6.10. Organic Origin of Petroleum
The most notable groups of chemicals used in the processes of living organisms include:
Proteins, which are the building blocks from which the structures of living organisms are constructed (this includes almost all enzymes, which catalyze organic chemical reactions)
Nucleic acids, which carry genetic information
Carbohydrates, which store energy in a form that can be used by living cells
Lipids, which also store energy, but in a more concentrated form, and which may be stored for extended periods in the bodies of animals.
Silicon has been a theme of noncarbon-based life since it also has four bonding sites and is just below carbon on the periodic table of the elements. This means silicon is very similar to carbon in its chemical characteristics. In cinematic and literary science fiction, when artificial machines cross from nonliving to living, this new form would be an example of noncarbon-based life. Since the advent of the microprocessor in the late 1960s, these machines are often classed as “silicon-based life.” Another example of “silicon based-life” is the episode “The Devil in the Dark” from Star Trek: The Original Series, where a living rock creature's biochemistry is based on silicon.
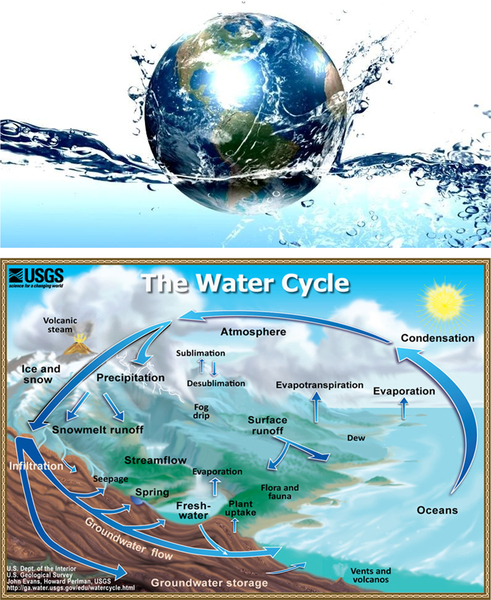
Figure 6.37 Water cycle, involving energy and mass.
Scientifically, natural processing of organic materials into petroleum products is entirely environment-friendly as each stage of processing is well balanced through proper use of characteristic frequencies (Islam et al., 2014). It is commonly agreed that petroleum has an organic origin. Organic materials are transformed by heat and pressure into a complex mixture, known as kerogen. Depending on the initial ingredients and the geologic conditions, kerogen can produce either coal (a solid carbon-rich fuel derived mostly from woody plants) or hydrocarbons (a relatively hydrogen-rich substance that comes from algae and various lipid-containing plant parts). Oil is formed from kerogen, a mixture of organic compounds in sedimentary rocks. It is most abundant in shales. Shales are analyzed and characterized as potential “source rocks” for oil based largely on their total organic content.
For kerogen to be transformed into oil, it must be buried to a depth where the temperature and pressure are sufficiently high to convert the kerogen into oil. The place where the depth is sufficient to achieve this, is called the “oil window.” Oil shale which contains only kerogen was never buried deeply enough to generate oil. Therefore, humans must artificially convert the kerogen in oil shale into oil at high temperatures. The processing time of petroleum is longer than a million years. Such long processing time makes petroleum fluids the most stabilized and environmentally benign fluid other than water. Petroleum is equivalent to natural batteries that pack solar energy in the most efficient and environment-friendly way known to mankind. In contrast, pyrolysis is inherently artificial as it uses artificial sources of energy (heat and pressure) and often contains catalysts (artificial chemicals). As a consequence, pyrolysis leaves behind a trail of heavy foot prints and sets off negative impact on the environment, even though the final products of pyrolysis and natural processing are similar (e.g., methane, ethane, propane, and others). In terms of environmental impact, naturally processed petroleum products are completely benign to the environment. History tells us that the usage of petroleum products in their natural state does not endanger the environment. At the onset of modern age, starting with the mechanization of natural science and engineering, a bifurcation between economic index (driver of the modern civilization) and environmental index has taken place. Using 2000 as the reference point, Vassilis et al. (2013) presented the following graph that shows the nature of this bifurcation (Figure 6.38).
It is, therefore, unfathomable that such modus operandi had been in place in previous civilizations that were no less glamorous than the modern age. The most remarkable distinction is in the use of petroleum as a fuel. Today, 85% of petroleum use in the United States is as a fuel. Nonfuel applications include lubricants for cars, asphalt for roads, tars for roofing, waxes for food wrapping, as well as solvents for paints, cosmetics, and dry cleaning products. Petrochemicals also provide the building blocks for a vast panoply of plastics and foams. Pharmaceutical industry also uses petroleum plastics as well as chemicals derived from petroleum. In each application, however, numerous stages of refinement and processing are involved.
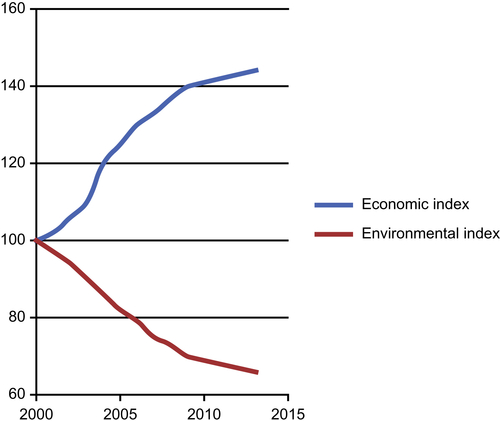
Figure 6.38 Even is short-term, modern age is synonymous with decoupling of economic index from environmental index.
Originally, petroleum played an entirely different role. There were oil pits near Ardericca (near Babylon). the Chinese drilled for “rock oil” to provide heating and lighting, and the Byzantines sprayed “Greek fire” as an incendiary weapon.
Many cultures have also employed petroleum as a medicinal cure, giving it names such as “St Quirinus oil,” “Barbados tar,” and “Seneca oil.” Our modern society continues to use petroleum jelly as a skin ointment.
Petroleum became a significantly valuable commodity in the mid-ninteenth century, when modern techniques of chemical distillation were developed to separate kerosene from crude oil. The refined kerosene could be used in lamps, replacing the more expensive whale oil.
Natural state of petroleum fluids has numerous qualities that make it an ideal antidote for many applications, including medicinal. It is difficult to characterize these qualities within a single index because the only commonality they have is natural processing. Figure 6.39 shows wide range of variability in crude oil characteristics.
The use of petroleum products in its natural state has been there for thousands of years. Petroleum has been used for thousands of years. The ancient Babylonians built walls and towers with asphalt. City walls were constructed to include gates and watchtowers and usually a ditch running around the outer perimeter of the wall, which could be filled with water. King Hammurabi surrounded his city of Babylon with more impressive walls than usually seen shortly after he assumed the throne in 1792 BC, but the credit for transforming the city of Babylon into an awe-inspiring wonder belongs to King Nebuchadnezzar II. Nebuchadnezzar built three walls around Babylon at heights of 40 feet and so broad at the top that chariots could race around them. The Ishtar Gate in the wall of Nebuchadnezzar II's Babylon was claimed by some to be greater than any of the listed Wonders of the Ancient World. Heavy petroleum components are effective cementing materials and this property of crude petroleum is rarely exploited today.
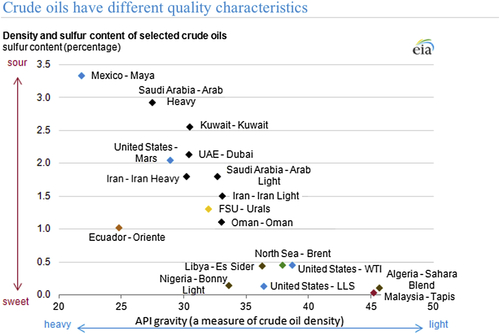
Figure 6.39 Crude oil characteristics vary widely, making it difficult to characterize with a single criterion.
Archeologists have uncovered tools used by Neanderthals dating back some 40,000 years ago. Neanderthals used oil from naturally occurring oil seeps to adhere stones to sticks.
As human society developed, so did the use of oil and natural gas. Around 3000 BC, man was using oil for the construction of buildings and waterproofing canoes, baskets, baths, and drains.
In ancient Mesopotamia, natural asphalt was used in the construction of the walls and towers of Babylon.
Around 1272 BC, Marco Polo, while traveling through the city of Baku (Azerbaijan), witnessed oil seeps being worked in hand-dug wells.
Natural gas was being used too.
Around 1000 BC, a goat herdsman in Greece came across what looked like a burning stream at the top of Mount Parnassus. It was a flame rising from a crack in the rock. Most likely, a lightening strike ignited the natural gas.
The ancient Greeks, believing the flame to be of divine origin, built a temple around it. This temple housed a priestess who was known as the Oracle of Delphi, giving out prophecies she claimed were inspired by the flickering flame.
Fast-forward 2000 years to 1821, when the first natural gas well was drilled in the United States.
William Hart drilled a 27-foot well in Fredonia, New York. How did he know where to drill? He asked the Native Americans who worshipped the naturally occurring gas flowing out of “burning creek.”
Hart's success lead to the first-ever utility company in America, called the Fredonia Gas Light Company.
And ever since, oil and gas companies have used the observation of naturally occurring seeps to find massive oilfields.
That's because typically where you see a seep, you find a highly-pressurized reservoir below. The high quality oil is literally being pushed out of the ground.
How did Captain Drake know where to drill in Titusville? Well it was actually quite easy for him. Since Drake did not have the technological advantages of satellite images or 3D seismic studies, he took the easiest path for discovering oil: He looked down at the ground.
For centuries, the Seneca tribe used the oil that seeped into the creek for many things, including cures for sickness.
For the next 40 years after Drake struck oil, Pennsylvania would supply the world one-third of its oil.
Then came Spindletop in 1901.
Before the legendary Spindletop field was drilled in 1901, Texas residents had for years witnessed thousands of “tar balls” and miles-long oil slicks right off shore in the Gulf of Mexico.
Spindletop quickly became the largest oilfield in America, larger than all of the previous oil fields in the United States, combined.
And it occurs all around the world. The second-largest oilfield in the world—Kuwait's Burgan—was discovered by British geologists who observed hundreds of oil seeps at the surface in 1912.
To this day, oil seeps are spewing crude in deserts, mountains, rivers, and oceans.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
