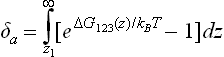Introduction to medical materials and devices
Abstract:
This chapter introduces medical materials, especially from the viewpoint of their surface attributes and interactions with living matter, considered at various length scales ranging from molecular through cellular to tissular. The basic biophysical chemistry of interaction is covered in sufficient detail to allow the reader to estimate the interactions when appraising novel materials. Consideration is given to how welding and other forms of joining might affect the interactions. The metrology of biocompatibility is also covered in order to acquaint the reader with practical approaches to assessing the interactions, which are, of course, of crucial importance in determining the functional success of any artificial material in contact with living matter.
1.1 Introduction
Medical materials are defined as materials used in medicine. Since medicine itself is defined as ‘the science and art concerned with the cure, alleviation, and prevention of disease, and with the restoration and preservation of health’ (Shorter Oxford English Dictionary), the compass of medical materials would appear to be rather wide: even the material used for tubing in a food-processing factory would fall into the definition, the material being specially designed to prevent colonization by pathogenic bacteria. The scientific issues underlying the application of materials in medicine are, however, clearly circumscribed and can be grouped under the heading of biocompatibility, which in turn is typically defined as ‘tolerant of life, or of biomolecular function’ (PAS 132, 2007). Regardless of the specific use to which the material is put, it must be biocompatible. The bactericidal or, more generally, biocidal surfaces that play a valuable role in keeping the walls, floor, furniture, etc. of a hospital free from infectious agents are not, of course, biocompatible – rather just the opposite – but the same principles that provide a framework for understanding biocompatibility apply to the biocidal surfaces. These principles may be collectively described as the science of the bio/nonbio interface. Thus, regardless of properties such as density, stiffness, etc. required for a particular application, it is essential to ensure that the surface of the material object is biocompatible. This implies that the special features of the science of medical materials are those associated with surfaces; properties such as density and stiffness are common to materials science as a whole. Hence, the specific focus of this chapter will be on the surface properties of materials relevant to medical applications that bring them into contact with biological matter.
Despite the acknowledgment above that materials used for production and packaging in the food and pharmaceutical industries and in water supply, etc. also have a bearing on health, in the remainder of this chapter medical materials will be defined more narrowly as the materials used for making medical devices. Nevertheless, it should be emphasized that the principles governing the bio/nonbio interface, which provide the theoretical framework for understanding biocompatibility, are also fully applicable to those other sectors.
1.1.1 An ontology for medical materials
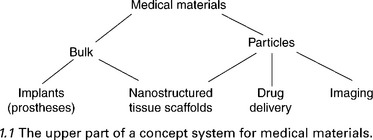
Figure 1.1 gives the first three levels of a concept system for medical materials. From the viewpoint of surface properties, the division between the bulk materials and particles is of little consequence; particles are essentially all surface (Ramsden and Freeman, 2009). The key thing to note about the concepts appearing at the lowest level are that the objects constituting their extensions interact with both biofluids (e.g. blood) and tissues.
1.1.2 An ostensive definition of medical materials
Table 1.1 lists the 24 most abundant materials used in implanted medical devices. Note the dominance of titanium. Its surface is invariably coated with a thin film of titanium dioxide, which governs its biocompatibility. Titanium is attractive because of its combination of excellent biocompatibility (of its oxide) and mechanical properties (relatively lightweight and strong). Polymers of various kinds are also prominent. On the other hand, composites have as yet found little favour in clinical practice. This compilation does not include the (still mostly experimental) materials used to fabricate drug delivery particles.
Table 1.1
Relative importance (ranked by numbers of occurrences N of the listed words) of the materials used in implanted devicesa
| Rank | Material | N |
| 1 | Titanium | 2492 |
| 2 | Steel | 607 |
| 3 | Chromium | 391 |
| 4 | Polyethylene (PE), ultra-high molecular weight polyethylene (UHMWPE) | 389 |
| 5 | Silicone | 375 |
| 6 | Cobalt | 235 |
| 7 | Polytetrafluoroethylene (PTFE), Teflon, fluoroplastic | 220 |
| 8 | Molybdenum | 216 |
| 9 | Vitallium | 198 |
| 10 | Polypropylene (PP) | 160 |
| 11 | Polymethylmethacrylate (PMMA), acrylic | 111 |
| 12 | Polyester | 107 |
| 13 | NiTiNOL | 101 |
| 14 | Polyurethane (PU) | 72 |
| 15 | Platinum | 58 |
| 16 | Premilene (a lightweight polypropylene mesh) | 55 |
| 17 | Aluminium, alumina | 52 |
| 18 | Carbon | 51 |
| 19 | Rubber | 49 |
| 20 | Hydroxyapatite (HAp) | 45 |
| 21 | Silicon | 42 |
| 22 | Polylactic acid (PLLA) | 39 |
| 23 | Polyethyletherketone (PEEK) | 32 |
| 24 | Tantalum | 18 |
aWords are from the Prostheses List of the Australian Health Insurance Association (AHIA), 2005.
1.1.3 Surface attributes of medical materials
As a general rule, it can be stated that materials in contact with the bloodstream should not interact with blood; that is, with its proteins and cells. Materials of devices such as hypodermic needles and surgical knives, intended for very transient contact with the living substance, form a subcategory of non-interacting materials. Conversely, materials intended to remain in contact with solid tissue (e.g. a bone implant) for an indefinite duration should become assimilated with it (Kutsevlyak et al., 2008).
1.1.4 The nature of interaction
We are concerned with two materials, that of the artificial device (the medical material) and the biological tissue. They will sense each other’s presence through various forces. For example, if the materials have mass, the gravitational force will act between them (this is usually weak enough to be neglected, but even a single living cell will sediment when suspended in a fluid). Electrostatic forces are far stronger. Matter is, normally, electrostatically neutral but especially in a medium such as water, which has a high dielectric constant, atoms may separate from each other, becoming ions, as in
and the ions may adsorb onto a neutral surface, electrifying it; water itself is also weakly ionized:
but at neutral (physiological) pH the concentration of each ion is only 100 nM. The surfaces of metal oxides such as titanium dioxide are typically hydroxylated through reaction with water and the hydroxyl groups can themselves ionize:
The small, mobile hydrogen ions will tend to diffuse away (thermal motion competing with the electrostatic interaction pulling them back to the surface), electrifying the interface, a tendency which is exacerbated if the water is moving relative to the metal oxide.
‘Ionization’ means that an atom has lost or gained one or more electrons. Even if there is no ionization, atoms can interact electrodynamically because, being formed of a heavy, compact, positively charged nucleus and a light, extended, negatively charged electron ‘cloud’, they have a transiently dipolar character and these dipoles can interact (engendering the van der Waals, London and Keesom forces, collectively called the Lifshitz–van der Waals (LW) force (van Oss, 2006)). This provides a universal attractive force between all materials, which suffices to enable creatures such as the gecko to climb vertical walls and cross ceilings upside down. A corollary of the existence of this universal force means that it is not possible to make a truly non-interacting material. Fortunately there is another set of forces, also based on electrodynamics, which depends on the fact that when atoms combine to create chemical compounds, the electrons are not usually equally shared and, hence, some compounds are electron donors (they have a slight electron excess) and some compounds are electron acceptors (they have a slight electron deficiency). These are called Lewis bases and Lewis acids, respectively. Water, incidentally, is both a Lewis acid and a Lewis base. Two monopolar surfaces (van Oss et al., 1987) of the same polarity (e.g. two Lewis bases) will repel each other. Fortunately many natural materials, both biological and non-biological, have a pronounced electron-donating tendency and will, therefore, tend to repel each other. Hydrocarbon waxes and synthetic polymers such as polyethylene are essentially apolar – they can only interact with the LW force.
Materials of devices, such as stents, intended to remain in contact with the bloodstream for prolonged periods must strongly interact with macro-molecular blood components – but repulsively. As will be covered in more detail in Section 1.2, this is achievable by more strongly interacting with the majority component of the blood, namely water, than anything else. This interaction is modified by the presence of small molecules (ions and osmolytes) dissolved in the biofluid (Cacace et al., 1997). It follows that a material characterized as bioinert, defined as ‘not evoking any significant response from the host organism or biological system’ PAS132, 2008), is not unreactive as are the gases helium, argon, etc., but must be strongly reactive – in the right sense.
Given that the characteristic time for macromolecular adsorption is typically of the order of tens of seconds (Ramsden, 1998), there is no need to consider such adsorption for the surfaces of devices (e.g. needles and knives) whose contact with the tissue is of the order of one second. Rather, the requirement here is to diminish the coefficient of friction as much as possible (see Section 1.2.4). Conversely, assimilation demands strong adhesion. Although from the viewpoint of the surgeon it is the cells that must strongly bond to the permanently implanted material, this in turn depends on macromolecules, mostly proteins and glycoproteins, expressed on the surface of the cell, which must be attracted to the artificial material’s surface.
1.1.5 The concept of surface free energy
In order to make the notion of ‘interaction’ more precise and amenable to quantification, we introduce the concept of surface energy. If a solid substance (e.g. a block of titanium dioxide) is placed in a vessel containing a liquid substance (e.g. water) and then the block is cut in two, work must be done against the forces holding the atoms of the solid substance together, counteracted by the work arising through any attractive forces between the solid and the liquid. The net work can be expressed as the interfacial energy per unit area of interface created. Note that the units of interfacial energy per unit area are the same as that of surface tension (force divided by distance); these concepts are essentially interchangeable. This opens a convenient experimental route to the measurement of interfacial energy. At the beginning of the nineteenth century, Thomas Young established his famous law, according to which the contact angle θ of a droplet of liquid 2 sitting on a planar surface of solid 1 is defined by the condition of stability of the droplet shape with respect to small perturbations or, equivalently, the fact that the droplet is stationary implies that, within the plane of the surface, the sum of the three forces meeting at the line at which the edge of the droplet contacts the surface is equal to zero, namely
where γ12 is the interfacial surface tension (or surface energy per unit area) between substances 1 and 2 and analogously for the other terms, neglecting the vapour phase surrounding the surface and the liquid droplet. The surface tension γ2 of a liquid alone can be determined by measuring the shape of a hanging droplet of the liquid. Since γ12 can be considered to be the geometric mean of γ1 and γ2, we can then solve Equation [1.4] to find the unknown γ2. If one wishes to determine the separate components of the surface tension (i.e. LW and Lewis acid/base) the measurement will have to be done three times with three different liquids to obtain three Young’s equations, which can then be solved simultaneously to yield the LW component and the electron donor and electron acceptor components of the Lewis acid/base contribution. An exhaustive discussion is given by van Oss (2006), and some typical values are given in Table 1.2. Note that it is possible to prepare a protein (or other biomacromolecule) surface by repeatedly allowing droplets of a solution of the molecule to dry on a substrate.
Table 1.2
Surface tension parameters of some artificial and biological materialsa
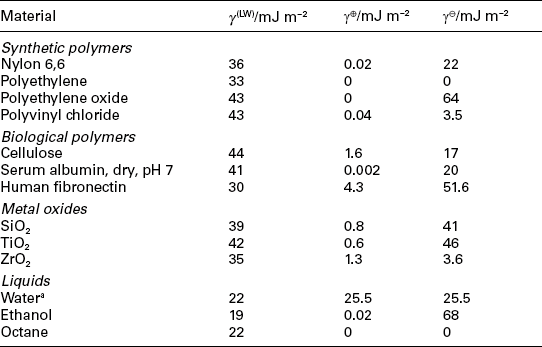
aData from van Oss (2006).
The interfacial tensions can be considered to be the geometric means of the single-substance tensions of the two substances that come together to form the interface. This is straightforward for the LW interaction:
but more complicated for the Lewis acid/base (ab) interaction because this has both electron donor (![]() ) and electron acceptor (
) and electron acceptor (![]() ) components, and the mean therefore contains cross terms:
) components, and the mean therefore contains cross terms:
The cross terms correspond, respectively (from left to right on the right-hand side of the equation), to the polar cohesive interaction energy between the electron donors and acceptors of the artificial surface, the polar cohesive interaction energy between the electron donors and acceptors of the aqueous medium in contact with the surface, the polar adhesive interaction energy between the electron acceptors of the surface and the electron donors of the medium, and the polar adhesive interaction energy between the electron donors of the surface and the electron acceptors of the medium. Due to the presence of the cross terms, the Lewis acid/base interaction can be either repulsive or attractive (Fig. 1.2).
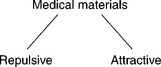
1.2 The division of medical materials into those that interact repulsively and those that interact attractively with the host organism – ultimately at the molecular level. The repulsive interaction is generally required for materials characterized as ‘bioinert’.
The sign of the interfacial free energy (ΔG) can be readily estimated using the concept of surface tension. A useful relation, derivable from Equations [1.5] and [1.6] and Dupré’s convention that ![]() (Cacace et al., 1997), is:
(Cacace et al., 1997), is:
This expression encapsulates the fact that the interfacial free energy depends positively on the cohesive energy ΔG22 of the liquid medium and the interfacial free energy ΔG13 of the artificial material and the biological material in the absence of intervening liquid, and negatively on the solvation free energies ΔG12 and ΔG32 of the artificial and biological materials. The superscript || denotes that the interfacial free energies are for plane parallel surfaces of infinite extent at the distance of closest approach (‘contact distance’), which will be mentioned again in the next section. This equation clearly shows that the minimum requirement for attraction between an artificial and a biological material is the absence of any specific interactions (including solvation) – the cohesive energy of the solvent, which is large and negative in the case of water, will ensure that the interfacial free energy is attractive. Conversely, the only way to overcome attraction is to ensure that the artificial material or the biological material or both are well solvated, in which case either or both of the last two terms on the right will make significant positive contributions to ΔG123. Note that the LW and ab contributions are simply added together.
Since the proteins present in the bloodstream and at the surfaces of living cells may undergo conformational change once present at the interface (Section 1.2.1), the overall behaviour is likely to be much more complicated than that suggested by Equation [1.7], which should therefore only be used to provide an initial indication.
1.1.6 How interactions are changed due to welding and other forms of joining
It is of course of relevance to understand how material processing may change biointeractions. This understanding may be achieved within the formalism of surface free energy; one needs to understand precisely how the surface energetic properties of the material have been changed. This can be investigated experimentally by ex situ experiments in which the material is subject to the same joining processes and then its surface morphology and energetics are measured. Welding is likely to involve heating of the material, and gluing involves the introduction of additional chemical species.
1.2 The anatomy of interaction
In the previous section we considered the rather idealized situation of plane parallel surfaces. In reality, the artificial medical material is typically hard relative to the biological material, which is conformal or soft. A liquid ‘tissue’ such as the blood represents the ultimate degree of condensed matter conformability. Even solid tissue (e.g. the epithelium) is mostly composed of water. Nevertheless, small areas of the interface can still be considered to be plane parallel in nature. In any case, it should be emphasized that the most important aspect of the interfacial interaction, regardless of what kind of biological material is involved, concerns the behaviour of the proteins. For a medical material in contact with the blood, the adsorption of blood proteins is of primordial importance in determining the fate of the artificial material. But even cells are densely populated with proteins on their outer surface. Hence, understanding how proteins interact with artificial materials will take us a long way towards understanding the behaviour of medical materials in contact with biological tissue.
Let us consider a single protein molecule at a distance z from an artificial surface. At infinite distance from the surface, the molecule will not experience any force. In reality, given the relatively short range of the relevant forces (LW, Lewis acid/base and electrostatic), a molecule might only have to be a few tens of nanometres distant from the medical material in order not to experience any force of attraction to or repulsion from it. In order to compute the interfacial free energy, one integrates the force on moving from effectively infinite separation to the distance of closest approach (van Oss and Good, 1984) – about 1.5 Å. The free energies of interaction thereby obtained correspond to those discussed in the previous section (cf. Equation [1.7] and others). The free energy of interaction, ΔG123, itself depends on z (Cacace et al., 1997). A typical profile is shown in Fig. 1.3. Now, considering the adsorption event to be a reaction, and denoting the protein or other biomolecule as B and the medical material as M, we have for the transition from solution:
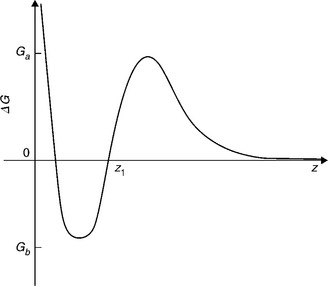
1.3 Sketch of the typical variation of interfacial free energy ΔG123 with separation z. The interface is, for example, that between a medical material and a biological fluid such as the blood, in which case the distance z is that between the surface of the medical material and a protein molecule. Ga is the height of the energy barrier hindering approach of the biological object to the surface of the artificial material, and Gb is the depth of the potential energy well in which a biological object, having arrived at the surface of the artificial material, finds itself.
The forward rate constant kf can be written as the quotient of the diffusivity of the protein D and the adsorption interaction length δa
where δa is in turn given by Spielman and Friedlander (1974)
The lower limit z1 of the integral can, practically speaking, be taken to be the point at which the curve of ΔG123(z) first crosses the axis as z diminishes from infinity (Fig. 1.3). Expressions for the distance dependence of the components of ΔG123 can be found in van Oss (2006) or Cacace et al. (1997). The decay of the dominant ab interaction is exponential, that is,
where ΔG0 is the value of ΔG123(z) for infinite parallel planar surfaces separated by the ‘distance of closest approach’ (van Oss, 2006) and zd is the characteristic decay length for the interaction – typically of the order of 1 nm under physiological conditions.
The overall adsorption rate of a protein at a surface will be
where Γ is the surface excess (i.e. the number or mass of adsorbed proteins per unit area), c* is the effective concentration of the proteins in the medium contacting the artificial material; it depends on the actual bulk concentration (or activity) of the adsorbing species and the prevailing hydrodynamic regime, and ϕ is the available area function (which gives the probability that a molecule arriving at the surface finds space to adsorb). A good deal of the discussion about adsorption rates focuses on appropriate expressions for c* and ϕ. For multilayer adsorption, ϕ always equals 1. Otherwise a random sequential addition (RSA) model might be appropriate (Ramsden, 1998), especially for small buoyant objects such as protein molecules. Cells sedimenting onto a horizontal substratum are best modelled as a ballistic deposition process (Lavalle et al., 1997). If the liquid medium in which proteins are dissolved is stagnant, their transport to the surface will be purely diffusive. Otherwise it will take place via convective diffusion, and it may be useful to characterize it with the Damköhler number Da, the quotient of reaction rate and convective mass transport rate, which here becomes, after cancelling c* in both numerator and denominator:
where δh is the thickness of the hydrodynamic boundary layer, which depends on the flow rate and geometry (Levich, 1962). If one is most interested in the initial events when the protein first comes into contact with the artificial material, ϕ may be taken as unity.
The desorption rate can be given, to a first approximation, as
where kb is the rate coefficient of desorption and Fig. 1.3 suggests that an Arrhenius expression
might be an acceptable first approximation to kb. Due to conformational change (or even simple reorientation) kb is, in reality, typically time-dependent (see Ramsden (2003) for more discussion).
1.2.1 Interactions of a protein molecule
The formalism developed in the previous section is still very rudimentary for use with real protein molecules. It has been tacitly assumed that the protein is a uniform sphere characterized by a particular value of single-substance surface tension parameters (Table 1.1), obtainable from contact angle measurements with a layer of protein molecules deposited on a substrate. This kind of measurement averages out atomic detail. In reality, we know above all from protein crystallography that the surface of a protein is highly heterogeneous (Calonder et al., 2001). The polypeptide backbone constituting the protein is itself rather polar, but the side chains (‘residues’) of the individual amino acids may be either polar or apolar. If this true nature of the protein surface is taken into account, the net interfacial free energy will be highly dependent on heterogeneity (especially of Lewis acid/base components) of the biomaterial. That the simple model averaging the various electron donating and accepting components is inadequate can already be seen by the behaviour of the abundant protein serum albumin, which according to the single-substance surface tension data should be repelled from pure silica, whereas it is a well-known experimental fact that it is strongly adsorbed. Similarly theoretically unexpected behaviour is observed with adsorption on heterogeneous materials (Aggarwal et al., 2009).
Furthermore, it must be borne in mind that the native conformation of a protein is principally due to intramolecular hydrogen bonds between the backbone peptide bonds. Note that the hydrogen bond is a particular kind, the most important and ubiquitous in biology, of Lewis acid/base interaction. Since proteins are suspended in water (as are those parts of membrane proteins involved in adsorption to a medical material), which is itself a strong hydrogen bond donor and hydrogen bond acceptor, the main structural challenge that a protein has to meet is how to prevent denaturation through the attack of its intramolecular hydrogen bonds by water. Proteins have evolved a three-body structural motif in order to overcome the danger (Fernández and Scott, 2003), namely the intramolecular hydrogen bond with its donor and acceptor together with apolar residues to desolvate the hydrogen bond, thereby stabilizing it. An underdesolvated hydrogen bond (i.e. one with insufficient apolar residues in its vicinity) is called a dehydron, and the presence of dehydrons on the surface of a protein make it susceptible to bind to surfaces that can accomplish the missing desolvation. Artificial surfaces can be probed using atomic-force microscopy in order to ascertain their desolvation ability (Fernández, 2006).
Even if the adsorption of a protein on the surface is enthalpically neutral (i.e. any new bonds formed between the protein and the surface of the medical material would be compensated by breaking intramolecular bonds), there is nearly always the danger of an entropic penalty. Let us consider the representation of the three-dimensional conformation of a protein molecule by the two dihedral angles of successive peptide bonds. The Ramachandran diagram plots, for each amino acid, those combinations of the two angles corresponding to compact, native conformations and to extended, denatured conformations. Far more combinations are associated with extended states than with the native state. This means that there is inevitably an entropic gain, hence a decrease of free energy, upon denaturation (Fernández and Ramsden, 2001). Hence, in order to obviate denaturation, it is essential to design the surface of a medical material to strongly repel the protein.
Most globular proteins have more than one stable compact conformation. This feature is usually an essential part of their operation as enzymes, motors, channels, etc. (Blumenfeld, 1981). Under some given condition (such as in a protein crystal), one would expect that one of the several possible compact confirmations is predominant. It is an unfortunate feature of the main source of structural information about proteins, namely X-ray crystallography, that the contributions of the minor structural component(s) to the X-ray diffractogram will be lost during computational refinement of the structure (Frauenfelder, 1984). It is highly likely that adsorption on a surface will cause a switch from one stable conformation to another. There is considerable evidence for such structural transitions upon adsorption (e.g. Ramsden, 1993) which, in the absence of definite knowledge about the different stable compact conformations, has sometimes been merely ascribed to reorientation (since globular proteins are rarely perfectly symmetrical but usually have some ellipsoidal character) or even to denaturation. Further evidence comes from observations of dramatic deceleration of enzyme operation, typically by many orders of magnitude, upon adsorption (e.g. Cacace and Ramsden, 2007). However, it is generally not possible to distinguish between deceleration due to the ‘wrong’ conformation being energetically favoured in the adsorbed state and the alternative explanation of a large fraction of the proteins being completely inactivated by denaturation and the remaining activity resulting from a few unchanged molecules. Figure 1.4 shows how a conformational change – or even merely an orientational change – can strongly change the affinity of a protein for a surface.
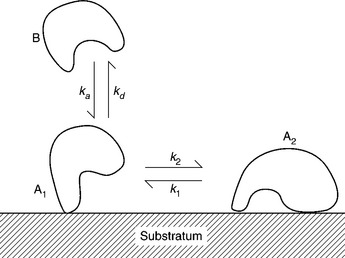
1.4 Sketch of a protein (species B) dissolved in a biofluid in contact with some medical material (‘Substratum’). The protein reaches the surface at a rate characterized by the rate coefficient ka (which is related to the energy barrier Ga in Fig. 1.3). Immediately after arrival at the surface, the protein (species A1) might differ very little from its dissolved counterpart, possibly being oriented in a fixed fashion and possibly retaining most of its native conformation while making some hydrogen bonds with the surface. While in residence (the duration of which will be proportional to the depth of the potential energy well, Gb in Fig. 1.3), it may undergo more extensive orientational and conformational changes, becoming transformed to species A2 with a rate proportional to the coefficient k2. In general, the reverse transformation would also be possible, with a rate proportional to the coefficient k1. In this diagram it is supposed that A2 is too strongly bound to the surface to be able to depart directly into the solution. Were it able to do so, it would very likely then be a different species from the original B.
The final point we want to consider is the possibility of incorporating specific receptors into an artificial surface for certain motifs of amino acids present in the protein. Nature makes extensive use of such specificity. Examples are antigen–antibody interactions, the affinity of cell membrane-embedded integrins for the amino acid triplet arginine-glycine-aspartic acid (RGD), and so forth. Typically these specific interactions, even though they are themselves dependent on hydrogen bonds, are much stronger than the chance interactions between a randomly oriented protein and a more or less homogeneous artificial surface, since within a small area there is a large number of hydrogen bonds whose strength is assured by an appropriate geometry of binding. On the other hand, it is problematical to manufacture medical materials incorporating fragile biological components such as amino acids. Even if they survive processing and storage, they are likely to be quickly destroyed or altered in use. Therefore, there is considerable interest in mimicking such specificity. One way of achieving it using purely artificial materials is through molecular imprinting (Mosbach, 1992). A molecularly imprinted material is prepared by mixing a precursor, which should be a polymer or polymerizable monomer with plenty of hydrogen bonding possibilities, together with the motif (e.g. the tripeptide RGD) to which it is desired to create affinity. The artificial material is polymerized around the guest, which is then washed out using a strongly hydrogen bond-breaking solution, leaving a material having cavities with highly specific affinity for the guest.
1.2.2 Interactions of living cells with medical materials
The classic mode of interaction of an isolated, suspended cell with a substratum on which it arrives is the transformation from a sphere to a segment (‘cell spreading’). An extensive literature on the subject going back at least 50 years reveals that the nature of the spreading is highly dependent on the cell type, the substratum and on the bathing medium (Li et al., 1994). This kind of approach has recently been updated, making use of experimental advances in nanofabrication, genomics and stem cells. The possibility of the creation of microscale and nanoscale features in a variety of materials has led to many studies of cell response (e.g. Curtis et al., 2001; Teixeira et al., 2004; Khor et al., 2007). Observations range from simple microscopical ones to immunochemistry for determining the presence of protein markers characteristic for certain cell types on the cell surface. The latter is of particular value when investigating the effect of substratum on stem-cell differentiation (e.g. Dalby et al., 2007). The relative ease, nowadays, of investigating gene expression has led to corresponding studies (e.g. Dalby et al., 2008). Despite the great volume of published work, it is difficult to draw general inferences. Knowledge of the effects of microscale and nanoscale topography, for example, is still limited to the fairly uninteresting observation that cells tend to align with parallel grooves. The difficulty of generalization is not least due to the fact that practically every experiment is carried out under different conditions, with different substrata or different cell types; particularly with stem cells, a very particular culture medium must generally be used for each type of cell, making it difficult to assess the effects of components in the medium on the behaviour. There is still a need for a far more extensive set of systematic studies coupled with rapid, quantitative cytometry (see also Section 1.4). Given that the number of possible combinations of variables is so vast, it is very necessary to develop testable hypotheses for the behaviour.
The native substrata of cells are, of course, the basement membranes constituted from extracellular matrix (ECM) proteins like laminin and tenascin (e.g. Bökel and Brown, 2002). It might therefore be supposed that the best medical materials are those that somehow mimic the ECM. On the other hand, it is known that if a cell comes into contact with an artificial material, it will secrete ECM proteins which coat the substratum (e.g. Li et al., 1994). This is somewhat discouraging for research into developing ideal material surface coatings, since it would appear that no matter how carefully the material is surface-engineered, its features will rapidly be obliterated by the cell carrying out its own engineering. A promising line of research is to attempt to artificially engineer surfaces such that the signals transmitted by the cell from its surface to its nucleus are controlled by the material. This represents a considerable challenge, on which very little work has hitherto been done, but not, hopefully, an insurmountable one.
Although the overall shape of the cell can be idealized as a sphere or a segment, in reality the cell is constantly creating protrusions (filopodia) with which to explore the surface (Aref et al., 2010). The tips of these filopodia are known to be rich in particular proteins, emphasizing the importance of considering and understanding the interactions of proteins with medical materials as the foundation of knowledge of the field.
Overall, the interactions of cells with medical materials can be considered as an adaptive response taking place on different timescales and with correspondingly different mechanisms. Even if we just look at the spreading transition, phenomenologically there is the rapid spreading, taking place over tens of minutes (e.g. Aref et al., 2010), and the classic or slow spreading, taking place over tens of hours (e.g. Li et al., 1994). It might be supposed that the rapid spreading is a purely mechanical (viscoelastic) response of the cell to a constraint, whereas the slow spreading involves restructuring of the cytoskeleton, which requires internal signalling processes involving, for example, the phosphorylation and dephosphorylation of enzymes, which could take place without altered gene expression, but if ECM proteins have to be exuded, it is very likely that the enzymes involved in the synthesis and exudation would themselves have to be synthesized, hence involving the DNA of the cell. The differentiation of stem cells presumably results from considerable epigenetic activity. Once cells have differentiated, they typically cease to divide and, hence, there is no opportunity for investigating whether genotypes can be altered through residence on a substratum. Studies with bacteria have clearly shown changes in phenotype (e.g. Vilain et al., 2004).
The majority of work appears to be carried out using eukaryotic cells, which are of course of immediate interest for the development of medical materials. Nevertheless, interactions with bacteria are also important and hopefully in the future more researchers will be attracted into that area. Certain parts of the human body, such as the colon, are rich in bacteria and devices intended for such parts need to interact appropriately with the bacterial population. For example, an implanted sensor should probably not become coated with a bacterial biofilm. Also of importance is the problem of persistent colonization of implants by bacteria. Very often they are resistant to antibiotics and act as a constant source of inflammatory irritation, which can often only be eliminated by removing the implant. The challenge is to create a material that is hostile to bacterial colonization, although, given the powerful capabilities of bacteria to modify their immediate environment (e.g. through biofilm formation) the challenge seems to be very great. As with the control of eukaryotic microexudates, influencing bacterial gene expression in a controlled fashion is a key goal.
1.2.3 Interactions with tissues
Tissues are of course made up of cells, the main difference between the topic of the previous section being that most of the laboratory research is carried out with individual cells, whereas in a tissue the cells are strongly mechanically constrained, which has a great effect on their behaviour (Nishihara, 2011). One implication is that the possibility of the implant to influence the cells within the tissue must principally work through influencing gene expression (Fig. 1.5).
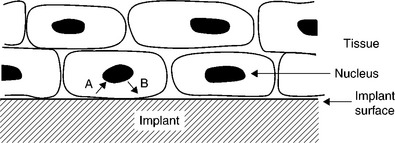
1.5 The interface between an artificial implant (hatched) and biological tissue represented by closely packed cells. Information characterizing the implant surface is transmitted (via signalling pathways) to the cell nucleus (arrow A), engendering changes in gene expression, resulting in fresh information being sent from the nucleus to the cell surface (arrow B), which might (for example) result in the excretion of some proteins to modify the implant surface.
1.2.4 Transient interactions: the tribology of medical materials
The two principal challenges in tribology are: (i) minimizing the coefficient of friction of surgical objects such as knives and needles; and (ii) minimizing the coefficient of friction of the materials in devices with rubbing surfaces, such as a ball-and-socket joint. Coefficients of friction depend on both material and morphology.
The purpose of (i) is to minimize collateral damage caused by a device as it passes through tissue. Similar considerations apply to permanently implanted devices that have to be passed through tissue in order to reach their final position. The purpose of (ii) is to minimize wear, which typically causes the release of particles. These particles may have significant deleterious biological effects (Revell, 2006), hence it is highly desirable to minimize their generation. The most common effect is inflammation caused by the adsorption of proteins (if the material is high density polyethylene, commonly used in prostheses, the hydrophobic nature of polyethylene – see Table 1.1 – promotes denaturation of proteins that come into contact with it, transforming them into non-self objects recognized as such by the immune system, which then works to eliminate them). One promising approach to minimizing wear is the introduction of ultra-hard materials such as monocrystalline sapphire (Mamalis et al., 2006, 2007). Sapphire-sapphire contacts have low coefficients of friction and the great hardness of the material means that it can be machined into very precise and stable shapes, which in itself minimizes wear. Nevertheless, some aspects of such novel materials still need further investigation. For example, the mass of ultra-hard material removed through wear during a given interval might indeed be one or more orders of magnitude less than in the case of traditional materials, but if the particles produced are very fine nanoparticles, they may engender a different set of biological responses, which might also be undesirable (Revell, 2006). Furthermore, if the particles are very small, they may be just as numerous as a much larger total mass of larger particles.
1.3 Materials for drug delivery
A very active area of research and development is the use of nanotechnology to engineer sophisticated small particles (as well as nanotubes and nanorods) for a variety of diagnostic and therapeutic applications (e.g. Arshady and Kono, 2006). The basic idea is to take a nanoparticle that is easy to image (e.g. a gold particle) and functionalize it with biomolecules having a specific affinity for some medically interesting cell or tissue (e.g. a tumour). The functionalization could be based on nucleic acids, peptides or oligosaccharides. In every case, the particles would be administered systemically. For diagnosis, one would then search (e.g. using X-rays) for any zone in which they have concentrated. It is easy to administer different particles (e.g. of different shape or size, each one being differently functionalized) simultaneously, thereby increasing the efficiency of diagnosis. By adding functionality, therapy can be carried out once the particles have reached the target. This might be achievable using external stimulation (e.g. radio frequency excitation, heating up the particles to locally destroy tissue, such as a tumour, by heating) or by arranging for the particles to release drugs from a reservoir (e.g. the particles could be hollow) in order to produce some therapeutic effect.
The main reservation of this kind of approach is that there is uncertainty over possibly adverse biological effects of the particles themselves (e.g. Revell, 2006). What is their fate after they have carried out their diagnostic or therapeutic mission? If they are rapidly excreted without causing any significant immune response, then there would appear to be little to worry about. At present, however, there is a great deal of uncertainty regarding the general health effects of nanoparticles (Hunt and Riediker, 2011).
Some of the most popular materials for nanoparticles used in biological research, such as the cadmium chalcogenides used as fluorescent markers, are highly chemically toxic and it is inconceivable that they would ever be diagnostically or therapeutically useful. But for the majority of materials found in the research literature, present knowledge of their biological effects is simply inadequate. There are myriads of compounds that can be made into nanoparticles, nanoshells, nanotubes, nanorods and so forth, each of which can be made in a range of sizes covering the entire nanoscale (considered to be 1–100 nm).
Nevertheless, some of the diseases potentially treatable by this kind of nanomedicine are so life-threatening that one might be prepared to take some risks with toxicity. The main generic challenge is to prevent the nanoparticles, which are of course non-self and recognized as such by the immune system, from being rapidly eliminated before they have done anything of medical utility. This challenge brings us back to the issues discussed in the earlier sections devoted to the interaction of proteins with artificial materials. The nanoparticles need to be coated with strongly hydrated materials that will repel proteins, while at the same time retaining their specific affinity for their target. If the target is intracellular, the particles then need to be taken up by the cell, by endocytosis or pinocytosis (Maitra, 2010), which requires different surface properties from those that are needed to ensure their safe passage to the vicinity of the target.
The ultimate medical nanodevice is the nanoscale robot or ‘nanobot’ (Hogg, 2007), envisaged to be the size of a typical bacterium. Serious work on such devices has until now been confined to careful consideration of requirements concerning power, information reception, processing and transmission, the need for on-board sensors and, of course, the nature of the materials with which the device must be covered in order to prevent undue interference from the immune system. The principles governing the surface coating specification are, of course, those that have been already discussed.
1.4 The metrology of biocompatibility
From the above, it is clear that the main metrology challenge is to determine the degree of protein adsorption to a medical material. Many techniques for accomplishing this exist, and have been reviewed by Ramsden (1994). The most basic measurement is simply to determine the quantity of adsorbed biomolecules. A further step in sophistication can be achieved if information about the structure of the adsorbed biomolecules can be obtained. It may also be very illuminating to characterize the kinetics of adsorption and desorption.
Ideally the technique should be able to accomplish comprehensive characterization of the adsorption phenomenon in situ under conditions of use. No existing technique is able to achieve this. An implant can be inserted into the body, left for some time, and then removed and examined. Such tests are frequently carried out using animals, but given the subtlety of protein adsorption, when even the substitution of a single amino acid can dramatically change the adsorption characteristics (e.g. Ramsden et al., 1995), ultimately tests on human beings have to be carried out in order to have confidence in the results. To avoid, or minimize, the need for such in vivo experiments, it is highly desirable to develop laboratory simulations of the natural processes. In any case, the implant procedure cannot monitor the processes as they occur.
Fortunately, several optical techniques are now available that allow rather detailed quantitative, structural and kinetic characterization of the adsorption of proteins at the surface of a material. They are based on the phase shift of a beam of light totally internally reflected at the material/biofluid interface due to the accumulation of proteins (Ramsden, 1994). The most sensitive and informative technique (optical waveguide lightmode spectroscopy – OWLS) has the material in the form of an optical waveguide, which means that it is restricted to optically transparent substances. This is less of a restriction than might be imagined, since many ceramics used as medical materials (Table 1.1) are indeed transparent in practically useful regions of the optical spectrum, and most metals used for implants are coated with a layer of transparent oxide. If it is necessary to examine an opaque material, less sensitive techniques such as scanning angle reflectometry, ellipsometry or surface plasmon resonance (Ramsden, 1994) must be employed. The sensitivity of OWLS can be further augmented by configuring it as an interferometer (optical waveguide lightmode interferometry – OWLI), although fewer structural parameters can then be extracted from the measurements.
Chemical characterization of the adsorbed biomaterial can be achieved using vibrational spectroscopy in attenuated total internal reflexion mode, although this is less useful than in small-molecule chemistry because of the complexity of the absorption spectra of biomolecules.
In use, these techniques involve interrogation of the material in an appropriate form (e.g. a planar optical waveguide) while in contact with blood or any other relevant biofluid. Other techniques, useful for delivering complementary or corroborative data, include scanning probe microscopy and X-ray or neutron diffraction. The former is attractive because it allows high-resolution observation of the surface under biomimetic conditions, although the slow, raster-type interrogation of the surface generally precludes meaningful kinetic investigations. The latter techniques are generally only available for ex situ investigations of a preformed layer of adsorbed biomaterial.
Some of these techniques can also be adapted to directly investigate the behaviour of living cells in contact with medical materials. The optical waveguide-based techniques are especially convenient in this regard (Ramsden and Horvath, 2009). The advanced application of OWLS enables successive slices of the cell parallel to the adsorbing substratum to be interrogated in the fashion of confocal microscopy (Horvath et al., 2008). A significant advantage of these optical waveguide-based techniques is their ability to yield quantitative structural parameters in situ in real time, thus enabling rapid screening of the material for its compatibility with living cells (e.g. Aref et al., 2010). Scanning probe microscopies can also be adapted to observe living cells. In order to prevent the brutal contact of the scanning probe with the living cell in a conventional scanning probe microscope, the new technique of scanning ion conduction microscopy (SICM) has been developed (Gorelik et al., 2008). In this technique, the scanning probe is a miniature electrode. A large counter-electrode in the medium bathing the cells completes the circuit. When the miniature electrode is very close to the surface of a cell, the electrical resistance is high and this provides the information required to generate an accurate topography of cell surfaces without ever coming into actual content with them.
1.5 Conclusion
Considerations of the biological aspects of medical materials lead one to a hierarchy (Fig. 1.6): interactions between artificial material and biological matter mainly depend on interactions between the artificial material and proteins, which should therefore form the focus of research and development.
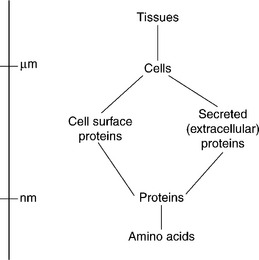
1.6 The hierarchy of interactions determining biocompatibility. Chemical functional groups (such as amino acid residues) determine the interfacial forces between proteins and artificial implants, and the ensemble of proteins expressed on the surface of cells determines the interaction between tissue and implant.
1.6 Future trends
The main development in recent years has been the opening of new possibilities in materials design and fabrication through nanotechnology. While we are still a long way from achieving such complete engineering control to enable any material of arbitrary shape and chemical composition to be created (but see Ly et al. (2011) for an encouraging sign of progress in this direction), there is already considerable fabrication capability in the submicrometre size domain, especially for regular structures, although whether their manufacture can be carried out cost-effectively at an appropriate scale remains to be seen. Above all, the problem is knowing what to make. There is a great need for more comprehensively systematic investigations of the responses of living cells and tissues to materials. Thanks to important advances in molecular biology, biological responses can be characterized in considerable detail and this capability, when coupled with comparable advances in creating precisely engineered material surfaces, should enable progress to be made. One difficulty seems to be the very large parameter space that is now available. It is, therefore, essential that more attention is paid to formulating hypotheses for the biological response to medical materials, which can then be rigorously tested.
1.7 Sources of further information
A basic introduction to biomedical surfaces is provided by Ramsden (2008). An encyclopaedic reference compendium for biomaterials is the work edited by Ratner et al. (2004). A good general work on materials is Ashby et al. (2007). For an overview of nanotechnology, including nanomaterials, one may consult the book by Ramsden (2011). The review by Ramsden et al. (2007) covers issues related to the fabrication of biomaterials.
1.8 References
Aggarwal, N., Lawson, K., Kershaw, M., Horvath, R., Ramsden, J.J. Protein adsorption on heterogeneous surfaces. Appl. Phys. Lett.. 2009; 94:083110.
Aref, A., Horvath, R., Ramsden, J.J. Spreading kinetics as a means of quantifying cell state during stem cell differentiation. J. Biol. Phys. Chem.. 2010; 10:53–54.
Arshady R., Kono K., eds. Smart Nanoparticles in Nanomedicine. London: Kentus, 2006.
Ashby, M., Shercliff, H., Cebon, D.Materials: Engineering, Science, Processing and Design. Oxford: Elsevier, 2007.
Blumenfeld, L.A.Problems of Biological Physics. Berlin: Springer, 1981.
Bökel, C., Brown, N.H. Integrins in development: moving on, responding to and sticking to the extracellular matrix. Dev. Cell. 2002; 3:311–321.
Cacace, M.G., Ramsden, J.J. In situ monitoring of adsorbed enzyme activity. J. Biol. Phys. Chem.. 2007; 7:6568.
Cacace, M.G., Landau, E.M., Ramsden, J.J. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q. Rev. Biophys.. 1997; 30:241–278.
Calonder, C., Talbot, J., Ramsden, J.J. Mapping the electron 34 donor/acceptor potentials on protein surfaces. J. Phys. Chem. B. 2001; 105:725–729.
Curtis, A.S.G., Casey, B., Gallagher, J.O., Pasqui, D., Wood, M.A., Wilkinson, C.D.W. Substratum nanotopography and the adhesion of biological cells: are symmetry or regularity of nanotopography important? Biophys. Chem.. 2001; 94:275–283.
Dalby, M.J., Gadegaard, N., Curtis, A.S.G., Oreffo, R.O.C. Nanotopographical control of human osteoprogenitor differentiation. Current Stem Cell Res. Therapy. 2007; 2:129–138.
Dalby, M.J., Andar, A., Nag, A., Affrossman, S., Tare, R., MacFarlane, S., Oreffo, R.O.C. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J. R. Soc. Interface. 2008; 5:1055–1965.
Fernández, A. Wrapping a hydrogen bond with a molecular force probe: the mechanical equivalent of dehydration propensity. J. Biol. Phys. Chem.. 2006; 6:3–7.
Fernández, A., Ramsden, J.J. On adsorption-induced denaturation of folded proteins. J. Biol. Phys. Chem.. 2001; 1:81–84.
Fernández, A., Scott, R. Dehydron: a structurally encoded signal for protein interaction. Biophys. J.. 2003; 85:1914–1928.
Frauenfelder, H. From atoms to biomolecules. Helv. Phys. Acta. 1984; 57:165–187.
Gorelik, J., Ali, N.N., Sheikh Abdul Kadir, S.H., Lab, M., Stojkovic, P., Armstrong, L., Sviderskaya, E.V., Negulyaev, Y.A., Klenerman, D., Bennett, D.C., Lako, M., Harding, S.E., Stojkovic, M., Korchev, Y.E. Non-invasive imaging of stem cells by scanning ion conductance microscopy: future perspective. Tissue Engng C. 2008; 14:311–318.
Hogg, T. Evaluating microscopic robots for medical diagnosis and treatment. Nanotechnol. Perceptions. 2007; 3:63–73.
Horvath, R., Cottier, K., Pedersen, H.C., Ramsden, J.J. Multi-depth screening of living cells using optical waveguides. Biosens. Bioelectron.. 2008; 24:805–810.
Hunt, G., Riediker, M. Building expert consensus on problems of uncertainty and complexity in nanomaterial safety. Nanotechnol. Perceptions. 2011; 7:82–98.
Khor, H.L., Kuan, Y., Kukula, H., Tamada, K., Knoll, W., Moeller, M., Hutmacher, D.W. Response of cells on surface-induced nanopatterns: fibroblasts and mesenchymal progenitor cells. Biomacromolecules. 2007; 8:1530–1540.
Kutsevlyak, V.I., Starikova, S.L., Starikov, V.V., Mamalis, A.G., Lavrynenko, S.N., Ramsden, J.J. Influence of implant surface modification on integration with bone tissue. J. Biol. Phys. Chem.. 2008; 8:147–150.
Lavalle, P., Stoltz, J.-F., Senger, B., Voegel, J.-C., Schaaf, P. Red blood cell adhesion on a solid/liquid interface: comparison of two models. Clin. Hemorheol. Microcirc. 1997; 17:307–313.
Levich, V.G.Physicochemical Hydrodynamics. Englewood Cliffs, NJ: Prentice Hall, 1962.
Li, S.-Y., Ramsden, J.J., Prenosil, J.E., Heinzle, E. Measurement of adhesion and spreading kinetics of baby hamster kidney and hybridoma cells using an integrated optical method. Biotechnology Progr.. 1994; 10:520–524.
Ly, D.Q., Paramonov, L., Davidson, C., Ramsden, J., Wright, H., Holliman, N., Hagon, J., Heggie, M., Makatsoris, C. The matter compiler – towards atomically precise engineering and manufacture. Nanotechnol. Perceptions. 2011; 7:1–20.
Maitra, A. Nanotechnology and nanobiotechnology – are they children of the same father? Nanotechnol. Perceptions. 2010; 6:197–204.
Mamalis, A.G., Ramsden, J.J., Grabchenko, A.I., Lytvynov, K.A., Filipenko, V.A., Lavrynenko, S.N. A novel concept for the manufacture of individual sapphire-metallic hip joint endoprostheses. J. Biol. Phys. Chem.. 2006; 6:113–117.
Mamalis, A.G., Lytvynov, K.A., Filipenko, V.A., Lavrynenko, S.N., Ramsden, J.J., Soukakos, P.N. Perfection of contemporary hip joint endoprostheses by using a sapphire-sapphire friction pair. J. Biol. Phys. Chem.. 2007; 7:3–5.
Mosbach, K. Preparation of synthetic enzymes and synthetic antibodies, and use of the thus prepared enzymes and antibodies. US Patent. 1992; 5(110):833.
van Oss, C.J. Interfacial Forces in Aqueous Media, 2nd. New York: Taylor & Francis, 2006.
van Oss, C.J., Good, R.J. The ‘equilibrium distance’ between two bodies immersed in a liquid. Colloids Surf.. 1984; 8:373–381.
van Oss, C.J., Chaudhury, M.K., Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci.. 1987; 28:35–64.
Nishihara, K. Great medical discoveries of the 21st century. Part I: Revitalizing stagnant medicine by establishing energy-based bioscience. Disclosure of the aetiological factors of three major intractable maladies at the subcellular level: immune diseases, carcinoma and mental illness. J. Biol. Phys. Chem.. 2011; 11:63–85.
PAS (Publicly Available Specification)Terminology for the Bio-nano Interface. London: British Standards Institution, 2007. [132].
Ramsden, J.J. Concentration scaling of protein deposition kinetics. Phys. Rev. Lett.. 1993; 71:295–298.
Ramsden, J.J. Experimental methods for investigating protein adsorption kinetics at surfaces. Q. Rev. Biophys.. 1994; 27:41–105.
Ramsden, J.J. Kinetics of protein adsorption. In: Malmsten M., ed. Biopolymers at Interfaces. New York: Dekker; 1998:321–361. [Chapter 10].
Ramsden, J.J. Protein adsorption kinetics. In: Malmsten M., ed. Biopolymers at Interfaces. 2nd. New York: Dekker; 2003:199–220. [Chapter 8].
Ramsden, J.J.Biomedical Surfaces. Norwood, MA: Artech House, 2008.
Ramsden, J.J.Nanotechnology: An Introduction. Oxford: Elsevier, 2011.
Ramsden, J.J., Freeman, J. The nanoscale. Nanotechnology Perceptions. 2009; 5:3–25.
Ramsden, J.J., Horvath, R. Optical biosensors for cell adhesion. J. Receptors Signal Transduction. 2009; 29:211–223.
Ramsden, J.J., Roush, D.J., Gill, D.S., Kurrat, R.G., Willson, R.C. Protein adsorption kinetics drastically altered by repositioning a single charge. J. Am. Chem. Soc.. 1995; 117:8511–8516.
Ramsden, J.J., Allen, D.M., Stephenson, D.J., Alcock, J.R., Peggs, G.N., Fuller, G., Goch, G. The design and manufacture of biomedical surfaces. Annals CIRP. 2007; 56/2:687–711.
Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., eds. Biomaterials Science: An Introduction to Materials in Medicine. San Diego, CA: Academic Press, 2004.
Revell, P.A. The biological effects of nanoparticles. Nanotechnol. Perceptions. 2006; 2:283–298.
Spielman, L.A., Friedlander, S.K. Role of the electrical double layer in particle deposition by convective diffusion. J. Colloid Interface Sci.. 1974; 46:22–31.
Teixeira, A.I., Nealey, P.F., Murphy, C.J. Responses of human keratinocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res.. 2004; 71A:369–376.
Vilain, S., Cosette, P., Zimmerlin, I., Dupont, J.P., Junter, G.A., Jouenne, T. Bacterial proteome: homogeneity of versatility? J. Proteome Res.. 2004; 3:132–136.