The effects of sterilization on medical materials and welded devices
Abstract:
The effects of a sterilization method on medical materials such as polymers are diverse. The number of sterilization agents is few without significant adverse effects to materials and polymers. Effects of sterilization methods consider not only chemical and physical effects, but also some effects on applications such as welding and device compatibility. Selecting the right method for a particular or specific product or procedure can be challenging in some situations, but beneficial and critical within other cases.
Note: This chapter contains material previously published in Sterilisation of biomaterials and medical devices, eds. S. Lerouge and A. Simmons (Chapter 7 Sterilisation techniques for polymers by W. J. Rogers), Cambridge, Woodhead Publishing Limited, 2012, ISBN: 978-1-84569-932-1.
4.1 Introduction to sterilization
Sterilization is a like a magic wand that eliminates and removes all microbes and biological organisms from medical materials and medical devices. It inactivates extremely resistant bacteria spores that may have survived and recovered after thousands of years, since the pyramids.
Sterilization is used to create a germ-free environment and products, reducing the risk of microbial infections for the ultimate promotion or preservation of health. Sterilization is basic to the processing and manufacturing of sterile medical materials and medical devices.
‘An ounce of prevention is worth a pound of cure.’ Without sterilization, infectious disease would exist everywhere in a hospital or healthcare facilities, and it is debatable that antibiotics could ever control the onslaught of infections everywhere. Consequently, sterilization has tremendous value to medicine surgery and healthcare facilities. Sterilization is typically performed by dry heat, ethylene oxide (EO) and hydrogen peroxide with and without plasma, irradiation, and ozone. The number of sterilization methods are few that are effective and efficacious without adverse effects to medical materials and devices, such as polymer degradation, melting, and oxidation of some metals.
The effect of sterilization on medical materials and devices can provide reasons why one method of sterilization is applied, employed and another is not, and why there is ultimately a sterilization method available and right for your medical material and device.
Heat sterilization (dry and moist) are traditional methods, but alter, corrode damage, distort, or melt many materials. Moist heat like steam can wet, distort, soften, expand and affect product functionality. Dry heat can melt and cannot sterilize aqueous exposed materials. Dry heat can sterilize some powders. Steam and dry heat have many similarities, including being easy to control and monitor, being inexpensive, and not having toxic residues or wastes as EO and gamma radiation may have. Ethylene oxide is a standard method that is able to sterilize many materials, but not liquids, and craze some materials, leaving toxic residues and by-products. Radiation is another standard method, but it can change molecular structure (cross-link or scissor), cause odors, change pH, discolor, embrittle, stiffen and degrade a few materials, or affect bond strengths and cause changes over shelf life. The development of sterilization has changed over the past few years. Hydrogen peroxide and ozone are two newer terminally accepted sterilizing methods. They can both oxidize some materials. Hydrogen peroxide cannot sterilize cellulosics. The potential disadvantages of oxidizing agents include their oxidizing reactivity with certain materials (e.g., rubber, celllu-olistics, and polyurethanes). Neither hydrogen peroxide nor ozone have the penetration capability of traditional methods of dry heat, ethylene oxide, irradiation, or steam.
4.2 Sterilization methods
Sterilization is defined as killing or eliminating of ‘all’ types of biological organisms and is achieved by either physical or chemical processes. It is an absolute term as well as a probability function.
Processes that do not kill or eliminate all types of microbes are not deemed to be sterilization methods, but may be techniques such as antiseptics, decontamination agents, disinfection, germicides, high level disinfectants, or sanitizers. Current acceptable, available and recognized ‘terminal’ sterilization methods are dry heat, ethylene oxide, hydrogen peroxide with or without plasma, irradiation, steam, and ozone.
4.2.1 Dry-heat sterilization
Dry-heat sterilization is one of the oldest sterilization methods from the time of the ancient Egyptians, but it is infrequently applied in medical-device industry, except in the pharmaceutical area where it is used as part of aseptic processing.
It was originally used to preserve and sterilize items that were moisture sensitive. Today it is used to sterilize items such as powders, glass, non-aqueous materials, electronics, and silicone prosthesis. In hospitals high-temperature dry heat (e.g., 150–180 °C) should only be used for materials that might be damaged by steam or impenetrable to steam. Dry heat has continued to be used in sterilizing dental instruments to minimize the corrosion of sharp items, and depyrogenation of pyrogens. It has been used more recently as the method of choice for spacecraft sterilization in the United States. The Russians used an EO/methyl bromide gas mixture, instead. With its further evolvement for sterilization of spacecraft component, etc. dry-heat sterilization became more useful. As a result it is used to sterilize silicone prosthesis (e.g., mammary glands).
Classically, dry heat has meant very high temperatures which have destroyed many items. In the pharmaceutical industry it is used to depyro-genate or inactivate pyrogens (typically cell walls of dead microbes that can elicit a febrile response from the patient).
Dry heat requires significantly more time or higher temperature to process and to inactivate resistant microbes (e.g., spores) on products than steam. Consequently dry-heat sterilization has been generally reserved for materials and products that cannot withstand steam (e.g., glass, powders, and depyrogenation) or penetrate with steam (e.g., silicone and prosthesis).
Dry-heat sterilization (Table 4.1) is as simple as baking in an oven. Dry-heat sterilization is a time- and temperature-dependent variable. With dry heat, the best sterilization occurs with elevated temperature (e.g., 105–190 °C) and under dehydrated conditions. Typical dry heat (160–180 °C) is the simplest, least expensive method, with fewer parameters and excellent penetration capabilities; however, it has long exposure:
Besides long exposure time it has a slow rate of heat penetration. New infrared can sterilize faster, but is not necessarily approved for hospitals. Infrared, however, is used in the pharmaceutical industry for depyrogenating, sterilizing glass vials.
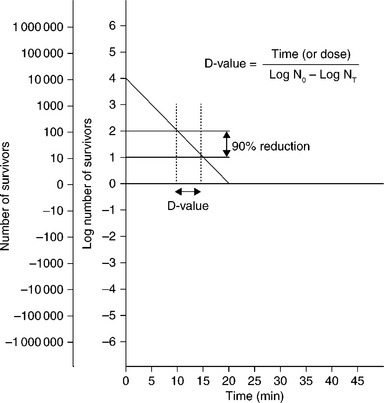
At a lower temperature (e.g., 105–135 °C), more materials and devices become compatible. Lower sterilizing temperatures (e.g., <105 °C) have been demonstrated with more than a day or year D-value. A D-value is defined as the time to kill one log or 90% of a population at a given temperature. The time to kill 90% of a Bacillus atrophaeus population at 120 °C may be 1 h. Times also will vary depending upon the spore history, environment, substrate of the population, and heat-penetration needs of a product. The D-value (Fig. 4.1) is the backbone of microbial death kinetics.
The classical method is the Stumbo method, as follows:
where N0 is the initial number of organisms (the bioburden or biological indicator population) and NT is the number of survivors after the exposure time or dose of the sterilant.
Bioburden is an estimate of what is on the product or item being sterilized. The biological indicator (BI) is a solution or carrier consisting of a known concentration (population) of spores (typically) that is highly resistant to and challenges the sterilization method (e.g., steam, EO, and dry heat).
Two typical commercial dry-heat sterilizers (Fig. 4.2) run exposure times of, for example, 6 min at 190 °C and 30–120 min between 160 °C and 180 °C. At its highest temperatures (e.g., 330 °C) dry heat becomes virtually an ‘absolute method’ and fastest (1.15 s) sterilization (Rhodes, 19661) by breaking down all organic matter down to carbon. At its lowest possible temperatures (37 °C or lower), dry heat can take the longest exposure time (~45 days D-value) of virtually any method to cause sterilization inactivation (12 × 45 days = 540 days). Theoretically at 0 °C, under extreme dehydration, the dry heat D-value would be about four years. (Molin, 19772). To achieve an overkill sterilization (e.g., 12 × D-value) this would require ~48 years.
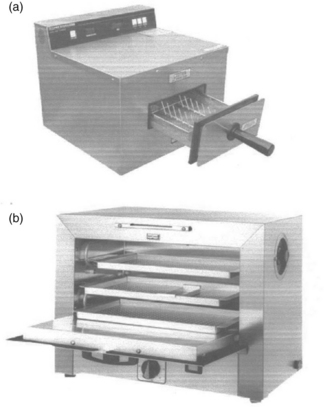
4.2 Two commercial dry-heat sterilizers: (a) Cox fastest dry-heat sterilizer (6 min unwrapped at 375 °F (190 °C) temperature; 12 min wrapped) and (b) Wayne S1000 dry-heat sterilizer (standard 160–180 °C oven for instruments).
Sterilization by dry heat, however, occurs primarily by dehydration and oxidation, but at temperatures typically at the same or higher than steam, which limits the types of heat-sensitive materials and polymers, unless there is a willingness to sterilize at lower temperatures and process for extremely long periods under dehydration conditions. So at its highest temperature it could virtually destroy everything (and would even temper metals) that came in contact with it; however, at its theoretically lowest level it would take too much time to achieve sterility.
There is a chemical vapor dry-heat sterilizer that may sterilize in 20 min at 132 °C. Its effects on polymers, however, is unknown. It does sterilize metal instruments without dulling, rusting, or corrosion, as steam sterilization may, and it is much faster than standard dry-heat sterilization with just heated air.
4.2.2 Ethylene oxide sterilization
Ethylene oxide (Table 4.2) is a mild sterilant to most medical materials and polymers. It is a long process with preconditioning and/or prehu-midification, gas introduction, sterilization, evacuation air washes and aeration (Fig. 4.3) and the later step (aeration) is required to reduce EO toxic residues, when necessary. Ethylene oxide requires typically temperature control such as +/− .5 C ambient to 63 °C; relative humidity control within +/− 5% RH within a range of 40–80%, EO gas concentration typically higher than 400 mg/L. Lower EO concentrations have been used with expertise and specialized processing. Further control of evacuations and preconditioning to place moisture to the bacterial site and post-cycle vacuums to remove the toxic gas and reduce EO residuals within polymers. Because of the use of toxic gas, it requires post-sterilization aeration, special handling, control, scrubbers, and meeting a large number of regulations.
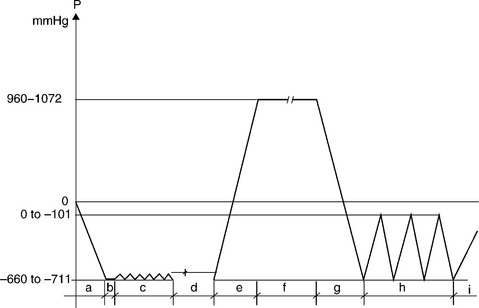
4.3 A prehumidification EO sterilization cycle. Steps: (a) initial evacuation, (b) leak check, (c) prehumidifying, (d) humidity dwell, (e) ethylene oxide gas mixture injection, (f) ethylene oxide exposure, (g) post-evacuation, (h) 3 air washes, (i) final air inbleed to atmospheric pressure.
EO evolved for sterilizing heat-sensitive materials such as plastics, rubber, and metals upon continual exposure. EO was not meant as an alternative to steam sterilization, but as an alternative process for temperature-sensitive items. It is an ideal gaseous sterilant because of its characteristically high diffusivity, permeability, and gentleness to most medical materials and medical devices.
While EO sterilization probably has been the predominant method of gaseous chemical sterilization, it has remained second to steam sterilization in the hospitals, but not in the medical-device industry.
The EO process can be lengthy where medical materials and medical devices need to be preconditioned before sterilization and aerated after sterilization to remove toxic residues before using. While it can penetrate long lumens and (some) mated surfaces and sterilize papers or other materials that other methods such as hydrogen peroxide, steam, and ozone cannot, EO sterilization has been challenged by less dangerous, toxic and wasteful chemical methods, such as ozone and hydrogen-peroxide sterilization in hospitals, and irradiation in industry.
4.2.3 Hydrogen-peroxide sterilization
Hydrogen peroxide (Table 4.3) is relatively new and an alternative to EO for many materials and devices in the hospital.
Hydrogen peroxide (with plasma) sterilization provides a dry, low-temperature (e.g., 37–44 °C) and nontoxic method, at the end of the process; however, extremely deep vacuums and a high concentration of H2 O2 exists at the start of the process. It is not highly penetrable, but is increasingly used in hospitals for surface devices. With low temperature hydrogen peroxide gas plasma, the following process parameters need to be applied, considered and evaluated, such as H2O2 concentration (e.g. 6–18 mg/L), temperature (e.g. 37 °–44 °C, < 55 °C), the presence and duration of plasma, and pressures (e.g. rate, level or both), vacuums (e.g. <50 Pa, <−0.5 Torr), exposure (time to sterilise). Exposure and process times continue to be reduced such as 73–52 min and further to a possible 32 min exposure with removal of most water from the H2O2.
With hydrogen peroxide with plasma, a very deep vacuum is drawn initially to vaporize the H2O2 and sterilize; then an electrical field is created by radio frequency to create a gas plasma; then the gases are removed and in the final stage vented to atmospheric pressure with-high efficiency filtered air. Newer and improved processes include consecutative sub-processes to improve penetration and inactivation.
Hydrogen peroxide with plasma and ozone are considered nontraditional sterilization methods yet are readily acceptable by regulatory agencies such as the FDA, because they do not have any toxic residuals per se.
Hydrogen peroxide (without plasma) can exist in vapor and liquid form. But the discussion here is of the vapor not liquid state. In the vapor phase it generally follows the ideal gas law.
Hydrogen peroxide has excellent antimicrobial properties against a wide range of microorganisms including bacterial endospores. Under carefully controlled process conditions hydrogen peroxide is also safe for use with many materials. Hydrogen peroxide may be decomposed into water and oxygen rendering it environmentally safe.
Hydrogen peroxide is a strong oxidizing agent. Hydrogen peroxide primarily reacts with cysteine-containing proteins, creating disulfide crosslinks between proteins. This oxidation of amino-acid moieties within a given protein may cause secondary damage as the radicals modify other amino acids within that protein or other proteins. Another inactivation mechanism of damage may be through intracellular reactions. For example, reactions may occur when iron (II), present in heme groups or in other forms, reacts with peroxide forming hydroxyl radicals. Such hydroxyl radicals would be highly reactive and can oxidize most organic molecules within a cell.
While hydrogen peroxide with plasma may have additional excellent microbiocidal properties, it has poor penetration. And while highly toxic in form before sterilization it may be environmentally acceptable under process control. H2O2 is typically used in the vapor phase for medical materials and devices. While it is compatible with many polymers, there are some materials that are damaged (e.g., acrylics, cellulosics (includes paper), natural rubbers, and bioadsorbables such as polyglycolides and polyesters).
Due to the oxidative nature of hydrogen peroxide vapor, some materials are not recommended for instruments intended for this sterilization method. The low-temperature hydrogen peroxide gas plasma method uses the plasma phase to further eliminate residuals; therefore, most commonly used materials for medical instrument fabrication do not retain enough sterilant residuals to affect biocompatibility and post-sterilization aeration usually is not required, with plasma process; however, with straight H2O2 without plasma may require aeration.
4.2.4 Radiation sterilization
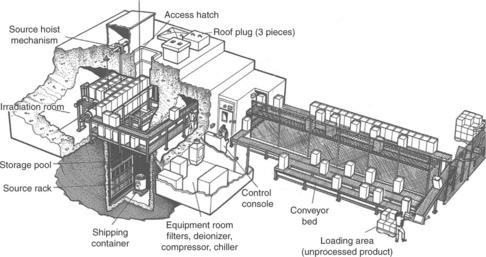
Radiation (Table 4.4) is an excellent sterilization method for many materials and single-use devices. There are typically no cleared hospital irradiators; because of its high cost in hospitals it has not been been favored. Large irradiators are used in industry (Fig. 4.4).
Ionizing radiation requires strong irradiation doses (e.g., 11–40 kGy), much higher than for body scanning. Radiation may be provided from gamma source (e.g., Co60), electron beams or X-rays. It can be very quick (seconds–minutes) to hours. It can have deep penetration, except for dense materials, metals, and water. Irradiation is particularly available industrially, for manufacture of disposable, but not for sterilizing in hospitals. Because of Co60 radiation source, radioactive wastes, X-rays, or electron beams, expensive facilities, equipment, and special handling and control of the process are necessary.
Radiation has long been recognized as a means of sterilization since X-rays were first demonstrated in 1896 to inactivate microorganisms. However, its practical application followed the use of EO because of the continuous improvement of plastic materials and medical devices, the availability of improved electron-beam accelerators and radioactive materials, and increased regulations on the use of EO.
1. Gamma irradiation provides protons with high penetration, longer exposure; but has toxic radioactive waste.
2. E-beam uses electrons which have limited penetration, are fast, and have no toxic waste.
3. X-ray delivers protons which have deep penetration, are moderately fast, and have no toxic waste.
Radiation can sterilize medical devices, pharmaceuticals, treat cosmetics, spices, biologics, foods, and consumer products. Radiation processes are very effective, but they are still very expensive and have not been put into use for general-hospital sterilization of supplies on site. Also, radiation would not be able to resterilize a number of polymer multiple resterilizations without effects on medical materials and polymers over exposures and time. Also, radiation is not typically useable in hospitals, due to costs and reusables.
Irradiation has been developed as means of sterilizing single-use (disposable) devices. It has tremendous penetration and ‘killing’ effectiveness, but it is costly for hospitals and not compatible with every product or material, multiple times.
4.2.5 Steam sterilization
Saturated steam (Table 4.5) is a simple, available, easy, environmental, and traditional sterilization process. In healthcare facilities, most medical materials and devices are made of materials that are heat stable for steam sterilization, but there are some medical materials and devices that are made of polymers which are heat and moisture sensitive.
Steam sterilization can inactivate all biological entities including prions, Pyronema domesticatum, and extremely small target viruses. With steam (moist-heat) sterilization, saturated moisture conditions elevated pressures, and elevated temperature (e.g., 104–138 °C) are optimal for sterilization. The lower the temperature, the longer the exposure; however, more materials tolerate lower temperatures. Steam sterilization is available and traditional to healthcare facilities. Heat sterilization without steam or moisture is dry-heat sterilization.
Steam sterilization has long been recognized for its simplicity, efficiency, effectiveness, low cost, and speed of operation. In the 1880s Koch recognized that dry heat was relatively inefficient compared with moist heat; however, he did not rule out dry heat because of the need to decontaminate silk thread.
Steam sterilization is currently considered as a more ideal candidate because of its compatibility with the environment (not toxic agents or wastes) and health safety, and its capacity to sterilize the most resistant biological entity, the prion. But the number of plastic materials, chemicals, and some metals capable of tolerating its high temperature and moisture (corrosion and hydration) are few. In hospitals and laboratories where reusable materials are frequently used, steam sterilization is predominantly used. It is also widely used in decontamination of infectious waste materials. Now, however, with emphasis on the environment, there is renewed interest in this method of sterilization. Unlike most other sterilization methods, steam is compatible with most aqueous liquids. Steam can sterilize most metals, glass, and some heat-resistant plastic materials.
The number of plastic materials capable of being steam sterilized will vary considerably with the selected temperature of sterilization. Standard steam sterilization is generally carried out at 250 °F (121 °C) for 15–20 min. Faster or immediate to use (flash) sterilization is generally carried out at 270 °F (134 °C) and greater for 3–4 min, typically without wrap or packaging. Burns can occur from such a process if the product being sterilized is not cooled before use. Longer sterilization or lower steam sterilization is carried out at 240 °F (115 °C) (e.g., 30 min). Lower steam sterilization can be performed at approximately 212 °F (100 °C) (fractional) but lower-temperatures approaches are marginal and possibly questionable, unless other aspects are provided. Some alternative approaches to classical steam sterilization for lower temperatures are combination of steam and formaldehyde, and steam sterilization of liquids with acids, etc.
Steam sterilization ‘overall’ is more widely employed and available than other methods, but it can be deleterious to heat labile medical materials and healthcare products. It is the work horse in must hospitals. It has multiple processes for different situations. Low and high temperatures: removal of air by gravity or vacuum or pulsing. Gravity allows lighter steam to drive air out at the bottom of the chamber (Fig. 4.5). However, it may not remove all air which could result in failure to sterilize completely. The vacuum and steam pulsing processes are more ideal for removing air (Fig. 4.6).
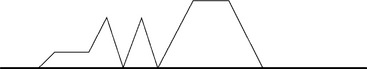
4.5 A dynamic gravity pulsing sterilization cycle curve. With a series of steam purge and pressure pulses with heat up phase to pressure, and a pressure hold period followed by cool down phase as shown above standard atmospheric pressure line. Simple gravity method will show only pressure displacement of air, hold period and cool down.
4.6 A dynamic evacuation pulsing sterilization cycle curve. With a series of steam purge and pressure/evacuation pulses with heat up phase to pressure, and pressure hold period followed by evacuation and drying period before returning back to the atmospheric standard pressure line. Simple high evacuation steam cycle would have neither the initial steam purge nor the pressure/evacuation series of pulses.
Other processes typically not seen in hospitals are steam–air mixtures (SAM) and air overpressure (AOP). These are special processes adapted in pharmaceutical and medical-device industries to prevent certain products (containers) and packaging from bursting.
Steam autoclave is used today to eliminate and reduce outbreaks of the spore Clostridium difficle in hospital and other nosocomial contamination and infections. Because of its deleterious effects on heat-liable medical materials and medical devices it is frequently replaced by EO, hydrogen peroxide, irradiation, and ozone sterilization.
However, steam sterilization is both a classical and traditional method of sterilization, continuing today and into the future. Steam remains the preferred method of sterilization in healthcare facilities, including the pharmaceutical industry, despite availability of low-temperature processes of increasing complexity for temperature-sensitive items. Steam remains a powerful weapon in the sterilization arsenal, including elimination or inactivation of prions.
There is a steam–formaldehyde process that will not be fully discussed here. It may be more compatible with heat-sensitive polymers than steam because it runs at 70–75 °C cycles. However, it has toxic formaldehyde present which must be eliminated with aeration or neutralization, and it is a hotter cycle than other alternative heat-sensitive sterilization processes such as EO and hydrogen peroxide with plasma and ozone. It will not have the penetrating capability of EO sterilization.
4.2.6 Ozone sterilization
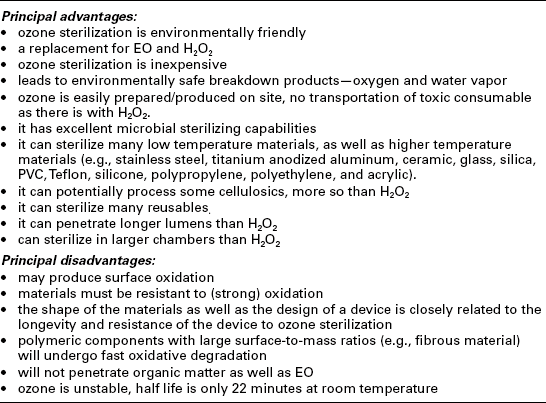
Ozone sterilization (Table 4.6) of medical devices has recently been introduced to healthcare facilities. It is a safe process by introducing the ozone in situ into a sterilizing chamber (Fig. 4.7). There are no toxic residues, and it may be more penetrable than hydrogen peroxide vapor (with plasma), but not as penetrable as EO, steam, dry heat, or irradiation.
In gaseous low-temperature ozone sterilization, the typical process variations or parameters required are vacuum, time, temperature, ozone concentration, humidity, and pressure (rate, level or both). The ozone concentration is typically at 85 mg/L for 15 min at a temperature of 30–36 °C. The process temperatures are generally low, making it suitable for temperature-sensitive materials. Ozone sterilization can be achieved in water as well as gaseous form. Ozone is a very strong oxidizer, making it an efficient sterilizing agent. It is relatively new (gaseous) technique for medical devices, making it a nontraditional and acceptable process, although it has been traditionally used to sterilize water, etc.
This section relates only to ozone in the gaseous state. The sterilant must be able to penetrate all portions of a load and product areas intended to be sterilized. Materials must be resistant to oxidation. Gaseous ozone requires high humidity to be effective.
Ozone is produced by mean of an electrical discharge passing through oxygen (O2). Due to its thermodynamic properties, ozone is a metastable product; it decomposes slowly (in minutes) at ambient temperatures and rapidly (in seconds) at higher temperatures.
Since ozone is a strong oxidizer it is an efficient sterilizing agent. But because ozone is a metastable product, it cannot be stored and is therefore produced in situ. Ozone-sterilization processes are particularly suited for sterilizing heat-sensitive materials because temperatures within the load currently do not exceed 36 °C (97 °F). The process is considered safe because there are no toxic emissions (just O2 and water), no residues to aerate, and a low processing time will not result in accidental burns.
Hydrogen peroxide (H2O2) and ozone (O3)
There is a newer ozone process that combines ozone with hydrogen peroxide: the microbiocidal efficacy of the action of free radicals formed by the two sterilizing agents in this process, hydrogen peroxide (H2O2) and ozone (O3). The mode of action for these two chemicals on microorganisms may be complex and specifically relates to the formation of free radicals such as HO•, O2•-, HO2•, HO3•, HO4• and other reactive species. In this oxidative-sterilization process, the radicals are formed first by the hydrogen peroxide and then by the reaction of ozone with the hydrogen peroxide, water, and radicals already present in the chamber. The combined process is fast, efficacious, low cost, has a large load capacity, and potentially higher material compatibility than just hydrogen peroxide vapor.
Ozone has a similar effect to plasma on the hydrogen-peroxide molecule in competitive technologies. However, the effect of ozone on hydrogen peroxide is not limited to only the accessible surface of devices, as in the case of plasma; ozone can penetrate lumens and hard-to-reach geometries.
Vaporized hydrogen peroxide and ozone have been used as agents for sterilization and disinfection for many years. When mixed together, they become a very powerful oxidizing agent, often referred to in scientific literature as a peroxone or perozone process. During the combining process, highly reactive particles such as hydroxyl radicals (HO•) are formed. These radicals react by oxidizing a wide variety of organic compounds. These processes are also called advanced oxidation processes. The advanced oxidative processes are characterized by the generation of hydroxyl radicals via various combinations of chemical and physical agents such as ozone, UV, and hydrogen peroxide. It will not be possible to fully discuss their effect on polymers until more information is acquired, but they may be more similar to just effects of ozone rather than hydrogen peroxide with plasma; however, the presence of ozone will allow for greater penetration of lumens than hydrogen peroxide with plasma. Nonetheless, this combination of peroxide and ozone may be more damaging to celluloses as hydrogen peroxide is.
Oxidizing agents such as ozone and hydrogen peroxide were evolved to replace EO that was extremely regulated, toxic, time consuming, and left toxic residues; however, oxidizing agents oxidize some materials, and they do not have the penetrating ability or capacity of EO.
4.3 The effects of sterilizing on different materials
Several types of materials are addressed within the following classes of materials: plastics and elastomers, metals, ceramics, and glass.
4.3.1 Plastics
The effects of sterilizing modalities on different plastics and elastomers are addressed for each sterilization modality (e.g., EO, heat, hydrogen peroxide, irradiation, ozone). In Section 4.3.2, the effects of different sterilization modalities on specific plastics are rated and described as excellent, good, poor to fair.
Effects of ethylene oxide on plastics
EO sterilization is compatible with nearly every polymer (Table 4.7), except those that may be particularly sensitive to humidity, low temperature, and high EO gas concentration EO sterilization is nearly always very gentle with most polymers, if used wisely. EO is compatible with nearly every polymer, and if there is a problem with the polymer because of the technique, there typically is an expert solution. EO has sterilized many polymers that could not be irradiated or heat sterilized. Some of the limitations related to EO may also relate to polymers’ ethylene oxide absorptivity to accumulating residuals, but this will vary significantly depending upon humidity, EO gas concentration, temperatures, and aeration. There may be some sensitivity to humidity, such as hydrophilic coatings, but there are typically solutions to this type of problem. Users also need to be careful with EO sterilization when applying polymers as carriers for drug delivery, and its EO residues reactivity, temperature, and humidification. A drug applied through a plastic such as Taxol based-formulations cannot withstand high temperature and high humidity EO cycles.
Table 4.7
Polymers compatible with the ethylene oxide technique
| Thermoplastics | Effects |
| Acrylic | Good. Some loss in tensile properties, no discoloration reported on multiple cycles with HCFC-124/EO blends There may be some crazing Excellent with low EO/CO2 concentration gas mixture, except at high sterilizing temperature >63 °C. Low EO cycle with EO/CO2 gas mix had low absorbency and very short aeration |
| Acrylonitrile butadiene styrene copolymer (ABS) | Compatible High absorbance of EO and long aeration for desorption Excellent with low EO/CO2 concentration Gas mixture with low EO concentration had low absorbance and short aeration |
| Nonplasticized polyvinyl chloride (PVC) | Compatible EO/CO2 concentration Gas mixture with low EO concentration had very short aeration |
| Plasticized polyvinyl chloride (PVC) | Compatible Plasticized PVC absorbs more EO than non-plasticized PVC Excellent with low EO/CO2 concentration Gas mixture with low EO concentration had very short aeration |
| Polyacetal | Compatible, no degradation Low EO concentration with EO/CO2 gas mix had short aeration |
| Polyamide (Nylon, all classes) | Compatible Increased residuals with high humidities; but low residuals with low EO concentration with EO/CO2 mix |
| Polyarylsulfone | Compatible |
| Polycarbonate | Compatible. Some formulations may be subject to stress cracking and some loss of tensile properties after multiple cycles and an extended time post-processing, no discoloration |
| Polyether sulfone | Compatible |
| Polyetheretherketone (PEEK) | Compatible |
| Polyethylene (PE, UHMWPE, LDPE, LLDPE, HDPE) | Generally compatible. HDPE may lose some tensile properties, no off-gassing Excellent with low EO/CO2 gas concentration mix; absorbs and desorbs EO well, very short aeration EO is excellent with UHMWPE for hip and knee implantation |
| Polyethylene terephthalate glycol copolymer (PETG) | Compatible |
| Polymethyl methacrylate (PMMA) | Compatible, no discoloration; EO acceptable for contact lenses |
| Polyphenylene oxide | Compatible |
| Polypropylene (PP) | Compatible. May be some long-term effect on tensile modulus. Excellent with 100% EO. Good with HCFC, no brittleness Can sterilize unstablized PP in syringes with no brittleness Excellent for 100% (pure) ethylene oxide gas. Good for HCFC-124 blend. Excellent with EO/CO2 gas mixture Absorbs and desorbs EO well |
| Polystyrene | Typically poor. Some embrittlement and loss of tensile strength for some formulations has been reported However, polystyrene petri dishes have been easily sterilized (excellently) with EO/CO2 gas mixtures and with moderate humidities; many European IV sets with styrene were compatibile with polystyrene parts Polystyrene tissue ware will absorb EO and will not desorb well enough For cell culture growth, unless low EO concentration in EO/CO2 gas mix. No crazing and no residuals with low EO concentration with EO/CO2 gas mix |
| Polysulfone | Compatible |
| Polytetrafluoroethylene (PTFE) | Compatible |
| Polyvinyl chloride | Compatible. Rigid PVC may decrease impact resistance after exposure. Medical-grade plasticized tubing may contain significant residual levels until aerated EO/CO2 gas mixtures had little EO residuals with low EO concentration EO and CO2 have the same molecular weight |
| Styrene acrylonitrile copolymer (SAN) | Generally OK for one cycle, but may embrittle and lose tensile properties on multiple cycles. May exhibit surface cracking and stress cracking on multiple cycles. Standard EO cycles have high EO absorbency and poor desorption, requiring long aeration Compatible with low EO/CO2 gas concentration. Low EO concentration cycle with EO/CO2 mix; had low EO absorbency and very short aeration |
| Styrenic block copolymer | Compatible |
| Polyester | Compatible With low EO concentration, EO/CO2 mix had low EO absorbency and very short aeration time |
| Polyetherimide (PEI) | Depending on formulation and application. Very thin tubing may present compatibility issues. Bulk structural materials are generally compatible |
| Polyurethane | Performance depends on formulation, cure conditions, material thickness and end use stresses. PU has high affinity for EO but releases with aeration Low EO concentration with EO/CO2 gas mix had short aeration |
| Silicone (RTV) | Excellent; no cross-linking |
| Butyl rubber | Butyl is even stable in liquid EO |
| Ethylene propylene diene (EPDM) | Generally compatible, but changing curing method to sulfur cure from peroxide cure may result in formation of small amounts of polyethylene oxide inside the matrix of the material |
| Latex | Compatible, but may be limited to the number of repeat cycles |
| Neoprene® | Compatible |
| Polyvinylidene fluoride elastomer | Compatible |
| Silicone elastomer | Compatible; no cross-linking High absorbency or EO desorbs well for short aeration with low EO concentration EO/CO2 mix had low EO absorbency, and very short aeration Nonelastomer prosthesis requires long aeration at high EO concentrations; but at very low EO concentrations with EO/CO2 gas mixtures, EO residuals may be much lower |
| Teflons® | Good to excellent materials. There may be low EO absorbency, but very slow desorption in some types (e.g., PTFE), but not in PVDF. Low EO concentration in EO/CO2 mix may result in very little EO absorbency |
Notes: EO residuals will vary between polymer types, polymer designs, thickness, formulation changes, packaging, etc. Typical aerations vary between 2 and 7 days.The above very short aeration was <12 h at ~50 °C with initial low EO concentration with EO/CO2 gas mixture.
EO will sterilize most polymers and materials for medical devices. However, because EO is deemed a potential human carcinogen and reproductive toxicant, its use is limited and controlled. It also has toxic byproducts such as ethylene glycol and ethylene chlorohydrin.
Post-sterilization evaluation for toxic residuals (ethylene oxide and ethylene chlorohydrin) must be performed before release or validation of product. Long exposure times and post-sterilization aeration times as well as post-processing biological indicator testing may reduce the use of this process on a practical basis.
Effects of heat (dry-heat and steam) sterilization on polymers
Heat sterilization, weather by dry heat or by steam, can cause thermal degradation of polymers and this may be due to the oxidation mechanism. Thermal degradation of polymers is typically a molecular deterioration as a result of overheating. At high temperatures the components of the long chain backbone of the polymer begin to separate (molecular scission); and react with one another to change the properties of the polymer. Thermal degradation can present an upper limit to the service temperature of plastics as much as the possibility of mechanical property loss. Indeed, unless correctly prevented, significant thermal degradation of polymers can occur at temperatures much lower than those at which mechanical failure is likely to occur. Consequently, many plastics or polymers selected for possible heat sterilization should be reviewed for their transition temperatures. The chemical reactions involved in thermal degradation may lead to physical and optical property changes relative to the initially specified properties.
It is of interest to find out what the effects of heat cycles will have on properties of polymeric materials. Thermal degradation generally involves changes to the molecular weight (MW); and molecular weight distribution of the polymer and typical property changes include reduced ductility and embrittlement, chalking, color changes, cracking, and general reduction in most other desirable physical properties. Radiation can cause cross-linking, scissoring, oxidative, or combination effects in polymer changes, which may result in cracking, disintegration, embrittlement, discoloration, stiffening, leaching, and pH change. These may result in change in MW or MW distributions. Thermal decomposition by exothermic heat or polymer breakdown of polymers by heat is predominately oxidative, causing breakage in chemical bonds, with property changes such as discoloration, hardening, softening, warpage, and/or melting. Excessive heat, however, may cause some polymers to burn, char, and totally disintegrate. Steam may result in wetting, hydrating, and even degrading. Many of the polymer property changes due to heat may similarly occur in over-irradiation. Dry heat is essentially an oxidative process, but is also dehydration. The effect of the steam is both a hydrolytic as well as an oxidative process, but is also dehydrating. The effects of steam are both hydrolytic and oxidative. Steam may also corrode metals. With steam sterilization there is not only potential thermal degradation and decomposition of a polymer as with dry heat but also the potential of hydrolysis.
Some polymers will lose structural integrity at the temperatures used for autoclaving. Some products made from such polymers may need to be supported to prevent slumping and distortion of the product. Even some polymers where the softening temperature is higher than the autoclaving temperature may suffer from the release of molded-in stresses and subsequent distortion. Where steam sterilization is to be used, the effect of multiple sterilization cycles may nee to be considered to prevent cumulative effects of the treatment on the plastic, when the product is not deemed as a single-use disposable product. If the products are to be packaged before autoclaving then the packaging material and packaging method need to be carefully chosen. The suitability of a package for steam sterilization will depend on the polymer, the size of the package, the wall thickness of the package, and the contents including any sharp corners which may pierce the package.
It is of interest to know what effects heat processes will have on the properties of polymeric materials. The number of polymers capable of tolerating moderate temperature and moisture are more numerous than often considered or recognized (Table 4.8):
Table 4.8
Polymer compatibility for dry heat and steam sterilization techniques
| Polymer | Comments (vary – consult authors or suppliers) |
| Acrylonitrile butadiene styrene (ABS) | Very unlikely, but some may be poor to possible, depending upon grade, filler Run low temperature process |
| Fluoropolymers Polytetrafluoroethylene (PTFE) |
Compatible up to 170 °C or higher Certain grades may allow for several cycles or long service; however, although PTFE has great thermal stability, once the activation energy for the rupture of the C-C bonds in the chain has been exceeded, it can unzip quantitatively releasing a potentially toxic monomer |
| Perfluoroalkoxy copolymer (PFA) | Working temperatures up to 204 °C or higher Long term up to 170 °C |
| Poly chlorotri-fluoroethylene (PCTFE) | Up to 150 °C continuous |
| Polyvinyl fluoride (PVF) Polyvinylidene fluoride (PVDF) |
Heat deflection temperature up to 134 °C; limited use Per use temperature is 150 °C (302 °F); however, some grades may only go to 125 °C Multiple, maximum operating temperature of 275 °F/130 °C |
| Ethylenechlorotri-fluorethylene | Compatible to 266 °F (131 °C); melt at 412 °F (211 °C) |
| Ethylene tetrafluoroethylene (ETFE) | Up to 150 °C |
| Fluorinated ethylene propylene (FEP) | Up to 170 °C or 200 °C (392 °F) |
| Polyoxymethylene (e.g., polyacetal) | Up to 121 °C or higher; may degas May use up to 100 cycles at 121 °C, but it may begin to degrade, emitting formaldehyde |
| Polyacrylic (e.g., PMMA) | Poor to fair; some highly resistant grades |
| Polyamide (e.g., Nylon) | Poor to excellent Absorbs moisture, some films will allow moisture to diffuse through |
| Polycarbonate | There are grades that can be sterilized at 134 °C Some formulations only allow a few cycles; other formulations allow up to 200 repeat cycles |
| Polyester | Possible to excellent; depends upon type, grade, form and function Some good PET films at 240 °F, PEN good Mylar resistant but will not allow steam penetration Aliphatic polyesters are sensitive to hydrolysis, while aromatic polyester (e.g., PET) may be less susceptible |
| Polyethylene (PE) – various densities; LDPE, LLDPE, HDPE, spun polyolefin® | Poor to fair HDPE fair and spun polyolefin fair High density better than low density Reinforcement of HDPE improves its temperature |
| Polyimides (PI) | Possible to excellent; depends upon grade, form and function PEI withstand up to 4000 cycles, 1000–2500 at 5 min at 134 °C |
| Polymethylpentene (PMP) | Excellent up to 235 °C; PMP withstands repeated autoclaving, up to 150 °C |
| Poly (ether) Ketone | High-temperature resistance; PEEK has heat resistance Good up to 2000 h of steam Typically long service |
| Polypropylene | Depends upon grade, form and formula Use heat-resistant grade with heat stabilizer for multiple cycles |
| Polypropylene copolymer (PPCO) | It is autoclavable; provides properties of polypropylene and polyethylene PPO replaces polyallomer |
| Polystyrene | Standard polystyrene not autoclavable; but syndiotactic polystyrene (S-SPS) is excellent, as is styrene |
| Polyphenyloxides (PPO) | Good, 215 °C; can be mixed with styrene |
| Polysulfones | Typically all types are excellent; however, polyether sulfone (PES) is less resistant Repeated autoclave cycle – PS up to 1500 cycles; but not PES |
| Polyurethane | Poor/possible, but some grades may be fair/good |
| Polyvinylacetates | Depends upon form, function, formulation and co-polymerization. Heat-stable PVA hot-melt adhesives used |
| Polyvinylchloride | Rigid PVC, not likely, unless PVC modified Plasticized (soft) PVC is good depending upon form, formulation and function |
| Styrene acrylonitrile copolymer (SAN) | Possible to fair; depends upon grade |
| Silicone | Has tremendous heat resistance, but is not a barrier to moisture vapor; dry heat may be better in some applications. If exposed to repeated steam, sterilization will eventually relax silicone and will become gummy Silicone is hydrophobic, it will resist moisture Diffusion, unless nano-channels exist |
| Thermoset polymers | |
| Epoxy reinforced plastics | Numerous types of reinforced epoxies Physical properties can vary. Heat distortion temperatures of up to 470 °F |
| Phenolics | Autoclaving can lead to phenolic degradation and extractable into fluids |
| Polyester, unsaturated | There are a variety of unsaturated polyesters (e.g., vinyl esters). C better cross-linked. Possible to good Isophthalic acid-based polyester High-temperature resistance |
| Polyimides (e.g., BI maleimides (BMI) and acetylene terminated polyimide (ACTP)) | BMIs and ACTP have use-service temperatures of 127–232 °C and 316 °C |
| Polyurethane (PU) | Typically possible; depends upon grade, form and function. There are heat-resistant cross-linked polyurethanes |
| Aliphatic | Radiation cross-linking increases its resistance |
| Aromatic | Aromatic thermoset PUR does not form 4,4’-methylenedianiline (MDA) in polyurethane |
| Adhesives | |
| Acrylic | Can tolerate autoclaving; depending upon grade and formulation, fair to good There is an acrylic adhesive film in a tape up to 280 °F |
| Epoxy | Depending upon grade and formulation, deflection temperature from 200 °F to 500 °F Some can lose retention of initial strength on only five cycles |
| Fluoroepox(y)ies | Epoxy adhesives; depending upon cure and formulation, good to excellent Epoxy adhesives cured with heat are more heat resistant than those cured at room temperatures |
| Silicone adhesives | Typically good; depends upon form, formulation and function, good to excellent Some may be good for only up to 6–8 cycles |
| Elastomers | |
| Butyl | Good, depending upon type and grade Resistant to water and up to 120 °C Multiple use – a halobutyl(halogenated poly (isobutylene)) |
| Ethylene propylene diene monomer (EPDM) Natural rubber-latex (synthetic cis 1–4 polyisoprene) |
Good up to 125 °C in water; up to 134–150 °C in air; continuous-use operation; temperature of 105 °C Possible to fair There are autoclavable grades Plastomers enhance thermal stability. Possible to fair Hardens with use Withstands repeated autoclaving at 250 °F for 20 min |
| Nitrile rubber (acrylonitrile butadiene) | Good resistance to moisture and water Tolerate temperatures of up to 120 °C at lower processing conditions, below 230 °F Better if hydrogenated nitrile rubber |
| Polyacrylic | Polyacrylate: it is a heat-resistant rubber; water resistance can be improved but with decrease in heat. Typically, resistance to water is poor. |
| Polychloroprene | Fair resistance to moisture, up to 230 °F; intermittent to 250 °F Fair to very good It is possible to resterilize at below 230 °F |
| Silicone elastomer | There are some representative polymers for steam sterilization technique |
| Styrene block copolymers, SBR | Depends upon grade, type, form and formulation Possible to fair It is possible to resterilize up to 100 °C |
| Thermal-based polyisocyanate, urethane (polyether/polyester) | There are some heat-resistant grades; depends upon type, form and formulation With silicone there is increased heat resistance Not likely to be multiple sterilizations |
| Thermoplastic elastomer (TPE) | Polyolefin that can be molded into autoclavable parts |
| Urethane elastomer aliphatic | Typically possible; some up to 135 °C. Steam autoclaving possible with selected grades |
| Urethane elastomer aromatic | Formation of 4,4’-methylenedianiline (MDA) with steam |
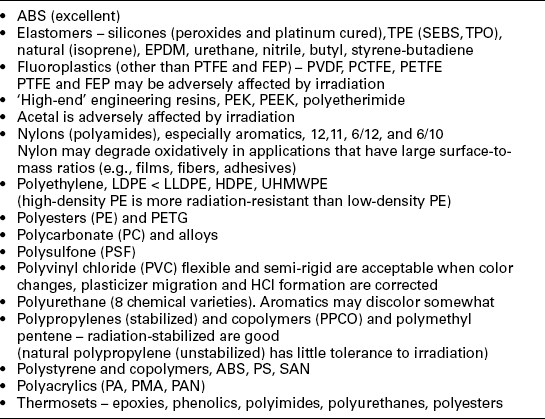
• Natural (isoprene) – EPDM, urethane, nitrile, butyl, styrene-butadiene.
• Fluor plastics (other than PTFE and FEP) – PVDF, PCTFE, PETFE.
• ‘High-end’ engineering – resins, PEK, PEEK, polyetherimide.
• Nylons(polyamides) – especially Aromatics, 12, 11, 6/12 and 6/10.
• Polyethylene HDPE, UHMWPE, but not low-density polyethylene.
• Polycarbonate (PC) and alloys.
• Polyesters (e.g., PET and PETG) and some polyesters are somewhat resistant to steam sterilization, but aliphatic forms are more vulnerable to hydrolysis than the aromatic form.
• Polysulfone (PSF) and polyphenyl sulfones may be virtually unaffected by thousands of autoclave cycles.
• Polyvinyl chloride (PVC); flexible and semi-rigid, color, plasticizer, and HCl creation. It may be corrected, where there is no load on them.
• Polystyrene (ABS, PS, etc.) typically melt with steam or dry-heat sterilization; however, syndiotactic polystyrene (S-PS) (where chemical groups are placed on one side of the polymer chain and then on the other side) is resistant and SAN can be heat resistant.
• Polyurethane (e.g., varieties) – some are very vulnerable to steam sterilization (e.g., moisture swelling); some vary with the hydrophobicity of the polyether segment of polyether urethanes.
• Polypropylenes (stabilized); and copolymers (PPCO); and polymethyl pentene – stabilized.
• Thermosets – epoxies, phenolic, polyimides, polyurethanes, and aromatic polyesters (aliphatic polyesters are more susceptible to hydrolysis attack).
• Silicones are heat stable, but more easily dry-heat sterilized, because moisture from steam cannot penetrate silicone prosthesis as dry heat can, for implantables.
A comparable number of polymers can be sterilized with dry heat without the adverse effects of moisture or hydrolysis (Table 4.9). Low-temperature dry heat may sterilize more polymers than steam, but the cycle or process times will be significantly longer (e.g., upwards to 12 times longer).
Table 4.9
Polymers and materials compatible with dry-heat sterilization technique (low and high temperatures)
Acetal (ACL), delrin, or polyoxymethylene up to 121 °C (dry)
Aluminum up to 190 °C (dry)
Cellulose acetate (non-load) up to 120 °C
Cellulose acetate butyrate (non-load) up to 130 °C
Cotton muslin up to 204 °C
Glass >190 °C
Grease (depends upon the type of grease) (dry)
Ethylene chlorotrifluoroethylene (ECTFE) up to 150 °C
Epoxies (vary up to 177 °C)
Ethylene propylene diene monomer (EPDM) up to 149 °C
ETFE up to 150 °C
Ethylene acrylic 149 °C
Fluorocarbon rubber 199 °C
Fluorinated ethylene propylene (FEP) up to 170 °C
Fluoro silicone 232 °C
High-density polyethylene (HDPE) up to 120 °C
Hydrogenated nitrile rubber 149 °C
Liquid crystal polymer (LCP) up to 275 °C
Metals (note some metal temper may occur above 160 °C) up to 190 °C (dry)
Muslin up to 160 °C
Natural rubber 104 °C, but low heat aging resistance
Neoprene/chloroprene rubber 121 °C
Nitrile rubber 100 °C, and low heat aging resistance
Nylon 4/6 (polyamide heat-stabilized grades) up to 130 °C
Nylon 6 <100 °C
Paper (varies depending upon paper) up to 160 °C (dry)
Perfluoroalkoxy (PFA) up to 170 °C
Petrolatum gauze up to 160 °C
Phenolics (vary) up to 150 °C
Polyacrylate (ACM) 149 °C
Polycarbonate (PC) up to 134 °C
Polyetherimide up to 134 °C
Polyetherketone (PEI, PEEK, etc.) up to 170 °C
Polyethylene (vary per molecular weight (e.g., 80–142 °C))
Polyethylene terephthalate copolymer (PETG) up to 170 °C
Polyimide 232 °C
Poly 4-methyl-pentene-1 (PMP) up to 170 °C
Polypropylene (PP) up to 135 °C, no stacking
Polyphenylene oxides (PPO) 100–148 °C
Polypropylene copolymer (PPCO) up to 120 °C
Polysulfone (PSF) up to 160 °C
Polytetrafluoroethylene (PTPE) up to 170 °C
Polyvinyl chloride tubing (flexible-non-load, varies) up to 120 °C
Polyvinylidene fluoride (PVF) up to 125 °C
Styrene-butadiene rubber 100 °C, but heat aging resistance
Silicones up to 200/232 °C
Teflons® up to 170 °C
Select a polymer whose temperature transition or melting temperature is comfortably ‘above’ the required, selected or chosen dry-heat sterilization operating temperature. Melting and/or deflection/maximum temperature can vary with formulation changes.
Note: Polymer responses may vary with the length of exposure to a temperature.
Effects of hydrogen-peroxide sterilization on polymers
Hydrogen peroxide and oxidizing agents can sterilize a multitude of polymers (Table 4.10). The numbers of polymers are more limited than with EO, because of the oxidizing effect of hydrogen peroxide; however, it is more attractive than EO sterilization because of its shorter process time and lack of residuals. Its very short processing time and no carcinogens make hydrogen peroxide very accessible. When designing for devices, it is best to avoid absorbers (such as silver, copper and copper alloys) and absorbers (such as polyurethane, nylon and cellulosic materials). Non-catalytic, non-absorbent materials such as PTFE, polyethylene, stainless steel, or low copper–aluminum alloys are recommended. Adhesives that use large proportions of amines as curing or cross-linking agents tend to be incompatible.
Table 4.10
Compatabilities of some polymers with hydrogen peroxide (with plasma*)

*Material compatibility with hydrogen peroxide vapor sterilization may not be the same as that with low-temperature hydrogen peroxide with plasma.
Low-temperature hydrogen peroxide with plasma has less effect typically on polymers than hydrogen-peroxide vapor without plasma, because plasma destroys or gets rid of peroxide residual than with aeration.
Plasma and oxidizing agents are generally applied only to small niche and minimal-sized loads of devices. Its use is predominantly in the general hospitals, and less in the medical-device manufacture industry. It is a surface sterilant and may not be used for implantables.
Effects of irradiation on polymers
Irradiation can cause deep changes in polymers that other methods will not, such as breaking bonds (scissoring), cross-linking or a combination of these ways, which may result in recombination. Radiation may cause odors, discolor, embrittle, and degrade a few materials, or affect bond strengths, which may cause changes to the life of a polymer during implantation.
Some polymers that may be particularly sensitive to radiation are unstabilized polypropylene, acetals, some Teflons (e.g., PTFE, PFA and FEP), polyglycolic acid and polylactide sutures, polymethylpentene, polyvinylidene fluoride, polymethyl methacrylate (PMMA), some acrylic adhesives, butyl rubber, some cellulose esters, natural liquid crystal polymer and cross-linking of silicone.
The effects of radiation on polymers may be influenced by:
• the chemical composition and formulation of the polymer;
• the morphology of the polymer (percentage of crystallinity, molecular weight, and density);
Understanding basic radiation chemistry may help to assess why a particular plastic is affected in a certain way. When a plastic is exposed to gamma radiation, in the case of Co60 with energy levels of 1.33 and 1.17 MeV, molecular bonds are broken. The polymer can either recombine into its original configuration or, if cross-scission occurs, the molecular weight of the molecules is reduced and the polymer is weakened. Conversely, where crosslinking occurs, a large three-dimensional matrix is formed and the polymer is strengthened.
The effects of radiation on polymers may also be influenced by the polymerization, molding, and process of plastics as well as the age and environment of the polymer.
• Some teflons (e.g., FEB and PTFE), despite their high heat resistance, are degraded by radiation, and generally not acceptable, although some thin films/coating and certain types of teflons have been demonstrated to be radiation compatible to low doses.
• ABS and polycarbonate are generally considered to be acceptable to one dose of radiation, but may be multiple sterilized up to 100 Mrad. Both may discolor; with ABS discoloring the most. ABS/Polycarbonate blend lose physical properties linearly with an increase in radiation dose.
• Acrylic polymers may be sensitive to radiation. The effect of scissoring of the ester chain is the main effect of radiation. Polymethylacrylate (PMMA) has been used for dosimeters, because it changes and is sensitive to irradiation. Radiation compatible acrylics, however, are available, but not typically for implantables or ophthalmic. Optical clarity of PMMA may be affected.
• Polyethylene is predominantly cross-linked, but may be acceptable to radiation, by sterilizing in nitrogen rather than in air (with oxygen). Slight odors may result, but they can be reduced through modification of the formulation. High-density polyethylene is more resistant than lower-density polyethylene.
• Polypropylene (unstabilized, natural) is both crosslinked and scissored. Embrittlement, breakage and discoloration can occur at sterilizing doses.
• Breakage of polypropylene syringe tips has been used for blood-borne-disease procedures to get rid of needles on tip.
• Radiation-stabilized polypropylene polymers, however, are available, using high molecular weights, co-polymerized and alloyed with polyethylene with additional stabilizers. Use of electron beam at high irradiation dose rate may further reduce the oxidative degradation of polypropylene.
• Polymethylpentene has similar effects as polypropylene, but using less irradiation improves possibilities.
• Polystyrene is very stable to radiation because of its benzene ring; however, it may begin to yellow above 50 kGy.
• ABS is much less resistant to radiation than polystyrene, but it may be suitable for a single dose of irradiation. High-impact grades are less radiation-resistant than standard grades.
• PVC can be compatible or tolerant to radiation, but may require squelching of HCl, prevention of discoloration and leaching of plasticizer(s). The addition of antioxidants and heat stabilizer help, as well as changing the plasticizer (DEHP or DOP) to one less toxic and non-carcinogenic.
• Resterilization with irradiation is not frequently used, as with other techniques. Single-use (irradiation) typically predominates. However, plasticized PVC may be resterilized. However, consideration of leaching of plasticizer should be made.
• Acetal or polyformaldehyde copolymers are sensitive to radiation and their chains are easily scissored (embrittlement); the material often changes from solid to dust, and the color changes from yellow to green.
• Polyamide or nylon are sensitive to radiation to cross-linking, but many are suitable for a single dose, and some for multiple doses. Nylon 10, 11, 12 and 6–6 are more stable than nylon 6. Nylon film and fiber are less resistant.
• Despite difficulties with irradiation, the number of polymers that are sterilizable are numerous (Table 4.11).
Effects of ozone sterilization on polymers
It should be noted that during ozone sterilization, ozone breaks down into reactive species, including hydroxyl radicals and atomic oxygen. Because of the strong oxidizing nature of ozone, polymers must be resistant to oxidation. Further, polymers and medical devices should be resistant to high relative humidity levels (>80%), which are required for the ozone to be effective as a sterilant. Consequently, materials should be resistant or tolerable to oxidation and moisture. This method of sterilization cannot be used for fluids or woven textiles. Ozone sterilization is a surface-oxidative process. Although many polymers may be satisfactorily used in the manufacture of a device intended for single use, they might not be effective for use with a reusable or refurbished device. For gaseous ozone, compatible polymers for its low-temperature sterilization should be resistant to oxidation and moisture, for one or more multiple processes (see Table 4.12).
Table 4.12
Compatibility of some polymers with the ozone sterilization technique
| Thermoplastics | Compatibility | Number of cycles that polymer may be compatible |
| Fluoropolymers | ||
| Polytetrafluoroethylene (PTFE) | Excellent | No change after > 100 cycles |
| Perfluoro alkoxy (PFA) | Excellent | No change after > 100 cycles |
| Polychlorotri-fluoroethylene (PCTFE) | Excellent | No change after > 100 cycles |
| Polyvinylidene fluoride (PVDF) | Excellent | No change after > 100 cycles. PVDF is considered a polymer of choice for ozone |
| Ethylene tetrafluoroethylene (ETFE) | Excellent | No change after > 100 cycles |
| Fluorinated ethylene propylene (FEP) | Excellent | No change after > 100 cycles |
| Polyacetals | Good | Color change and loss of gloss. Slight to significant change may occur after > 100 cycles Contact equipment manufacturer |
| Polyacrylates (e.g., PMMA) | Good | Slight to significant material change may occur after 10–100 cycles Contact equipment manufacturer |
| Polyamides (e.g., Nylon) | Good | Color change and loss of gloss. Significant material change after 10–100 cycles |
| Polycarbonate (PC) | Excellent | Slight surface change and loss of gloss. No significant change after > 100 cycles |
| Polyesters, saturated | Excellent | |
| Polyethylene (PE), various densities | Good | Color change and loss of gloss. Significant material change may occur after 10–100 cycles |
| Polyimides (e.g., PEI) | Excellent | Slight surface change. No significant change after > 100 cycles |
| Polyketones (e.g., PEEK) | Excellent | Unfilled PEEK only – avoid sharp edges. Color change and loss of gloss. No significant change after > 100 cycles |
| Polypropylene (PP) natural stabilized | Good | Color change and loss of gloss. Significant material change may occur after 10–100 cycles Polypropylene may not be good for multiple reuse |
| Polystyrene | Poor | Significant material or surface change < 3 cycles |
| Polysulfones | Good | Slight surface change and loss of gloss. No significant change after > 100 cycles |
| Polyurethane (PU) | Not likely | Significant material or surface change < 3 cycles |
| Polyvinylchloride (PVC) rigid | Excellent | Color change and loss of gloss. No significant change after > 100 cycles |
| Polyvinylchloride plasticised | Good | Surface change may occur after 5–25 cycles |
| Thermosets | ||
| Epoxies | Variable | Significant material change may occur after 10–100 cycles, check reliability and stability |
| Phenolics | Excellent | Loss of gloss. No significant change after > 100 cycles |
| Polyester, unsaturated | Excellent | |
| Polyurethanes | Not likely | Significant material or surface change < 3 cycles; not good |
| Adhesives | ||
| Acrylic | Good | Application specific. Contact equipment manufacturer |
| Epoxy | Variable | Application specific. Contact equipment manufacturer |
| Fluoroepoxy | Good | Application specific. Contact equipment manufacturer |
| Silicone | Good | Application specific. Contact equipment manufacturer |
| Elastomers | ||
| Natural rubber | Not likely | Significant material or surface change < 3 cycles |
| Butyl rubber | Not likely | |
| Ethylene propylene dienemonomer (EPDM) | Fair | Significant material or surface change with < 3 cycles |
| Silicone | Excellent | Slight material change after > 100 cycles |
| Styrenic block copolymers | Not likely | Significant material or surface change < 3 cycles |
| Polychloroprene | Poor | While in an ozone normal environment, it is OK, but under sterilization significant material or surface change may occur with < 3 cycles |
| Urethane | Not likely | Significant material or surface change < 3 cycles |
Some polymers remain unknown in terms of compatibility to ozone at this time. Woven materials polystyrene, polyurethane, butyl and natural rubber, polychloroprene, nickel, and silver are not likely to be compatible. Some cellulosic, however, may be compatible. The shape of the device and material as well as the design of a device may be closely related to its stability and resistance of the device to sterilization. Device and polymeric parts with wide surface-to-mass ratios (e.g., fibrous material) can undergo faster oxidative degradation. While such device and materials can be adequate for single use or used in the manufacture of a device that has limited reuse, such a condition might not be satisfactorily used for a device with a longer expiration period.
Ozone and oxidizing agents are generally applied only to small niche and minimal-sized loads of devices polymer. Their use is predominantly in the hospitals, and may be limited in industry. While ozone and hydrogen peroxide are both oxidizing agents, their damage is different.
Ozone may sterilize some cellulosic better than hydrogen peroxide, but hydrogen peroxide may sterilize butyl rubber, urethanes, and natural rubber better than ozone. Silicone may be sterilized better by ozone than hydrogen peroxide. Hydrogen peroxide is typically a surface sterilant with some diffusion of small lumens, while ozone should have the capacity to diffuse and penetrate deeper than peroxide, but not EO, dry heat, steam, or irradiation.
4.3.2 Effects of sterilization modalities on various plastics
In this section, different plastics (e.g., olfines, styrenes, vinyls, fluorinated polymers), and other plastics and elastomers are compared to different sterilization modalities.
This comparison device provides medical applications with each sterilization modality.5, 6 The following highlighted list incorporates a rating of effects (e.g., poor to excellent) of sterilizing different plastics and elastomers and their applications to different sterilization techniques.3–6
Olefin polymers
Polyethylene: radiation (good to excellent) – may ‘off gas’, low and moderately dense PE are more resistant and may be resterilized, but high-density PE can undergo oxidation (which has resulted in cracking under some circumstances); EO (excellent); moist heat (poor to good, high density more resistant); dry heat (poor to fair, but lower temperature improves for high density); hydrogen peroxide (excellent); ozone (excellent). Device applications: orthopedics, replacements in joints, ankles, elbow, shoulder and toe, tubing.
Polypropylene: radiation (poor to good, stabilized, but single-use only); EO (good to excellent); moist heat (good, and excellent with heat-stabilized grades and can be resterilized); dry heat (good and excellent at low temperatures (up to 135 °C), with heat-stabilized grades); hydrogen peroxide (excellent); ozone (excellent). Device applications: catheters, sutures, syringes, polypropylene surgical sutures, filaments, and mesh. Surgical meshes of this kind are used to reinforce soft tissue where weakness exists, such as in the repair of hernias and chest wall defects.
Poly methyl pentene: radiation (fair to good); EO (excellent); moist heat (good/excellent); dry heat (good/excellent up to 170 °C); hydrogen peroxide (unknown); ozone (unknown). Device applications: containers, covers for medical instruments, TPX film.
Copolymers (e.g., polyethylene/poly propylene, polyallomer): radiation (poor to good, stabilized, but single-use only); EO (excellent); moist heat (good, and excellent with heat stabilised grades and can be resterilized); dry heat (good and excellent at low temperatures (up to 135 °C); with heat-stabilized grades); hydrogen peroxide (excellent); ozone (excellent). Device applications: parenteral solution containers, containers for packaging applications; instrument, pneumatic and lubricant lines, tubes.
Styrene polymers
Polystyrene: radiation (excellent with benzene ring); EO (poor to good, but millions of parts and have been acceptably sterilized and some formulations can be resterilized 2–5 times); moist heat (poor to excellent, with syndiotatic styrene); dry heat (poor to excellent with syndiotatic styrene); hydrogen peroxide (excellent); ozone (fair). Device applications: containers, parts in IV sets, petri dishes, sputum cups.
Styrene acrylonitrile copolymers: radiation (good to excellent); EO (poor to good, but many parts are acceptable); moist heat (poor to fair); dry heat (poor to fair); hydrogen peroxide (excellent); ozone (may be unknown). Device applications: in dialysis devices, in IV connectors.
Acrylic polymers
Polymethyl methacrylate: radiation (fair to good); EO (good); moist heat (poor to fair at low temperatures but likely not resterilized); dry heat (poor to fair at low temperatures); hydrogen peroxide (fair); ozone (good). Device applications: bone cement, contact lenses, corneal prosthesis, grout for artificial joints, orthopedics, ophthalmology lenses, in membrane oxygenators.
Vinyl polymers
Polyvinyl acetate: radiation (good); EO (poor); moist heat (poor to fair); dry heat (poor to fair); hydrogen peroxide (excellent); ozone (unknown). Device application: film.
Polyvinyl chloride: radiation (good); EO (excellent); moist heat (poor to good up to 120 °C – good if no load on polymer; moist heat hydrates the PVC, but dry heat removes with heated aeration); dry heat (poor to good up to 120 °C*); hydrogen peroxide (excellent); ozone (good). Device applications: blood bags, catheters, containers, endotracheal tubes, films, hearing aid component, IV tubing, IV drip chambers, and packaging, shrink tubing, storage bags, in ventilation systems.
Vinyl chloride copolymers: radiation (good); EO (excellent); moist heat (poor to good (without load) up to 120 °C); dry heat (poor to good up to 120 °C); hydrogen peroxide and ozone unknown. Device applications: films, packaging, containers.
Polyvinylidene chloride: radiation (good); EO (excellent); moist heat (poor to fair up to 120 °C); dry heat (poor to fair up to 120 °C); hydrogen peroxide and ozone unknown. Device applications: PVDF syringe filters and PVDF membrane disc filters. Furthermore, PVDC is a thermoplastic that responds well to gas plasma treatment. Its inherently low surface energy and poor polarizability means the surface cannot provide enough energy to bond with adherents such as adhesives and inks, without gas plasma treatment.
Fluorinated polymers
Polytetrafluoroethylene (PTFE), PFA, PCTFE, PV, PVDF, ETFE, FEP: radiation has mixed results, some with poor compatibility (e.g., PFE, FEP, and PTFE); EO (excellent); moist heat (fair to excellent); dry heat (fair to excellent up to 170 °C); hydrogen peroxide (excellent); ozone (excellent). Note: PFA has a low-temperature transition fluoropolymer without additives and may not be steam or dry heat sterilized. Device applications: artificial bone joints and vasculature, fiber optics, surface treatments, stop cocks, tubing.
Miscellaneous polymers
Polyamides (nylons): radiation (poor/fair to good, varies depending upon if aromatic or aliphatic); EO (excellent); moist heat (poor to excellent); dry heat (poor to excellent); hydrogen peroxide (good, but only one use); ozone (good). Note: Nylon is polar, and will absorb moisture. Device applications: bags, catheters, films, kidney dialysis, in laparoscopy devices, special packaging, and nylon spikes.
Polyester: radiation (fair to good); EO (excellent); moist heat (poor to excellent); dry heat (poor to fair); hydrogen peroxide (excellent); ozone (excellent cover). Device applications: covers, films, IV infusion fluid containers.
Polyketones, polyaryletherketone, polyetheretherketone (PEEK): radiation (excellent); dry heat (excellent); EO (excellent); moist heat (excellent); hydrogen peroxide (excellent); ozone (excellent). Device applications: cardiovascular, orthopedic, dental implants, and tubing.
Polysulfone (PSF), polyphenylsulfone: radiation (excellent); EO (excellent); moist heat (excellent); dry heat (good to excellent); hydrogen peroxide (excellent); ozone (good). Device applications: handles for dental instruments, ophthalmic scopes and lenses, endoscopic device, dialyzers, can be autoclaved thousands of times.
Poly terephthalate copolymer (PETG): radiation (good to excellent); EO (excellent); steam and dry heat (good to excellent up to 134 °C); hydrogen peroxide and ozone (unknown). Device applications: packaging.
Poly terephthalate (PET): radiation (good to excellent); EO (excellent); steam and dry heat (good to excellent); hydrogen peroxide and ozone (unknown). Device applications: angioplasty balloons, woven vascular pros-theses, vascular grafts of both large diameters.
Cellulosic, cellulose esters, cellulose acetate propionate, cellulose acetate butyrate, cellulose (paper, cardboard): radiation (fair to good (note: esters degrade less than other cellulosics); EO (excellent); moist heat (poor to good at low temperatures and depending upon the cycle, some may be good); dry heat (poor to good but higher temperatures will char); hydrogen peroxide (poor); ozone (poor to good). Device applications: films, filters, hemodialyser, membrane, IV burette champers, packaging.
Thermosets
Epoxy: radiation (excellent); EO (good to excellent); moist heat (fair to excellent); dry heat (fair to excellent); hydrogen peroxide (excellent); ozone (fair to excellent). Device applications: case by case, adhesives for parts, fiber optics, etc.
Phenolics: radiation (excellent); EO (good); moist heat (fair to excellent); dry heat (fair to excellent); hydrogen peroxide (good); ozone (excellent). Device applications: case by case, implantable vascular medical device, semiconductor device fabrication, casters.
Polyimide: radiation (excellent); EO (excellent); moist heat (excellent); dry heat (good to excellent); hydrogen peroxide (excellent); ozone (unknown). Device applications: case by case, tubing such as cardiovascular catheters, urological retrieval devices, coated wires.
Polyurethanes: radiation (good to excellent-aromatic better than aliphatic); EO (poor to good); moist heat (poor to fair, may create toxic residue); dry heat (poor to fair/good, at low temperature, likely has no toxic residue); hydrogen peroxide (good); ozone (poor). Device applications: blood pumps, catheters, connectors containers, enteral feeding tubes, lipid resistant stopcocks, needless syringes, vials, balloons, pacemaker leads.
Acetal: radiation (poor, embrittlement); EO (excellent); moist heat (fair to good up to 120 °C); dry heat (good to excellent up to 120 °C); hydrogen peroxide (excellent); ozone (good). Device applications: engineering plastic, structural keel for a prosthetic device, stop cock.
Polycarbonate: radiation (good to excellent); EO (excellent); moist heat (fair to good); dry heat (fair to good/excellent up to 134 °C); hydrogen peroxide (excellent); ozone (excellent). Device applications: blood set, cases, covers, cardiotomy trocars, injection sites, in drug delivery devices, IV connectors, reservoirs, surgical instruments, safety syringes, valve occludes.
Acrylonitrile butadiene styrene (ABS): radiation (good); EO (excellent); moist heat (poor to fair); dry heat (poor to fair); hydrogen peroxide (excellent); ozone (fair). Device applications in administration IV sets (e.g. luers, roller clamps, Y connectors, etc.) and in dialysis units.
Elastomers (rubber)
Butyl: radiation (poor); EO (excellent); moist heat(fair to excellent); dry heat (poor to good); hydrogen peroxide (good, but only one cycle); ozone (poor). Device applications: tubing, butyl has been the most common choice for closures, but not implantable.
Ethylene propylene diene monomer (EPDM): radiation (good to excellent); EO (excellent); moist heat (good to excellent); dry heat (fair to good); hydrogen peroxide (fair to good); ozone (fair). Device applications: tubing, other uses, but not implantable.
Nitrile: radiation (good to excellent); EO (excellent); moist heat (fair to good); dry heat (poor to fair); hydrogen peroxide (fair); ozone (unknown). Device applications: gloves, including surgical gloves.
Polyacrylic: radiation (fair to good) (e.g., gamma radiation modifies the molecular weight of the PMMA while compression and bending strength may not affected by the sterilization process applied); EO (fair, but only one cycle); moist heat (poor, melts acrylic); dry heat (poor, melts acrylic); hydrogen peroxide (fair); ozone (good). Device applications: dental polymer, contact lens-methylacrylate.
Polychlorophrene: radiation (good); EO (good); moist heat (fair to good); dry heat (poor to fair); hydrogen peroxide (excellent); ozone (poor). Device applications: tubing.
Silicone: radiation (fair to good, can cross-link); EO (excellent); moist heat (fair to excellent); dry heat (fair to excellent up to 200 °C, many prosthesis processed by dry heat); hydrogen peroxide (excellent, but surface sterilant); ozone (excellent but surface sterilant). Device applications: catheters, membranes, prostheses (prosthetics), tubing.
What may physically/chemically appear to be a compatible polymer may not be biocompatible. A polymer listing (as above) for a specific polymer is not an indication that the polymer is compatible biologically. Polymer degradation biologically and failure may occur individually with some polymers. It is the responsibility of the ‘user’ to determine the suitability and biocompatibility of a polymer for its specific application.7
Note: the presence of additives, plasticizers, and stabilizers can significantly affect the stability properties of many polymers, including their suitability for a specific sterilization. Additionally, under some conditions a material that is generally thought to be compatible with a technique will not be compatible when evaluated under another condition (e.g., irradiation of high-density polyethylene under air will be different when processed under nitrogen). This incompatibility is often due to oxidation, stability, formulation, and/or processing changes in the polymer.
4.3.3 Metals: the effects of different sterilization techniques on metals
The number of metals capable of tolerating moderate irradiation, oxidizing, temperature and moisture are more numerous than often considered. Most metals are compatible with different sterilization modalities, except with repetitive sterilizations and a few variations with different sterilization modalities. It should be noted that some oxidative processes occur in some sterilization processes.
Dry heat
Metals can be subjected to dry-heat sterilization typically without adverse effects. While metals can be subjected to dry heat sterilization some material fibers can be damaged
Ethylene oxide
Most metals are compatible with multiple sterilization cycles, except for acetylides in copper that may cause it to polymerize. EO may react with water vapor to form glycols on the surface of some metals.
Hydrogen peroxide
Metals may not retain measurable residual peroxide, but aluminum is attacked. With hydrogen peroxide, it is best to avoid decomposers (such as silver, copper, and copper alloys).
Irradiation
Metals are typically very stable under the influence of irradiation, but they may attenuate full penetration of a low dose. Metallic bonds result in minimal ionizations and thus no significant change in properties. They do not ionize as a result of the low-energy radiation used in medical product sterilization; however, electrical (e.g., circuit) systems may undergo significant changes in resistance owing to changes in the electrical potential of the circuit. The electrons in the valance band might shift to the conductive band and lower the resistance of the circuit, a condition that is not reversible. However, there have been circumstances where the circuit has not been adversely affected enough to observe a problem. This may be due to shielding from irradiation, or lower irradiation dose. Also radiation hardening, an act of making electronic components and systems resistant to damage or malfunctions caused by ionizing radiation, can be implemented or used.
With high doses such as E-beam and metals’ low specific heats, localized elevated temperature rises might result. Also, in systems that use high-energy E-beam accelerators, neutron displacements and creation of measurable radioactive subspecies have been reported in some metals. Because of the very high rate of energy deposition in electron beam, care should be taken when product designs incorporate metals, as very large temperature gradients can develop. It is generally the case that metals are not negatively affected by radiation.
In general with gamma irradiation, the following metals response well with irradiation: aluminum, gold, brass, copper, magnesium, nickel, silver, stainless steel, and titanium, except in circuits. However, it should be noted that heavy metals may attenuate E-beam or electrons enough to prevent a 10−6 sterility assurance of some microbes.
Steam
Steam may sterilize many metals, but upon repeated reprocessing with, more than one, sterilization, oxidation and corrosion can occurs. Some metals (e.g., iron) may be subjected to steam corrosion but not all (e.g., stainless steel). If prudently applied (e.g., with inhibitors) and controlled (dry vs. wet steam), most metals may be sterilized. Moist heat may not corrode metals, except aluminum, copper, and iron, for example, but aluminum foil, when typically sterilized with single-use inhibitors, is acceptable. Corrosion may be limited if aluminum anodized. With brass it is used in steam traps. Copper has no reaction when heated in steam, but surface blackens when heated strongly in air. Copper and brass are acceptable with corrosion inhibitors, including triazole. Gold has no reaction when heated in air and no reaction when heated in steam. Magnesium metal is autoclavable, as is titanium, but not magnesium powder per se. Nickel is used in autoclaves. Silver has virtually no reaction when heated in air and no reaction when heated in steam. Autoclaving does not remove activity. Stainless-steel response to steam will vary with the grade of the steel and content of other inhibitors. With chrome there may be stainless-steel pitting and dulling of cutting edges after several sterilization cycles. Iron will corrode with steam. Titanium resists corrosion. Nickel-titanium alloy improves against corrosion. Titanium molybdenum is acceptable. Many metals may be steam sterilized if generated with pure water without dissolved gases (e.g., oxygen, carbon dioxide, and sulfur dioxide).
4.3.4 Ceramics and glass: the effects of sterilizing on ceramics and glass
Ceramics are usually a compound of metallic and nonmetallic elements. They are poor conductors of heat, but are excellent at retaining heat once heated. They are very resistant to corrosion and are nontoxic. Due to their ability to retain heat, ceramic materials will keep warm longer than metallic counterparts of comparable shape and size.
A common problem with most ceramics is their tendency to crack due to thermal stress. Since they are such poor conductors of heat, there may be a large temperature difference between one side of the ceramic and the other. In such a case, the warmer side expands more than the other, causing the ceramic to crack. Ceramics are seldom used for quick heating for this reason; dry-heat ovens allow even heating from all sides, preventing large thermal gradients, which steam heat may not.
Ceramics in healthcare products may be sterilized by the following techniques:
Some ceramic examples are aluminum oxides, silica, and zirconium oxide.3
Aluminum oxides are sterilized excellently with irradiation, EO, hydrogen peroxide, and ozone. Steam sterilizes aluminum oxides from good to excellent. Dry heat is good on aluminum oxide, but better at lower temperatures. Dry heat was selected for spacecraft sterilization because it could sterilize most ceramics between 105 and 135 °C. Dry heat was run in large ovens.
The silica ceramic is sterilized easily by all techniques. It withstands thermal gradients better than ceramics such aluminum oxides and zirconium oxides
Zirconium oxides are easily sterilized by irradiation, EO, hydrogen peroxide, and ozone. Steam and dry heat sterilize zirconium oxides from fair to good; however, again spacecraft sterilize ceramics with dry heat.
Steam, EO, dry heat, hydrogen peroxide, and ozone can sterilize most glasses.
Irradiation, however, will affect glass. It will discolor glass, but this effect can be used for manufacturing colored glasses.
Steam will affect some types of glass, but dry heat will not. Dry heat is the better modalitie for sterilizing glass. It can also be used to depyrogenate any pyrogen contamination on glass.
4.4 The effects of sterilizing on welded joints
The first consideration in sterilizing welded joints is to determine that the medical materials or polymers are compatible with the sterilization technique. One of the major difficulties in sterilization of welded joints is how to determine if sterility occurred within possible welds, particularly for implantables. To determine this result, resistant spores are inoculated in joints that will be welded. After sterilization, the welded joint can be assessed with immersion in sterility-test media to determine if the inoculated spores are recoverable or not. Resistant spores will vary depending upon the sterilization techniques employed. For example:
Hydrogen peroxide, ozone and steam sterilization: thermophile spores (Geobacillus sterothermophilus-ATCC 7953) are more resistant than ambient or mesophile spores. These may also be used as biological indicators (BI).
Dry heat and EO sterilization: Bacillus atrophaeus ATCC 9372 or NCTC 10073.
However, new bead and crevice-free welding helps to eliminate bacteria build-up. Weld areas can be easily inspected in translucent polypropylene and PVDF.
In most cases the effects of sterilization on welds will be no different than their effects on materials, except for possible results from ‘expansions’ resulting from the sterilization, such as heat expansion, pressure/vacuum expansions of EO, hydrogen peroxide with vapor or ozone sterilization.
With dry-heat and steam sterilization, differences in expansion rates, which could cause damage to welded mated parts, should be evaluated. Even with irradiation, differences in expansion rates, which could affect the bond strengths of welded mated parts, should be evaluated.7
Welding materials will not eliminate the effects of sterilization on the materials being welded.
4.4.1 Laser welding of joints
One of the better techniques for welding joints is laser welding, it does not have any toxic effects on the joints and is therefore biocompatible. However, if the material being welded was already toxic before welding, it will remain toxic and not biocompatible.
Laser welding minimizes any heat effect. For dry-heat and steam sterilization, lasers create high-strength and helium leak-tight welds in metals and polymers with pore-free surfaces and in this way make this joining technique the ideal solution, against migration of microbes or impurities. Excellent beam quality, high pulse-to-pulse stability and flexible pulse shaping are the preconditions for the finest seam and spot welds. Thus, laser-welded joints can be used for high-temperature sterilization and exhibit pore-free surfaces even without finishing. This is a crucial requirement for sterilizable biocompatible components, particularly implant systems.
Gamma irradiation may not have little effect on the laser weld of a joint whether in the manufacture of sterilizable implant systems or in the manufacture of surgical instruments, endoscopes or other devices. The particular properties of laser welding joints and seams are further recognized here for their special ideal qualities:
• welding narrow seams with high strength;
• welding gas tight bonding of seams;
• welding that is corrosion resistant;
• welding porous free with smooth surface, suitable for high-temperature sterilization;
• biocompatibility in accordance with the basic material;
• material suitability in accordance with the basic material with effects from sterilization technique.
The welding can be carried out with or without filler wires, according to the properties of the basic material. Neither the biocompatibility, the strength, toxicity, nor the porous-free nature of the materials are typically affected by sterilization of laser welds.
4.4.2 Heat welding (sealing)
Heat is another type of welding that is frequently used on polymers (e.g., PE, polyolefins) instead of solvent bonding. For example, metallocene polyolefins are suitable for heat sealing but not for solvent bonding or RF sealing, which are required steps in assembling some products. However metallocene PE not may be autoclaved because of its low melting point (Tm).
Rigid PVC parts that have been molded are suitable for ultrasonic bonding, while flexible PVC extruded or calendered films can be EO sterilized when they have been sealed using heat.
The method of joining together the ends of melt processible fluoropolymers such as Teflon are characterized by a high degree of chemical inertness and a continuous service temperature in the range of 300 °F to 500 °F. The joining of the ends together may be accomplished by the use of infrared heat emanating from an infrared heat source with temperatures in the range of 1250–2000 °F. and spaced from the ends for a period of time approximately up to one minute which may be gauged by the appearance of the material end as it becomes visibly molten to a depth. The infrared heaters are then removed and the ends may be put together under almost no pressure and held briefly, in one form, for less than a minute, to quickly establish a joint that may become at least as strong and probably stronger than the original ends. Heat sterilization continues to be the idea technique for sterilization of the fluoropolymer family, except PFA which has heat deflection temperature of 164 °F. The infrared fusion or thermal radiation heating is typically used as a substitute for hot-plate welding (contact heating) when the plate temperature has to rise above 260 °C. Above this temperature, coatings to prevent adhesion of molten plastic to the hot plate can no longer be used. Therefore using infrared radiation heating instead of a hot plate at a temperature of 400 to 500 °C or greater for fluorocarbons is preferred. Irradiation sterilization may be troublesome with some Teflon welding. The application of heat resistant fluoropolymers will likely continue to increase, providing more cost-effective solutions to the ever growing demands and diversity of joint-welding techniques and modern medical technology.
Infrared (IR) fusion is excellent toward welding PVDF and PP materials. PVDF may be a polymer of choice for steam and ozone. PP can be auto-claved up to about 121 °C, while PVDF can be steam sterilized up to at least 127 °C. PP, PVDF, and PVC may be H2O2 sterilized.
Thermal cycling can be particularly valuable in assessing designs that involve differentials in expansion coefficients, especially with welded joints.
4.4.3 Ultrasonic welding or bonding
Rigid PVC parts that have been molded are suitable for ultrasonic bonding, and may be EO, or irradiation sterilized.
Ultrasonic welding depends on materials softening not melting with increased temperature. Therefore, it is suitable only for thermoplastic polymers, not thermosets. For example, ABS, acrylic, polycarbonate, and PVC are amorphous polymers – with little or no crystalline structure, they are ideal for ultrasonic welding. Polyethylene, polypropylene, polyester and nylon are semi-crystalline – much more difficult to weld with ultrasonics. In general, best results are obtained when both components to be welded are made from identical material, but in some cases dissimilar materials can be welded using ultrasonics. For this to work the materials must be chemically compatible and have similar melting points (easier for amorphous materials – see the discussion of amorphous and crystalline materials above). One of the better combinations is acrylobutylstyrene (ABS) and acrylic (PMMA), though others are also possible to different degrees. Irradiation technique, however, may not work well with this joint because of the sensitivity of PMMA to irradiation. While polyethylene and polypropylene are both olefin the general consideration appears to be that these are chemically incompatible. Similarly, irradiation sterilization of these two materials would be disadvantageous.
4.4.4 Radiofrequency welding
Rigid flexible extruded or calendered PVC films can be sealed using radiofrequency (RF) sealing, and sterilizable with EO. Metallocene polyolefins (e.g., PE) are suitable for heat sealing but not for RF sealing. Metallocene (sealed) polyolefins may be heat sterilized.
RF welding can only occur between highly dipolar materials that are excited by an alternating magnetic field. The RF welding process uses radio frequency energy to produce molecular agitation in thermoplastic materials (e.g., thermoplastic polyurethane, PVC, PVDC, EVA, PET, nylon, and other customized resins) such that they melt and flow together, forming a bond that is as strong as the original materials. As long as the bond is as strong as the original materials the effects of sterilization of the original materials should continue to apply.
Variation, however, may occur with dissimilar materials, despite their both being highly polar. Dissimilar materials cannot be steam sterilized below their transitional temperature
4.4.5 Miscellaneous effects of sterilization on welded joints
With steam sterilization or other sterilization methods with pressure differentials (e.g., HO, EO and ozone), if the external pressure on a sealed soft bag or device space is greater than the internal pressure, the volume of the head-space will reduce due to compression of the air. In such a case, is difficult for a high-pressure differential in this direction to damage the bag because it does not stretch the material or compromise its welded joints. However, a low chamber pressure will cause the bag to inflate which, if it results in stretching beyond the elastic limit, may lead to nonrecoverable deformation or failure at a welded joint. Consequently, the ideal processing condition for this type of product is to always maintain the chamber pressure a little above the theoretical pressure inside the container, bag, or device space.
Thermal cycling is particularly valuable in assessing designs that involve differentials in expansion coefficients, especially with adhesive bonding
In the United States sterilizers are considered a medical device. Because sterilizers such as steam sterilizer have many welded parts and joints, there may be a number of standards required to evaluate these welded parts or joints by manufacturers. Some specific standards are provided as follows:
• BS 3970: 1990: Steam sterilizers.
• BS 2646: Autoclaves for sterilization in laboratories. Part 1: 1983; Part 2: 1990; Part 3: 1993; Part 4: 1991; Part 5: 1993.
Autoclave welds must be strong enough to withstand multiple changes in temperature and processing.
Radiation sterilization itself may be another possible means of welding materials. For example, incomplete cured silicone parts can be cross-linked together by irradiation.
Ultraviolet light can theoretically both sterilize and weld parts. UV is a limited sterilization technique because it cannot sterilize around corners nor penetrate many materials. Plastics are usually transparent in the visible spectrum, not UV. Ultraviolet light is both harmful to health and spatters. UV light range may occur from other welding techniques.
4.5 Selecting a suitable sterilization method
There are always tradeoffs when selecting a method of sterilization based on acceptable materials’ inherent properties to welding and processing.8
4.5.1 Determining the appropriate method of sterilization
Some qualities to consider when selecting a sterilization method are:
• Availability: steam autoclave is most available in a hospital; for in-house or contract sterilization EO is available; and irradiation is very available by contract. Hydrogen peroxide is becoming more widely used in hospitals followed by ozone.
• Cost: dry heat is the least expensive method, next steam, then hydrogen peroxide and ozone, and then irradiation and EO sterilization. This cost includes equipment, facilities, and consumables.
• Compatibility: EO is the most compatible to polymer materials.
• Disposability: deals with disposal of polymers after use, and sterilant wastes (e.g., EO and used gamma sources).
• Ease of control and monitoring: dry heat and irradiation are the easiest to control, followed by steam, then hydrogen peroxide, ozone, and EO.
• Environment: ability to recycle, reuse, and disposal of polymers. Dry heat, moist heat, and ozone are the most environmental friendly; followed by E-beam and hydrogen peroxide.
• In-house versus contract (external) sterilization: dry heat and steam at re most easiest to use in-house; while radiation is used predominantly under contract basis; EO is used more often either in-house or by contract. Irradiation is typically used in contract with a few exceptions, such as for small gamma and E-beam facilities in industrial applications, but not in hospitals.
• Lethality: steam has the greatest lethality, which includes ineffectivity of resistant prions; irradiation has excellent penetration and consequently lethality; dry heat is able to depyrogenate pyrogens.
• Packaging: irradiation can penetrate virtually every type of package.
• Regulatory: EO is the most regulated sterilization process and sterilizing chemical.
• Reusability: steam is the easiest sterilant to reuse; EO can be reused.
Besides the above qualities the following ‘steps’ should be taken into consideration when selecting the sterilization method to be applied or used:
1. Identify the method(s) of sterilization that appear compatible with product design and materials and joining approaches.
2. List options: Cost, in-house vs contract; regulatory issues, local codes and environmental regulations.
3. Perform feasibility studies to determine gross compatibility with selected processes.
4. Perform detailed pre-validation studies to demonstrate product, component, joint compatibility with the selected process and attainment of required sterility assurance level (SAL).
5. Based upon the above qualities and steps select the most suitable sterilization method to be applied or used.
4.6 Conclusions
Every sterilization technique must be what their own characteristics and qualities determine. Control of sterilization must of necessity begin with its environment and material compatibility. The qualities of different sterilization techniques vary. Heat sterilization may melt and corrode some materials, while irradiation may create radicals and cause embrittlement and cross-linking of materials. Hydrogen peroxide and ozone sterilization both oxidize some materials and polymers. Sterilization of polymers continues to be an important challenge of medical materials and polymers in products.
Welding materials will not eliminate the effects of sterilization on the materials being welded. Different welding techniques have their individual qualities.
There are always tradeoffs when selecting a method of sterilization based on acceptable materials’ inherent properties to welding and sterilization. Whatever the sterilizing technique, they must be able to (1) completely sterilize both surfaces and below surfaces or difficult to access locations; (2) the cost of heat sterilization is less costly, (3) there are more items being resterilizable by heat than by other means, (4) heat sterilization is more readily available and accessible in health care facilities than other techniques; (5) lower-temperature heat sterilization techniques will occur more easily in the future, allowing for more heat-sensitive polymers to be sterilizable (because the new sterilization techniques are more niche players, less penetrable, and are small sterilizers; (6) heat sterilization uses no toxic chemicals, or emits any toxic emissions or wastes, and is environmentally (green) safe; (7) polymers and packaging materials continue to be invented and improved to be heat stable, heat sterilizable, reusable, and less costly, paving the way to improved medical materials; (8) alternatives to EO sterilization continue to occur because of its toxicity and multiple regulations, inherent hazards and increasing costs; however, EO sterilization is likely the gentlest process on materials, polymers, and welded joints, and it is able to penetrate and can sterilize many joints and welds.
Experience with real-world polymers and materials is showing a need for careful and thorough evaluations when it comes to the effects of sterilization on medical materials, polymers, and welded joints. In general, the trends point toward a need for more careful understanding of the interaction between design, material interaction, sterilization, biocompatibility, environment, and final polymer product and welded joint performance.
4.7 Sources of further information
Anand, V.P., Cogdill, C.P., Klausner, K.A., Lister, L., Barbolt, T., Page, B.F.J., Urbanski, P., Woss, Casimir J., Boyce, J. Re-evaluation of EO hemolysis and irritation potential. Journal of Biomedical Materials Research. 2003; 64A:648–654.
Anes, J., Nase, R.S., White, C.H. Use of plastics for parenteral packaging. In: Avis K.E., Lieberman H.A., Lachman L., eds. Pharmaceutical dosage forms: Parenteral medication. 2nd. Munich: Marcel Dekker; 1992:387–444. [Vol. 1].
Berger, K., Doriff, D. Efficacy and sterility in flexibile packaging. Medical Device & Diagnostic Industry. 2001; 1–8. [August].
Canon Communications. Modern plastics worldwide world encyclopedia: The global plastics magazine, 2004. http://www.asiaing.com/modern-plastics-worldwide-free-subscription.html
Czuba, L. Medical plastics for PVC replacement in medical solution containers. Medical Device & Diagnostic Industry. 1999; 1–5. [April].
Czuba, L. Polymers: Paving the road of medical device progress. Medical Device & Diagnostic Industry. 2004; 1–4. [August].
Feldman, L.A., Hui, H.K. Compatibility of medical devices and materials with low-temperature hydrogen peroxide gas plasma. Medical Device & Diagnostic Industry. 1997; 19:57–62. [896].
Frissora, C. Trends in device design: Implications for materials selection. Medical Device & Diagnostic Industry. 2007; 80–90. [May].
Gil, F.J., Gnebra, M.P., Planell, J.A., Hermanson, N., Wessel, T. Syndiotactic polystyrene: A new polymer for high performance. Medical Applications. 1998; 1–4. [July].
Hemmerich, K.J., Polymer materials selection for radiation-sterilised products. Medical Device & Diagnostic Industry. 2002. [February].
www.devicelink.com/mddi/archive/00/02/006.html.
Lundy, Charles. Selecting polymers for medical devices. Medical Plastics and Biomaterials. 1994; 22–27. [Summer].
Marino, F., Floyd, B., Rogers, W. Industrial sterilisation: A review of current principles and practices. In: Avis K.E., Lieberman H.A., Lachman L., eds. Pharmaceutical dosage forms: Parenteral medication. New York: Marcel Dekker; 1992:1–54. [(technical assistance), Vol. 2].
Marczis, B. and Tibor, C., Polymer Joints, Department of Polymer Engineering and Textile Technology, Faculty of Mechanical Engineering, Budapest University of Technology and Economics.
Molin, G. Inactivation of bacillus spores in dry systems at low and high temperatures. Journal of General Microbiology. 1977; 101(2):227–231.
Modern Plastics (yearly) Modern plastics world encyclopedia, New York: Modern Plastics.
Perkins, J.J. Principles and methods ofsterilisation in health sciences. Springfield, IL: Charles C. Thomas, 1970; 54. [204, 261–262, 296–299, 301].
Plastics Design LibraryThe effect ofsterilisation methods on plastics and elastomers. Morris, NY: Plastics Design Laboratory, 1994.
Portnoy, R. Clear radiation, autoclavable polypropylene. Medical Plastics and Biomaterials. 1997; 1–9. [January].
Rhodes, A., Fletcher, D. Principles of sterilisation, sterility tests and asepsis. In: Principles of industrial microbiology. Oxford: Pergamon; 1966:45–57. [Chapter 5].
Rogers, W. Steam (moist heat) and dry heat sterilization. In: Lerouge Sophie, Simmons Anne, eds. Sterilisation of biomaterials and medical devices. Cambridge: Woodhead Publishing, 2012. [Chapter 2].
Rogers, W. Sterilisation techniques for polymers. In: Lerouge Sophie, Simmons Anne, eds. Sterilisation of biomaterials and medical devices. Cambridge: Woodhead Publishing, 2012. [Chapter 7].
Rubin, I.Handbook of plastic materials and technology. New York: John Wiley & Sons, 1990.
Steward, R. New polymers offer advantages for medical devices and packaging. Plastics Engineering. 2005; 20–27. [October].
Sturdevant, M. Sterilisation compatibility of rigid thermoplastic materials, 1998. [Dow Chemical Company report].
Szycher, M.High performance biomaterials. Lancaster, PA: Technomic Publishing, 1991.
Williams, D.F. Tissue-bio material interaction. Journal of Material Science. 1987; 22:3421–3445.
Woo, L., Sherwin, S. Selecting materials for medical products. In: Kutz Myer, ed. Handbook of materials selection. New York: John Wiley; 2000:1195–1222. [Chapter 38].
4.8 References
1. Rhodes, A., Fletcher, D. Principles of sterilisation, sterility tests and asepsis. In: Chapter 5 of Principles of industrial microbiology. Oxford: Pergamon; 1966:50.
2. Molin, G. Inactivation of Bacillus spores in dry systems at low and high temperatures. Journal of General Microbiology. 1977; 101:227–231.
3. AAMI, Technical Information Report 17Compatibility of materials subject to sterilisation. American Association of Medical Instrumentation, 2008. [Technical Information Report (TIR) 17].
4. Rogers, W. Steam: uses and challenges for device sterilisation. In: Medical Device & Diagnostic Industry. Los Angeles, CA, USA: Canon Communications; 2006:80–87.
5. Rogers, W. Sterilisation of polymer health care products. Shrewsbury, UK: RAPRA Technology, 2005; 26. [298–302].
6. Plastics Design Library. The effect of sterilization methods on plastics and elastomers. Morris, New York: Plastics Design Library, 1994; 1–338.
7. Tang, F.W., Lambert, B.J., Rogers, W.J. Polymers in medical applications. Shrewsbury, UK: RAPRA Technology, 2001; 1–15.
8. ANSI/AAMI/ISO 10993-7Biological evaluation of medical devices – part 7: ethylene oxide sterilisation residuals. Arlington, VA: Association for the Advancement of Medical Instrumentation, 2001.
9. ANSI/AAMIBiological evaluation ofmedical devices – part 1: evaluation and testing. Arlington, VA: Association for the Advancement of Medical Instrumentation, 2003.
10. Kowalkski, J.B. Selecting a sterilization method. In: Morrisey Robert F., Phillips C.Briggs, eds. Sterilisation technology: a practical guide for manufacturers and users of health care products. Kluwer Academic Publishers; 1993:70–79.
*Acceptable when there is no load on the polymer.