagent for adrenaline and noradrenalin: Reduction of the hetero poly acid complexes by organic reagents is basic principle for this reaction. More the number of poly acids in the complex, it readily undergoes the reduction. Among the various hetero poly acids, phosphomolybdo tungestic acid known as FC reagent for the determination of adrenergic drugs.
3H2O.P2O5.13WO3.5MoO3.10 H2O3H2O.P2O5.14WO3.4MoO3.10 H2O
Adrenaline and noradrenalin probably effect reduction of the 1,2 or 3 oxygen atoms from tungestate and molybdate, thereby producing one or more of several possible reduced species which have characteristic intense blue colour which is measured at 740 nm.
- Fe(III)-TPTZ for adrenaline, noradrenalin, salbutamol, terbutaline, orciprenaline and oxymetazoline: This is the an improved method for the determination of adrenergic agents based on 2,4,6-triphenyl-S-triazine selected from a group of iron reagents which are measured at 595 nm.
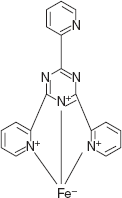
Coloured complex
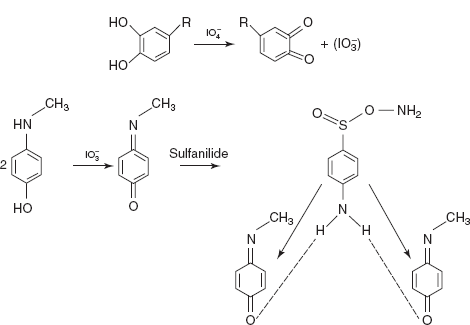
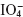 /Mo(VI)/metol-sulphanilide for adrenaline and noradrenalin:
/Mo(VI)/metol-sulphanilide for adrenaline and noradrenalin: 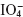 is a specific oxidant for compounds with vicinal diols results in the oxidation of metol from quinone and reduction forms iodate. This is a coloured complex and shows absorption maximum at 520 nm.
is a specific oxidant for compounds with vicinal diols results in the oxidation of metol from quinone and reduction forms iodate. This is a coloured complex and shows absorption maximum at 520 nm.
Reaction:
- Folin–Ciocalteu (FC) reagent for adrenaline and noradrenalin: Reduction of the hetero poly acid complexes by organic reagents is basic principle for this reaction. More the number of poly acids in the complex, it readily undergoes the reduction. Among the various hetero poly acids, phosphomolybdo tungestic acid known as FC reagent for the determination of adrenergic drugs.
- Oxidative coupling reactions: This reaction involves the use of MBTH, N, N-dimethylamino-paraphenylenediamine (DMPD) and PAP in the presence of appropriate oxidant or Gibb's reagent under slightly acidic, basic or neutral conditions to form highly coloured products which are used for the analysis of adrenergic drugs.
- Titrimetric methods:
- For adrenaline: Extract the drug with carbon tetra chloride and add starch solution and iodine solution. Then add sodium thio sulphate and finally titrate with sodium bicarbonate.
- For isoprenaline, adrenaline, noradrenalin, xylometazoline, terbutaline and salbutamol: Drug solutions are mixed with glacial acetic acid and titrate with 0.1N perchloric acid using crystal violet as indicator.
1 ml of 0.1N perchloric acid ≡ 0.5206 g of isoprenaline
≡ 0.3193 g of noradrenalin
≡ 0.05767 g of salbutamol
≡ 28.08 g of xylometazoline
- UV methods:
These methods are mainly based on the solubility of drugs in different solvents.
Drug Solvent Absorption maximum (nm) Isoxeprine Water 269 Adrenaline HCl 279 Noradrenalin HCl 279 Tetra hydroxyzoline Water 264 Dried bases 271
REVIEW QUESTIONS
- What is the principle involved in the analysis of the adrenergic drugs by the oxidative coupling method?
- Explain the mechanism of the reaction of the DMPD-CAT method for the analysis of the adrenergic drugs.
- What is the principle involved in the PAP-O2 method for the analysis of the adrenergic drugs?
- What is the method for the analysis of adrenergic drugs by the DCQC reagent?
- Explain the diazo coupling reactions for the analysis of adrenergic drugs.
- Explain the condensation reactions for the analysis of adrenergic drugs.
- Explain the redox reactions for the analysis of adrenergic drugs.
- What are the principles involved in the analysis of the adrenergic drugs by the titrimetric methods?
- Explain the procedures for the analysis of the adrenergic drugs by the UV method.