Tissue adhesives and sealants for surgical applications
Abstract:
This chapter reviews tissue sealants and adhesives used in surgery, which can be categorized as bioresorbable or biostable and synthetic or bio-derived. The fastest growing of these categories commercially is synthetic bioresorbable sealants, which form into solid gel in situ. Key characteristics of mechanical properties, application to tissue, mechanisms of bonding and biocompatibility are discussed. Finally, descriptions of the major commercial surgical sealants and adhesives in each category are described.
16.1 Introduction
This chapter will cover topical and implantable tissue adhesives and sealants. These materials differ from pressure-sensitive bandages and electrode adhesives, and each present distinctly different problems of bonding mechanisms and biocompatibility. They are also fundamentally different than hemostats, which are solely intended to coagulate autologous blood. Internal tissue adhesives and sealants depend on the formation of a bond at the interface with the substrate tissue. To establish a good bond, these agents are usually applied to the tissue as a low-viscosity liquid that then solidifies in situ, providing intimate tissue contact and a mechanically interlocking interface with secondary bonding interactions coming into play. Other methods of bond formation such as covalent bonding have been used with some success (Smith, 2004; Park and Lakes, 2007). The chemical nature of this bond is important to its strength and to the impact on the health of the tissue being sealed. The mechanism and chemistry of in situ bond formation will be discussed as will key considerations of efficacy and biocompatibility.
Sealants and adhesives have long been sought for surgical use (Cooper and Falb, 1968). Today they are used in many surgical arenas (Wheat and Wolf, 2009), notably in vascular, gastrointestinal, ocular, neural, spine and lung. Difficult to seal tissues such as liver (Berrevoet and de Hemptinne, 2007) have long been an area of investigation for fibrin adhesives. In vascular surgery (Schenk et al, 2003) sealants have been used to minimize bleeding and the use of sealants for femoral artery closure following angiography and angioplasty procedures has grown recently, with several products on the market. The use of adhesives in ocular tissues has long been a clinical interest (Singh et al., 2010; Wathier and Grinstaff, 2010). The use of fibrin glue and cyanoacrylate adhesives for corneal perforation repair (Lagoutte et al., 1989; Sharma et al., 2003) has been an ‘off label’ practice for decades. Sealants and adhesives have been used for prevention of epithelial in-growth after laser refractive surgery (Anderson and Hardten, 2003; Narváez et al., 2006). Corneal incision sealing has also been a growth area since the advent of ‘sutureless incisions’ in cataract surgery (Kalayci et al., 2003; Grinstaff, 2007). Sealing air leaks after lung surgery (Bayfield and Spotnitz, 1996; Wain et al., 2001; Reul and Walsh, 2005; Rathinam et al., 2009). Adhesives have also been used extensively in ENT procedures (Schneider, 2009). Fibrin glue has been used in sealing cerebrospinal fluid leaks (Shaffrey et al., 1990; Patel et al., 1996), but now new synthetic bioresorbable adhesives are indicated for this application (Cosgrove et al., 2007).
An important synthetic biostable material, commonly known as ‘bone cement’, is composed of a prepolymer of polymethylmethacrylate (PMMA) that polymerizes in situ to provide a rigid mechanical fixation of, for example, a hip prosthesis. These and related cement materials are not strictly adhesives or sealants, but rather grouts or potting compounds, so they will not be treated in this review.
16.2 Principles of tissue adhesives and sealants
The purpose of a tissue sealant is to form an adherent barrier over an opening in tissue that can prevent fluid or particulate ingress or egress through the tissue opening (Fig. 16.1). The sealant must be capable of adhering to the tissue substrate sufficiently to remain in place under the forces acting upon it for a duration sufficient to allow healing to take place. Typically, sealants rely on transformation from a low-viscosity liquid to a flexible or semi-rigid solid during which chemical bonds (covalent, ionic or other secondary bonds) are formed, leading to a molecular network formation.
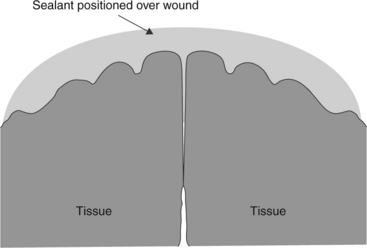
16.1 A surgical sealant or adhesive is placed over the incision to provide apposition and to prevent fluid ingress or egress. This application method allows healing by primary intention.
Tissue adhesives are materials that can be used as a ‘glue’ to hold tissue surfaces together. The adhesive can be placed between tissues to be held in apposition until healing occurs (Fig. 16.2). However, unless the adhesive provides a means to support tissue in-growth and penetration, the placement of adhesive between tissues can impede the healing process. Alternatively, an adhesive is placed across the outer surface of tissues on either side of a wound as in Fig. 16.1 to hold the tissue in apposition.
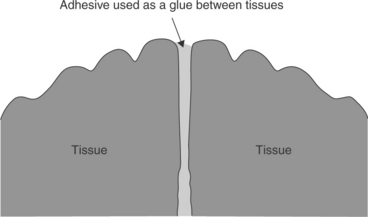
16.2 A resorbable tissue adhesive may be placed between tissues. This approach relies on healing by secondary intention.
The properties of the ideal tissue adhesive or sealant are listed below:
Safe: The product or metabolites must not present toxicological or mechanical risks that could impair healing or damage tissue.
Effective: The material must be able to establish and hold a bond with the intended substrate tissue. The material itself must have strength and flexibility sufficient to avoid failure under the forces acting upon it in the intended use.
Bioresorbable: Once the adhesive has done its job it should exit the area as soon as possible, and in a benign fashion.
Easy to use: The product must be quick and easy to prepare for use. Ancillary equipment or energy sources should be minimized since they take time to prepare for use and they require maintenance and storage. Storage of the material should require minimal special conditions, such as refrigeration or freezing, since these storage places limit its availability at short notice.
Inexpensive: Wound closure and leak prevention have predominantly been accomplished until now using fastening devices such as sutures, staples, clips and meshes. To achieve wide acceptance, the adhesive must not add a large additional cost to primary procedures.
The types of tissue adhesives and sealants can be categorized as follows:
Each type of tissue adhesive or sealant has its place under different medical circumstances. Synthetic biostable adhesives such as cyanoacrylates (‘crazy glues’) are most often used topically to close simple skin wounds (Spotnitz and Burks, 2010). Cyanoacrylate adhesives are also used ‘off label’ in situations where other solutions are unavailable, such as battlefield injuries and certain ophthalmic surgical procedures. Cyanoacrylates have the advantages of ease of application and rapid solidification times. The disadvantages of synthetic biostable sealants such as cyanoacrylates include significant heat generation and local toxicity. Heat is liberated from the exothermic polymerization reaction (Fig. 16.3), which can cause local tissue damage. Local toxicity results from any residual monomer present and the formation of degradation products such as formaldehyde in the case of the monomers with lower molecular weight side chains (Webster and West, 2002). Typically they form fairly rigid solids with excellent adhesion. If placed between apposing tissues, cyanoacrylates can act as a biological barrier and interfere with the healing process.
Synthetic bioresorbable sealants are generally hydrogels designed to incorporate hydrolyzable linkages into the molecular structure such that hydrolysis causes the deconstruction of the molecular network into water-soluble degradation products. Chemically these hydrolyzable linkages (usually esters) are similar to chemistries used in other synthetic bioresorbable devices such as degradable sutures (e.g. Vicryl®). Sealant materials developed in this category have been primarily hydrogels, due to their tissue-like compliance and to the very mild inflammatory tissue responses hydrogels elicit. These materials can be designed to degrade over a period of days to weeks, depending on the specific need for long-term wound reinforcement.
Bio-derived sealants fall into two major categories, biostable and bioresorbable. In many cases the bio-derived sealants utilize synthetic components in their formulation.
Bio-derived biostable materials have been developed, such as the early material known as GRF (gelatin resorcinol formaldehyde) adhesive used to treat aortic dissections (Chao and Torchiana, 2003). BioGlue® is an albumin–glutaraldehyde adhesive in commercial use today for sealing aortic dissections and vascular prosthesis–tissue junctions among other applications.
Bio-derived bioresorbable adhesives include mainly ‘fibrin glues’ which have been in use for over 100 years (Spotnitz, 1996), indicating a longstanding need for surgical sealants and adhesives. Tisseel® is generally considered a fibrin adhesive, but is actually indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent fluid leakage, for example, from colonic anastomoses following the reversal of temporary colostomies (Baxter Healthcare Corp, 2009b).
Hybrid adhesives or sealants are based on synthetic protein-based materials. Such materials include adhesives based on mussel glue (Brubaker et al., 2010; Lee et al., 2011) and a silk-elastin protein polymer (Capello, 1997; Rabotyagova et al., 2011). These technologies have long appeared promising as adhesives and sealants, although none have yet emerged from the laboratory to commercialization. Several other novel hydrogel materials are in various stages of research or development currently (Mai-ngam et al., 2005; McCrea et al., 2011; McKerracher, 2011; Murakami et al., 2009; Wang et al., 2010).
16.3 Definitions and general considerations
• Adhesive: agent that bonds with a surface or between two surfaces, for example tissue to tissue, or tissue to biomaterial.
• Sealant: agent that bonds to tissue surrounding an opening, such as a wound or incision, to halt the ingress or egress of liquid or gas.
• Adherend/substrate: material containing a surface to be bonded.
• Bond: area of contact between two surfaces that requires a force to separate.
• Bond strength (adherence): force to separate two surfaces at the bond.
• Adhesion: force required to separate two materials based on chemical interactions.
• Mechanical interlock: contribution to bond strength based on interdigitation of adhesive and adherend surfaces. (Cohesive failure of one or the other material determines force of mechanical interlock.)
• Cohesive failure: failure within the structure of the adherend or adhesive.
• Adhesive failure: failure at the interface of the adherend and adhesive.
16.4 Mechanisms of bonding
The strength of bonding between the adhesive or sealant and the tissue is determined by the mechanism of bonding. Several mechanisms of bonding for adhesives to adherend have been found to occur under various circumstances. Bond forces can be derived from several physical or chemical interactions and are most often a combination of mechanisms as listed below:
Mechanical interlock is the classical adhesion mechanism employed in general adhesives, and it is the most prevalent mechanism of adhesion for surgical adhesives and sealants. Mechanical interlock, depicted in Fig. 16.4, relies on the irregularities of the tissue-surface topography. When good intimate tissue content is achieved, the interdigitation of the tissue surface with the in situ solidified adhesive provides the mechanical interlock. Surface contaminants such as blood or other fluids can cause a weak boundary layer, effectively blocking access of the sealant to the tissue surface (Browdie and Cox, 2007). Contaminants can collect in depressions of the tissue surface as depicted in Fig. 16.4. This type of bond can be extremely strong, with bond separation tests usually exhibiting cohesive failure, where the tissue–material bond strength exceeds the strength of the adhesive material itself. The strength of this type of adhesion varies with the tissue type being bonded. In general, tissue having more irregular topography provides a stronger bond to the adhesive. Injured tissue where sub-epithelial tissue is exposed has been found to provide the best adherend (Reul and Walsh, 2005). This de-epithelialized tissue is typically found adjacent to incisions and wounds, or in areas of dissection, etc. Incisions or tissue injuries of different types, for example scalpel blade or electro-cautery, provide a wide variation in de-epithelialized surface area.
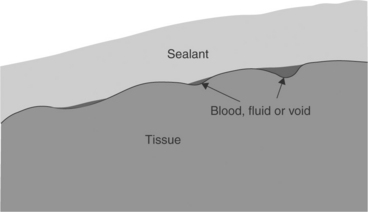
16.4 Tissue surface should be clean and dry to avoid weak boundary layers formed by collections of fluid, foreign matter, air voids, etc.
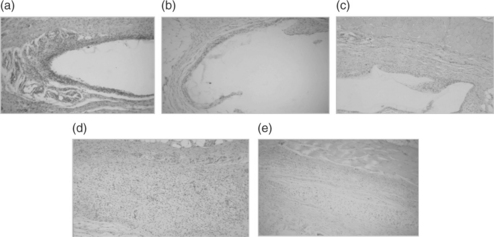
In addition to promoting mechanical interlock, the increased surface area of contact of irregular or roughened surfaces provides enhanced opportunity for other bonding forces to act in concert with mechanical interlock at the interface. In all cases surface preparation and application procedure are key components to achieving good bond strength. Figure 16.5 is a histological section showing a hydrogel sealant (FocalSeal S, a Dural Sealant developed by Focal, Inc.) (Alleyne et al., 1998) that has established a good mechanical interlock bond with the tissue surface.
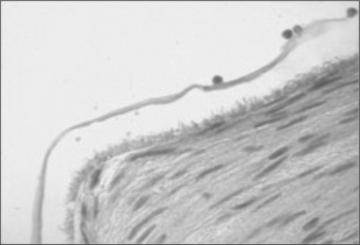
16.5 Stained tissue section showing FocalSeal S sealant on the luminal surface of an endothelium denuded artery. The irregularities of the sealant–tissue interface illustrate mechanical interlock bonding.
Some surgical procedures result in broad areas of exposed tissue, such as areas of dissection, which provide excellent areas for bonding if clear of blood or other contaminating fluids. Care must be taken in these tissues, however, to avoid interference with the healing process.
Most adhesives and sealants are not designed to promote rapid tissue in-growth and may interfere with healing. Bioresorbable sealants and adhesives are designed to gradually lose strength and mass over time. The strength and mass loss rate of the sealant must be considered in developing a surgical procedure that incorporates sealants applied to broad areas.
16.5 Application methods
The method used to apply a tissue adhesive or sealant is an important component to achieving a good bond. Applicators provide a method for conveying the material to the site as well as to prepare the site. Some agitation of sealant or adhesive on the surface improves the ability to achieve good intimate contact with the tissue by displacing or incorporating any fluid or foreign matter on the tissue surface. Brushing and spraying are the most commonly used methods to provide agitation at the interface.
Considerations for tissue surface preparation:
• Clean surface: The surface should be clear of any blood clots and other loose material.
• Fluid presence: The presence of fluid at the tissue site can interfere with the adhesive either by dilution at the interface – for water-based or water-soluble adhesives – or by creating a boundary layer trapped between the adherend and adhesive (Fig. 16.4). Any fluid at or near the site of application should be removed before the adhesive is applied. Alternatively, the sealant or adhesive formulation can be designed to function with some dilution by liquid without excessive loss of properties.
• Fluid flow: Bleeding or fluid infiltration can dilute water-based or water-soluble adhesives and can severely inhibit contact bonding of hydrophobic polymers. Flowing fluid can also dislocate or wash away the adhesive before solidification can occur. Scrupulous efforts to halt fluid flow should be made prior to application of the adhesive. In some cases it is possible to temporarily stop or reduce flow long enough for a fast-setting sealant to obtain sufficient adhesion to contain further fluid egress.
• Injured tissue: Adherence to intact epithelium is often difficult to achieve due to smooth surfaces that do not promote mechanical interlocking. Procedures such as dissection or electro-cautery result in roughened, dry surfaces at the wound margins that promote mechanical interlocking. Adherence to the injured tissue at the wound edges, therefore, is often stronger than adjacent uninjured tissue. In preparing a wound, efforts should be made to clean injured tissue and ensure good intimate contact with the adhesive or sealant to achieve the strongest possible bond. The injured layer of tissue is usually stable during the healing process and healing proceeds normally in the healthy tissue below and adjacent to the injury. Of course, the presence of necrotic tissue elicits an inflammatory response. Inflammation may cause significant local concentrations of oxygen-derived free radical species that can degrade biomaterials (Coury, 2004).
16.6 Synthetic bioresorbable sealants
The synthetic bioresorbable materials have been the fastest growing segment of surgical tissue sealant and adhesive technologies with several new sealants entering the market in the US and Europe in the last few years. This is primarily due to their biocompatibility, cost effectiveness, reproducibility and ease of manufacturing. In addition, synthetic materials can be tailored to achieve properties best suited to the surgical application for which they are designed.
First among the synthetic bioresorbable sealants are the polyethylene glycol-based sealants. The polyethylene glycol (PEG) based sealants are specialized to achieve properties best suited to the intended surgical procedure. Typical properties that are manipulated are:
• Gel time – the time required for gelation to occur. The gel time can vary from nearly instantaneous to minutes. The choice of gel time is usually a function of how it will be applied by the surgeon. A spray system is best suited to a fast gel time, whereas slower gel times are required for more meticulous application requirements such as ocular surgery or minimally invasive dural sealing. Viscosity control is mandatory to achieve optimal application properties for the various techniques.
• Swelling – the degree the gel swells once it is fully cured, usually over a specified period of time while soaking, for example in phosphate buffered saline (PBS) solution at 37 °C. In situations where the sealant is applied in confined spaces, such as in spinal surgery, low swelling is required to avoid unnecessary pressure on local tissues that may be injured such as nervous tissue. Clinical cases have occurred in which high-swelling materials used in the spine have resulted in patient injury (Mulder et al., 2009; Epstein, 2010; Thavarajah et al., 2010). High swelling can also cause injury by desiccating attached tissue.
• Modulus – the stiffness of the gel is inversely proportional to its compliance. Matching compliance to the tissue adherend is important for maintaining good bonding. In addition, compliance mismatch can cause local stresses on tissue, which may be injurious or may cause undesirable tissue remodelling responses.
• Strength – The strength of the gel determines its ability to avoid cohesive failure. This is a greater concern during the period shortly following surgery when the sealant must bear the full responsibility for leak prevention. As healing occurs under the sealant, the fibrin clot and developing tissue matrix add their strength, which offsets the loss of strength of bioresorbable sealants over time.
• Degradation rate – The degradation rate of the adhesive or sealant should be sufficient to provide time for adequate healing to occur, but prolonged material persistence should be minimized. Persistence of the material at the site can be problematic if a prolonged inflammatory response occurs.
The primary method for manipulation of properties such as swelling and modulus is via the structure of the crosslinked network. Several strategies have been employed in network design; specific strategies include PEG functionality (number of reactive end groups per molecule), crosslinker functionality, segmented PEG with hydrophobic and hydrophilic segments.
PEG is synthesized by the living polymerization of ethylene oxide, which provides the ability to precisely control molecular weight. In addition, these PEGs have very narrow molecular weight distributions, virtually monodisperse, which provides good control over the molecular network of the gel formed from PEG macromonomer. The structure of the macromonomer can be controlled to have a defined number of terminal ends.
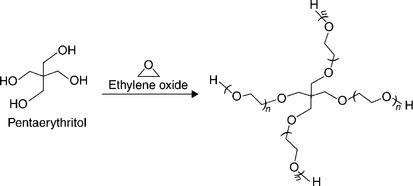
Multi-armed or branched PEG molecules can be made with two to several terminal ends that can be modified to provide reactive end groups. In these structures the ethylene oxide polymerization is initiated with a multifunctional molecule to form multiple arms of equal length; the number of arms is determined by the functionality of the initiator, for example pentaerythritol (four arms) (Fig. 16.6) or hexaglycerol (eight arms) (Harris and Zalipski, 1997).
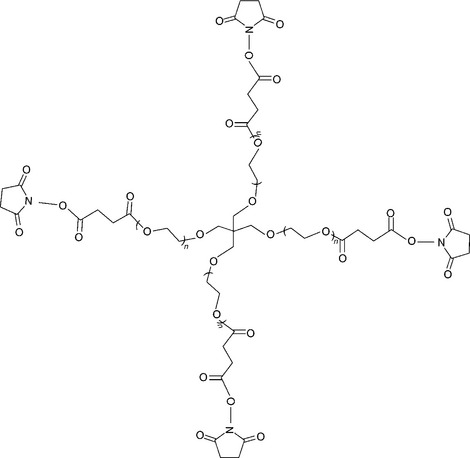
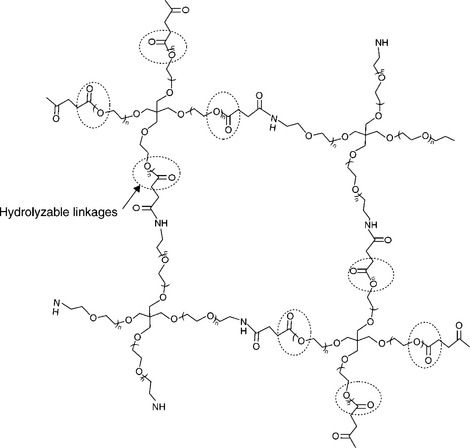
Reactive end groups are affixed to the PEG termini (Fig. 16.7). The reactive end groups are then used to crosslink the PEG molecules to form a molecular network. The branches or ‘arms’ of the PEG, combined with the molecular weight between crosslinks (Mc), determine the architecture and the crosslink density of gel. The moieties between crosslinks contain hydrolyzable linkages such as ester or carbonate groups for controlled degradability. Figure 16.7 depicts a PEG molecule bearing succinimidyl esters as hydrolyzable sites. Crosslink density is a controlling factor for properties such as modulus, swelling and degradation rate (Coury, 2004).
Dendritic molecular architectures (Grinstaff, 2007) employ a similar strategy to the multi-armed PEG structures, but accomplish it in a slightly different way – using multi-functional amines crosslinked with di-functional PEG. Dendritic structures progressively branch as the molecule grows from a central initiator by adding multifunctional segments to form a multiple of molecular termini with each successive ‘generation’ of branches. Once the desired number of termini is reached the ends are modified to enable crosslinking to form a gel. Dendritic amines crosslinked with difunctional reactive PEG molecules have been employed commercially (Wathier et al., 2005). Also, polyamines such as branched polyethyleneimines (PEI) have been used to form multifunctional reactants in a similar fashion (Carnahan and Butlin, 2010).
Segmented PEG architectures have been made which bear hydrophilic PEG segments and hydrophobic segments in a linear or branched arrangement (Sawhney et al., 2001). The hydrophobic segments provide a way to use secondary associative forces to achieve control over properties such as swelling and modulus. The hydrophobic segments can also contain hydrolyzable linkages to provide a mechanism for resorbability of the sealant.
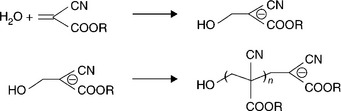
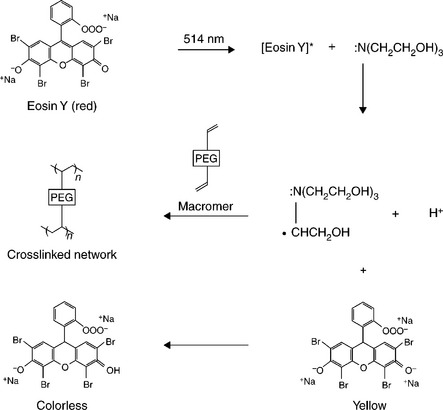
The crosslinking mechanism for these PEG architectures can be any high-efficiency reaction that does not produce toxic side (reaction) products. End groups such as acrylates or methacrylates provide double bonds, which act as sites for addition reactions. Figure 16.8 is a diagram of the macromonomer used in FocalSeal L (Genzyme), an FDA approved lung sealant (Focal Inc., 2000). Free radical polymerization is used to crosslink these groups (Sawhney et al., 1994). The free radical polymerization method can be accomplished by different chemical initiation schemes such as Fenton’s reaction (ferrous plus hydroperoxide form radical initiators) or by use of an energy source (e.g. UV light or heat) to activate a free radical initiator. Light activated polymerization of FocalSeal L is accomplished by a reaction scheme (Fig. 16.9) that utilizes visible light-sensitive dye to initiate the polymerization reaction.
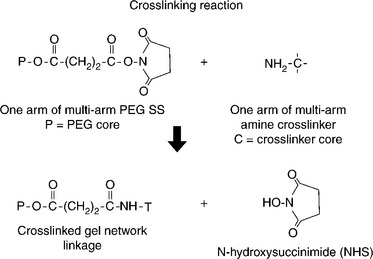
Another crosslinking method that has been employed is the reaction of electrophilic and nucleophilic groups (Bennett et al., 2003). Electrophilic groups such as N-hydroxysuccinimidyl (NHS) esters have been paired with nucleophilic groups such as primary amines or thiols to enable nucleophilic substitution reaction crosslinking. Figure 16.10 shows the crosslinking reaction between a multi-armed PEG terminated with N-hydroxysuccinimidysuccinate end groups when reacted with a multi-armed amine. In this case each crosslink results in the liberation of one NHS molecule, a water-soluble and non-toxic by-product. Double bonds have also been used as sites for crosslinking via Michael addition of nucleophiles such as thiols (Metters and Hubbell, 2004). A schematic structure of a crosslinked hydrogel formed from a four-armed PEG-NHS and a four-armed PEG amine is depicted in Fig. 16.11.
16.6.1 Bioresorption of synthetic hydrogel sealants
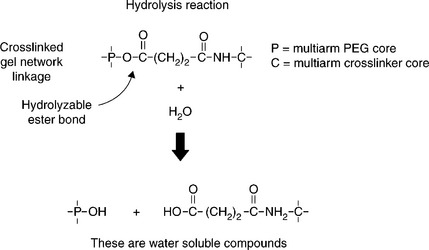
Degradation of synthetic bioresorbable hydrogel sealants or adhesives is accomplished by incorporation of a cleavable linkage (see Fig. 16.11). The linkages used with nearly all commercial synthetic resorbable materials are hydrolyzable esters. Esters are used because the rate of hydrolysis can be well controlled by the structure of the ester linkage or segments of ester linkages selected. Figure 16.12 shows a schematic of the hydrolytic degradation mechanism of a hydrogel having strategically placed ester linkages. The molecular network forms the structural members of the hydrogel, and incorporation of hydrolyzable linkages provides a means for disintegration of the molecular network. As these linkages are cleaved the gel weakens evenly throughout its bulk. This type of degradation is often termed ‘bulk degradation’ and is characterized by four phases. The first phase involves swelling to equilibrium accompanied by a proportionate loss of modulus. The second phase involves the gradual cleavage of hydrolytically labile bonds with a corresponding growth in volume and additional loss of modulus and strength. The third phase, actually a continuation of the second phase, shows an acceleration of swelling as the gel’s molecular network approaches disintegration. The final phase involves mass and volume loss until complete dissolution occurs (Fig. 16.13). Where compression of sensitive tissues in confined spaces is a concern, such as in spinal dura sealing (Mulder et al., 2009; Epstein, 2010; Thavarajah et al., 2010), the surgeon must consider the volumetric changes as well as the material property loss progression during the sealant residence time.
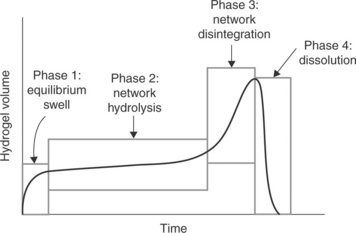
16.13 Phases of synthetic hydrogel degradation by hydrolysis. Hydrogel sealants swell, soften and weaken after implantation. This process generally follows a sequence of four phases. The surgeon should consider the volumetric changes as well as the material property loss progression during the sealant residence time.
Some biomaterials are designed for ‘surface erosion’, in which the material shrinks like a lozenge. Polymers exhibiting this type of behaviour are hydrophobic and generally contain freely hydrolyzable groups such as anhydrides so that degradation occurs at a rate faster than water permeation (Dang et al., 1996). The series of photomicrographs in Fig. 16.14 track the resorption of FocalSeal-S, a sealant developed for cranial dural incisions, over 60 days after subcutaneous implantation in rats. As the implant undergoes bulk degradation with solubilization of the degradation products, the implant shrinks, with tissue growth advancing as the material surface retreats. A mild inflammatory response to the degradation products is evidenced by macrophages present around the gel borders.
16.7 Commercial resorbable synthetic sealants and adhesives
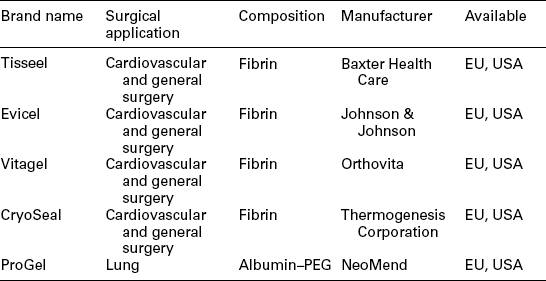
The availability of commercial sealants and adhesives has grown significantly in recent years. An impressive list of materials for use in various surgical applications is shown in Table 16.1. A brief description of each product is given below.
Table 16.1
Commercial bioresorbable synthetic tissue adhesives and sealants
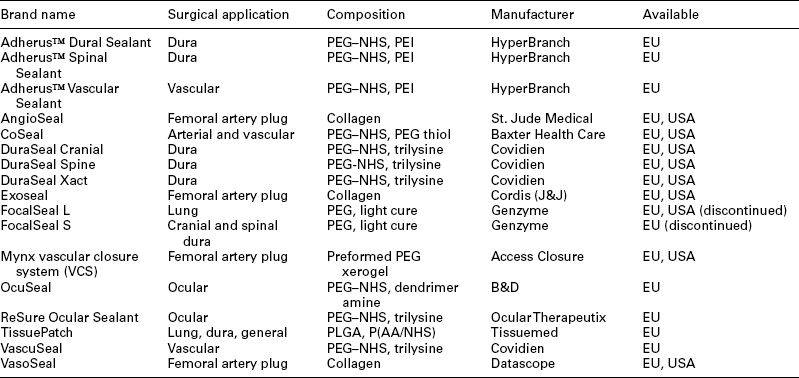
P(AA/NHS) refers to NHS modified poly (acrylic acid) and PLGA refers to a lactide-glycolide copolymer.
16.7.1 DuraSeal Dural Sealant System (Covidien)
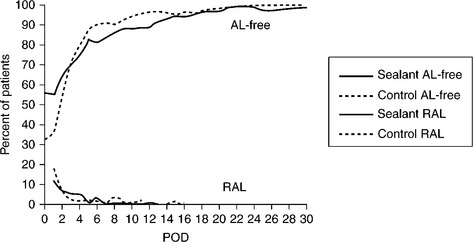
The DuraSeal Dural Sealant System consists of components for preparation of a synthetic absorbable sealant, and an applicator for delivery of the sealant to the target site (Fig. 16.15). The sealant is composed of two solutions, a high molecular weight polyethylene glycol (PEG) ester solution and a low molecular weight trilysine amine solution (referred to as the ‘blue’ and ‘clear’ precursors, respectively). When mixed together, the precursors crosslink to form the hydrogel sealant. The mixing of the precursors is accomplished as the materials exit the tip of the spray applicator. The hydrogel implant is absorbed in approximately four–eight weeks, sufficient time to allow for dural healing. The DuraSeal Dural Sealant System is indicated for use as an adjunct to sutured dural repair during cranial surgery to provide watertight closure (Cosgrove et al., 2007). Clinical testing was conducted in Europe (Boogaarts et al., 2005) in 46 patients (in cranial and spinal dural incisions) and in the United States (Cosgrove et al., 2007) in 111 patients (cranial) with demonstrated CSF leaks. In both studies 100% of patients were without leaks after sealing.
The DuraSeal material has also been adapted to spinal-cord sealing (DuraSeal Spine, Fig. 16.16) with the aid of a gas-assisted spray-delivery system (Wright et al., 2009). Clinical-sealing results for the US DuraSeal Spine study were significant: 100% watertight in the sealant group (102/102) vs 64% (36/56) in the standard of care group.
16.7.2 FocalSeal L (Genzyme)
FocalSeal L was approved as a lung sealant in the US in 2000 (Wain et al., 2001). The FocalSeal L product is shown in Fig. 16.17. It consisted of:
• a primer – a lyophilized powder, a diluent solution and a flow-through brush applicator;
• a sealant – a frozen solution in a syringe and a flow-through brush applicator;
• a xenon light source and a sterilizable fiber optic light wand.
Although the product performed well for its intended use, it was withdrawn from the market in 2003 due to a combination of factors including high manufacturing cost, slow adoption and a change in company business direction.
In this system a multiblock copolymer macromonomer (Fig. 16.7) composed of PEG and trimethylene carbonate formed a sealant in situ upon exposure to a high-intensity blue light (480–520 nm). Eosin Y and triethanolamine act together as a photoinitiator for the macromonomer polymerization reaction. The reaction scheme is shown in Fig. 16.8. A xenon light source was used to direct light through a sterile reusable fibre-optic wand to the wound site. Gelation occurred in a few seconds, although full strength was achieved by continuing illumination for approximately 40s.
The molecular structure of the macromonomer provided excellent elasticity and strength, allowing the material to function as a lung sealant where continuous expansion and contraction occur. To enhance bonding to lung tissue, a primer was included in FocalSeal L. The primer was a lower molecular weight PEG macromonomer with a lower viscosity and a higher concentration. The higher concentration allowed the primer to accommodate dilution from surface moisture and lower viscosity allowed improved penetration into tissue micro-topological features. The primer was designed to be applied in a thin layer just prior to application of the sealant layer. Before the light initiated polymerization reaction, a Fenton initiation reaction was employed to form a thin layer of gel at the tissue interface upon mixing the primer with the sealant on the tissue. Intermingling of the primer and sealant on a molecular level prior to gelation provided covalent coupling to sealant macromonomer. Application of additional sealant was followed by illumination to drive the photopolymerization reaction.
16.7.3 FocalSeal S (Genzyme)
FocalSeal S was approved as a dural sealant in the EU (Alleyne et al., 1998). The FocalSeal S product was similar to FocalSeal L, but was designed specifically for minimal swelling in the cranial and spinal area.
16.7.4 OcuSeal Liquid Ocular Bandage (B&D)
OcuSeal™ Liquid Ocular Bandage (Fig. 16.18) is intended for direct application on the surface of the eye to form an ocular bandage, thereby covering an opening in the eye and providing an immediate protective barrier (Johnson et al., 2009). This material uses a multi-armed polyamine that is crosslinked with a difunctional PEG N-hydroxysuccinimidylsuccinate. The material is provided in a multicomponent applicator that is assembled prior to use. The contents of two compartments in the applicator are mixed together to initiate the gelation reaction. The elongated gel time allows passage of mixed material through a flow-through brush, which aids corneal application. Gelation occurs on the tissue surface.
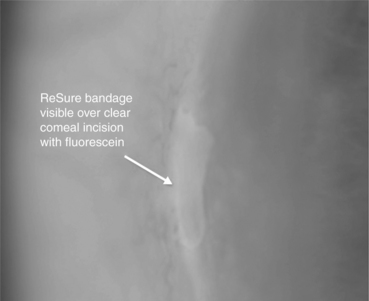
16.7.5 ReSure Ocular Bandage (Ocular Therapeutix)
ReSure Ocular Bandage (Fig. 16.19) is intended for covering ocular incisions and wounds on the surface of the eye. It is available in Europe, but is not currently available in the United States. The technology is similar to the DuraSeal Sealant described above. The system consists of components for preparation of the synthetic absorbable bandage, and an applicator for delivery of the sealant to the target site. The sealant is composed of two solutions, a multifunctionalized PEG ester solution and a tetrafunctional trilysine amine solution (referred to as the ‘blue’ and ‘clear’ precursors, respectively). When mixed together, the precursors crosslink to form the hydrogel sealant. A drop of each component is mixed together with the applicator just prior to use.
16.7.6 CoSeal Surgical Sealant (Baxter Health Care)
CoSeal Surgical Sealant (Glickman et al., 2002), for peripheral vascular reconstruction, is composed of two synthetic polyethylene glycols (PEGs), a dilute hydrogen chloride solution and a sodium phosphate/sodium carbonate solution (Fig. 16.20). These components come in a kit that includes an applicator(s). CoSeal is composed of (Hill et al., 2001) a four-arm PEG have been functionalized with N-hydroxysuccinimdylglutarate and a four-armed PEG thiol. Each has a molecular weight of approximately 10000 Da. Before use the polymers are dissolved separately in their respective phosphate/carbonate buffer or hydrogen-chloride solutions. Each solution is then drawn into a syringe and assembled into a delivery device. At the time of administration, the mixed PEG solutions form a hydrogel that adheres to tissue and synthetic graft materials (Baxter Healthcare Corp, 2003).
16.7.7 Mynx vascular closure system (VCS) (Access Closure, Inc.)
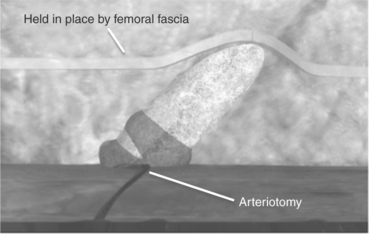
Mynx is indicated for use to seal femoral arterial access sites (Access-Closure, Inc., 2010), reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional endovascular procedures (Scheinert et al., 2007). The sealant is based on a freeze-dried polyethylene glycol (PEG) ‘xerogel’ that instantly expands in the tissue tract upon contacting blood and tissue fluids, forming a hydrogel clot (see Fig. 16.21). The sealant then dissolves within 30 days, leaving a healed artery.
16.7.8 AngioSeal
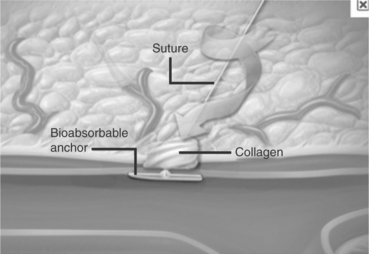
(St. Jude Medical) The Angio-Seal™ vascular closure device consists of an intravascular bioresorbable anchor and an extravascular bovine collagen sponge, which are connected by an absorbable synthetic suture. Figure 16.22 is an illustration of the deployment of the device (Abando et al., 2004).
16.7.9 Exoseal (Cordis, J&J)
The Exoseal vascular closure device (Wong et al., 2009) is a wound fibrous plug composed of poly(glycolic acid) (PGA) fibre. PGA is a bioresorbable material commonly used in bioresorbable sutures such as Dexon (Covidien). This device relies on blood clotting in the fibrous plug to form a seal. Figure 16.23 is an illustration of the device after implantation.
16.7.10 Adherus™ (HyperBranch)
HyperBranch has introduced a series of sealant products into Europe and has a clinical trial under way in the US (HyperBranch, 2010). The hydrogel is formed by reaction of a branched polyethyleneimine bearing multiple primary amines with an NHS-activated PEG. The resulting sealant is resorbed over approximately 90 days (Carnahan and Butlin, 2010).
The Adherus Dural Sealant is used (in the EU) as an aid in dural repair in cranial surgeries such as the treatment of intracranial aneurysms, tumours and spinal-disc disease.
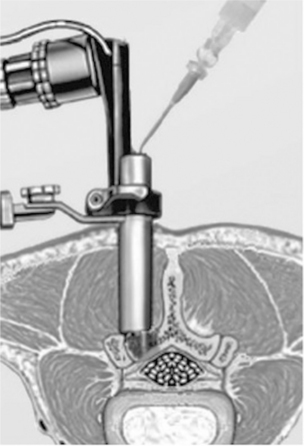
The Adherus Spinal Sealant is used (in the EU) as an aid in dural repair in minimally invasive spinal surgeries. This product is additionally claimed to act as an adhesion barrier. It is delivered by an applicator that is designed for endoscopic spinal procedures (Fig. 16.24).
HyperBranch’s Adherus Vascular Sealant mechanically seals the sutured anastomosis sites of blood vessels and artificial grafts to prevent blood leakage. It is applied from a ready to use delivery system.
TissuePatch (Tissuemed, Leeds UK) is a surgical sealant film with EU approval for general surgery, lung and dural applications (Puppa et al.,2010; Ferroli et al., 2011). The technology is based on a bioresorbable poly(lactide-co-glycolide) (PLGA) film laminated with a film of poly(acrylic acid-co-N-vinylpyrrolidone) partially substituted with N-hydroxysuccinimide (NHS). The NHS groups provide reactive groups that form covalent linkages with nucleophilic species on the tissue surface, specifically targeting proteins bearing primary amines.
Three variants of TissuePatch have been developed: TissuePatch3, TissuePatchDural and TissuePactchThoracic. TissuePatch3 is indicated for general and thoracic sealing. TissuePatchDural and TisuePatchThoracic are designed specifically for dural and thoracic sealing respectively. The patch is applied by pressing the reactive side of the laminate against the tissue for at least 30s. The stated mode of action (Tissuemed, 2012) is the formation of ionic and covalent interactions with the tissue. The material gradually degrades and resorbs over about 50 days.
16.8 Commercial resorbable bio-derived sealants and adhesives
This group of materials consists of two principal types: fibrin glue and albumin crosslinked with functionalized PEG (see Table 16.2).
16.8.1 Fibrin sealants
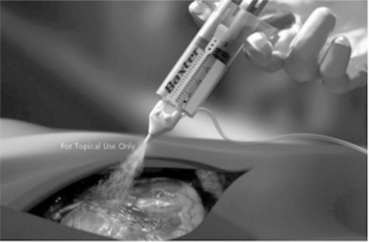
Fibrin sealants are composed of several components that are combined as two solutions to form the two-part adhesive preparation. The main components, fibrinogen and thrombin, are produced from pooled human blood. In many formulations Factor XIII, a crosslinking agent also from human origin, is included. The leading product in this sector is Tisseel (Baxter Healthcare Corp, CA), which is sold in different formats. The most advanced and easy to use format is the Tisseel Duo product with frozen pre-filled syringes (Fig. 16.25).
The Tisseel pre-filled syringe (frozen) with DUO Set contains a sealer protein solution and a thrombin solution with compositions as follows (Baxter Healthcare Corp, 2009b):
Mixing the sealer and thrombin solutions in equal proportions initiates the clotting cascade. A two-syringe sprayer can also be used to mix and spray, which is preferred when applying a thin layer over a large area.
Fibrin sealants are useful for their hemostatic properties. The resorption is a result of natural fibrinolysis, which can be controlled by formulation. The bond strength to internal tissues is limited when compared with synthetic sealants and adhesives.
16.8.2 PEG/albumin – NeoMend
NeoMend Inc. ‘ProGEL™ Surgical Sealant’ is intended as an adjunct to standard tissue-closure techniques for sealing or reducing air leaks incurred during pulmonary surgery (Neomend Inc., 2010). ProGEL is a two component hydrogel sealant, consisting of a human serum albumin solution and difunctional PEG N-hydroxysuccinimidylsuccinate, a dried powder reconstituted with sterile water. Mixed at the time of application, the liquid components quickly cure in situ to form a flexible patch for sealing and/or reducing air leaks in the lung (NeoMend Inc., 2012). After application, the body resorbs the patch material within 28 days. This material was tested clinically (Fig. 16.26), showing a marginal benefit in leak rates.
16.9 Commercial biostable bio-derived sealants and adhesives
This class of materials uses at least one component that is biologically derived, such as albumin or gelatin. Although these products contain natural components, the extensive reactions used to effect gelation render them biostable. BioGlue, for example, uses bovine serum albumin, but then crosslinks it by reaction with glutaraldehyde (Chao and Torchiana, 2003), creating a new chemical species that is unrecognizable by proteolytic enzymes. Depending on the degree of crosslinking, biodegradation or biodeterioration of such materials by enzymes is therefore minimal, that is, the material is biostable. There are two commercial products currently available in this class of adhesives/sealants as shown in Table 16.3.
16.9.1 Biological surgical G.R.F (MicroVal)
Gelatin-resorcinol-formaldehyde-glutaraldehyde (GRFG) is a contemporary version of an early bio-derived adhesive that uses gelatin and resorcinol crosslinked by formaldehyde. In this system, gelatin and resorcinol are dissolved in water and a second solution containing dissolved formaldehyde and glutaraldehyde initiates crosslinking when added. The mechanism of curing has not been fully described, but it is likely the gelatin and resorcinol do not intercrosslink, rather forming an interpenetrating network (IPN), each separately reacting with aldehyde.
The degree of crosslinking to achieve gel formation is low, so although aldehyde is largely consumed by crosslinking, presence of a small amount of residual aldehyde is likely. Formaldehyde, in particular, has known negative toxicity and carcinogenicity profiles, so partial or complete replacement of formaldehyde with less toxic aldehydes such as glutaraldehyde and glyoxal have been explored. The GRF adhesives have been surpassed by more advanced and less toxic materials. These alternative aldehydes are still quite toxic to nervous and ocular tissue and can cause local tissue necrosis and inflammation. Gluetiss® (Berlin Heart), which uses both glutaradehyde and glyoxal, was originally indicated for treating aortic dissections and remains commercially available in some countries.
16.9.2 BioGlue (CryoLife)
A tissue adhesive in current use with some similarities to GRF is the commercially available BioGlue® Surgical Adhesive (Fig. 16.27) (Chao and Torchiana, 2003; Friedman and Schalch, 2008; Rathinam et al., 2009; Chauvet et al., 2011). It is a two-component formulation composed of solutions of purified bovine serum albumin (BSA) and glutaraldehyde that mixes as it is extruded through a static mixer. The material reaches adequate strength in 2 min (Cryolife Inc., 2010). Originally approved for aortic dissections by the FDA under the Humanitarian Device Exemption (HDE) in 1999, BioGlue was later approved for vascular anastomosis sealing (CryoLife, Inc., 2001). The glutaraldehyde is claimed to react with tissue proteins in addition to the BSA, forming covalent adhesive-tissue bonds. Company literature (CryoLife, 2012) explains that BioGlue degrades via proteolysis and that it can be slow to resorb dependent on the quantity of adhesive applied. No reference could be found, however, demonstrating the material is actually resorbable so it is classified herein as biostable. As in the case of GRF, the toxicity of BioGlue is a serious concern as glutaraldehyde is toxic to ocular and nervous tissue.
16.10 Commercial biostable synthetic sealants and adhesives
Synthetic biostable adhesives function by undergoing a physical change from liquid to solid on the tissue surface, thereby engaging a physical bond with the underlying tissue surface irregularities. In the case of cyanoacrylate, this transformation is a moisture induced polymerization of the cyanoacrylate monomer. Biostability refers to the irreversibility of the transformation. Biostable materials are characterized by the lack of degradable molecular linkages that can be exploited to reduce crosslinking or molecular weight. The use of such materials should be limited to topical areas where the material eventually falls or wears off.
16.10.1 Cyanoacrylates
Cyanoacrylates are indicated for topical skin-closure applications, but have been used as adhesives in other areas where unusual circumstances or emergency situations presented, such as battlefield injuries and some ophthalmology procedures (Chan and Boisjoly, 2004). Cyanoacrylates present toxicity concerns due to three factors: local heat generation due to the highly exothermic polymerization reaction, residual monomer and hydrolytic degradation products. Internal use of cyanoacrylates can result in inflammatory response, delayed healing and necrosis. While low molecular weight side chain cyanoacrylates such as ethyl-2-cyanoacrylate degrade via hydrolysis, toxic degradation products such as formaldehyde are liberated, potentially causing local tissue toxicity (Webster and West, 2002). Higher molecular weigh side chains, such as n-butyl and n-octyl, are employed in commercial topical sealants due to their hydrolytic stability, higher viscosity and reduced exotherm from polymerization. These higher molecular weight variants overcome much of the toxicity concerns, but at the cost of becoming biostable, making them unsuited to internal use. The commercial cyanoacrylate adhesives are listed in Table 16.4.
16.11 Conclusion
The difference between adhesives and sealants is fundamental to the material-design considerations for the device. Adhesives can either join tissues together at the top edges or by being interposed between the separated tissue surfaces. In the latter case, the treatment must be evaluated for its potential to delay healing. Sealants, which cover a leaking wound, but do not penetrate the wound, must be evaluated for their ability to withstand pressure from these fluids, both along the sealant/tissue interface and through the sealant bulk. The chemistry of the in situ solidification reaction must be evaluated for local chemical or thermal effects on the tissue. Recent advances in synthetic bioresorbable materials, especially hydrogels, offer surgeons new opportunities to speed surgery and improve outcomes.
16.12 Sources of further information and advice
Anderson, J. Inflammation, wound healing and the foreign-body response. In Ratner B., Hoffman A., Lemons J., eds.: Biomaterials Science: An Introduction to Materials in Medicine, 2nd, Amsterdam; Boston: Elsevier Academic Press, 2004.
Anger, W.H. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. AORN. 2010; Vol. 92(3):351–352.
Avalos-González, J., Portilla-deBuen, E., Leal-Cortés, C.A., Orozco-Mosqueda, A., del Carmen Estrada-Aguilar, M., Velázquez-Ramírez, G.A., Ambriz-González, G., Fuentes-Orozco, C., Guzmán-Gurrola, A.E., González-Ojeda, A. Reduction of the closure time of postoperative enterocutaneous fistulas with fibrin sealant. World Journal of Gastroenterology: WJG. 2010; Vol. 16(22):2793.
Bachet, J., Goudot, B., Dreyfus, G.D., Brodaty, D., Dubois, C., Delentdecker, P., Guilmet, D. Surgery for acute type A aortic dissection: the Hôpital Foch experience (1977–1998). Annals of Thoracic Surgery. 1999; Vol. 67(6):2006–2009.
Berdahl, J.P., Johnson, C.S., Proia, A.D., Grinstaff, M.W., Kim, T. Comparison of sutures and dendritic polymer adhesives for corneal laceration repair in an in vivo chicken model. Archives of Ophthalmology. 2009; Vol. 127(4):442–447.
Bhatia, S.K., Arthur, S.D. Poly(vinyl alcohol) acetoacetate-based tissue adhesives are non-cytotoxic and non-inflammatory. Biotechnology Letters. 2008; Vol. 30(8):1339–1345.
Birth, M., Figueras, J., Bernardini, S., Troen, T., Günther, K., Mirza, D., Mortensen, F.V., others. Collagen fleece-bound fibrin sealant is not associated with an increased risk of thromboembolic events or major bleeding after its use for haemostasis in surgery: a prospective multicentre surveillance study. Patient Safety in Surgery. 2009; Vol. 3(1):13.
Bitton, R., Bianco-Peled, H. Novel biomimetic adhesives based on algae glue. Macromolecular Bioscience. 2008; Vol. 8(5):393–400.
Black, J.Biological Performance of Materials Fundamentals of Biocompatibility. Boca Raton, FL: Taylor & Francis, 2006.
Box, G.N., Lee, H.J., Abraham, J.B.A., Deane, L.A., Santos, R.J.S., Elchico, E.R., Khosravi, A., Abdelshehid, C.A., Alipanah, R., Li, K., Moskowitz, R.M., Philips, J.M., Edwards, R.A., Borin, J.F., McDougall, E.M., Clayman, R.V. Evaluation of the outcomes of electrosurgical induced bowel injury treated with tissue glue/sealant versus sutured repair in a rabbit model. Journal of Endourology. 2009; Vol. 23(3):535–540.
Buskens, E., Meijboom, M.J., Kooijman, H., Van Hout, B.A. The use of a surgical sealant (CoSeal) in cardiac and vascular reconstructive surgery: an economic analysis. Journal of Cardiovascular Surgery. 2006; Vol. 47(2):161–170.
Cabral, J., Moratti, S.C. Hydrogels for biomedical applications. Future Medicinal Chemistry. 2011; Vol. 3(15):1877–1888.
Chenault, H.K. Protein-based polymer tissue adhesives for medical use, 2010. [US 7837986].
Chenault, H.K., Figuly, G.D. Tissue adhesives with modified elasticity, 2008. [US 2008/0004421 A1].
Chew, A.C.Y., Tan, D.T.H., Poh, R., Htoon, H.M., Beuerman, R.W., Mehta, J.S. Effect of intracameral injection of fibrin tissue sealant on the rabbit anterior segment. Molecular Vision. 2010; Vol. 16:1087.
Confluent SurgicalDuraSeal Dural Sealant Instructions for Use. Confluent Surgical, 2005.
Confluent Surgical. SSED – DuraSeal Spine Sealant System – P080013, 2009.
Cordis CorpEXOSEAL Brochure. Cordis Corp., 2011.
Degoricija, L., Johnson, C.S., Wathier, M., Kim, T., Grinstaff, M.W. Photo cross-linkable biodendrimers as ophthalmic adhesives for central lacerations and penetrating keratoplasties. Investigative Ophthalmology & Visual Science. 2007; Vol. 48(5):2037–2042.
Duarte, A.P., Coelho, J.F., Bordado, J.C., Cidade, M.T. and Gil, M.H. n.d., ‘Surgical adhesives: Systematic review of the main types and development forecast’, Progress in Polymer Science, Available online 14 December 2011, viewed 26 March 2012, www.sciencedirect.com/science/article/pii/S0079670011001365.
Elvin, C.M., Brownlee, A.G., Huson, M.G., Tebb, T.A., Kim, M., Lyons, R.E., Vuocolo, T., Liyou, N.E., Hughes, T.C., Ramshaw, J.A.M., Werkmeister, J.A. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials. 2009; Vol. 30(11):2059–2065.
Elvin, C.M., Danon, S.J., Brownlee, A.G., White, J.F., Hickey, M., Liyou, N.E., Edwards, G.A., Ramshaw, J.A.M., Werkmeister, J.A. Evaluation of photo-crosslinked fibrinogen as a rapid and strong tissue adhesive. Journal of Biomedical Materials Research Part A. 2010; Vol. 93A(2):687–695.
Elvin, C.M., Vuocolo, T., Brownlee, A.G., Sando, L., Huson, M.G., Liyou, N.E., Stockwell, P.R., Lyons, R.E., Kim, M., Edwards, G.A., Johnson, G., McFarland, G.A., Ramshaw, J.A.M., Werkmeister, J.A. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials. 2010; Vol. 31(32):8323–8331.
Ethicon, IncEVICEL Fibrin Sealant (Human) package insert. Ethicon, Inc., 2009.
Figueras, J., Llado, L., Miro, M., Ramos, E., Torras, J., Fabregat, J., Serrano, T. Application of fibrin glue sealant after hepatectomy does not seem justified. Annals of Surgery. 2007; Vol. 245(4):536–542.
Floris, R., Salvatore, C., Fraioli, B., Pastore, F.S., Vagnozzi, R., Simonetti, G. Trans-sphenoidal treatment of postsurgical cerebrospinal fluid fistula: CT-guided closure. Neuroradiology. 1998; Vol. 40(10):690–694.
Fortune, D.H., Kettlewell, G., Mandley, D.J., Morris, D., Thompson, I. Tissue-adhesive materials, 2009. [US 2009/0044895 A1].
Fortune, D.H., Kettlewell, G., Mandley, D.J., Thompson, I., Cook, D. Tissue-adhesive formulations, 2010. [US 7727547].
Fransen, P. Reduction of postoperative pain after lumbar microdiscectomy with DuraSeal Xact Adhesion Barrier and Sealant System. The Spine Journal: Official Journal of the North American Spine Society. 2010; Vol. 10(9):751–761.
Fukuchi, T., Sueda, J., Usumoto, N., Okuno, T., Kalayci, D., Sawhney, A., Hirose, T. Hydrogel tissue adhesive: feasiblity study for intraocular use ERG and histology. Investigative Ophthalmology & Visual Science. 2003; Vol. 44(5):3015.
Geister Medizintechnik GmbH. GLUETISS Product Brochure, 2012. www.geister.com
Gong, G., Wartchow, C., Schlosser, A. Tissue adhesive compositions and methods thereof, 2010. [US 2010/0297218 A1].
Guilmet, D., Bachet, J., Goudot, B., Laurian, C., Gigou, F., Bical, O., Barbagelatta, M. Use of biological glue in acute aortic dissection: preliminary clinical results with a new surgical technique. Journal of Thoracic and Cardiovascular Surgery. 1979; Vol. 77(4):516–521.
Hadba, A.R., Stopek, J.B. Tissue adhesives and sealants and method for their use, 2010. [US 7858079].
Hammerslag, J.G., Quinn, J. Controlled viscosity tissue adhesive, 2009. [US 2009/0036921 A1].
Hidas, G., Kastin, A., Mullerad, M., Shental, J., Moskovitz, B., Nativ, O. Sutureless nephron-sparing surgery: use of albumin glutaraldehyde tissue adhesive (BioGlue). Urology. 2006; Vol. 67(4):697–700.
Jackson, M.R. Fibrin sealants in surgical practice: an overview. American Journal of Surgery. 2001; Vol. 182(2):S1–S7.
Jankowitz, B., Atteberry, D., Gerszten, P., Karausky, P., Cheng, B., Faught, R., Welch, W. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. European Spine Journal. 2009; Vol. 18(8):1169–1174.
Kim, K.D., Wright, N.M. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine. 2011; Vol. 36(23):1906–1912.
Koranyi, G. Cut and paste: a no suture, small incision approach to pterygium surgery. British Journal of Ophthalmology. 2004; Vol. 88(7):911–914.
Küçükaksu, D.S., Akgül, A., Çağli, K., Ta![]() demir, O. Beneficial effect of BioGlue® surgical adhesive in repair of iatrogenic aortic dissection. Texas Heart Institute Journal. 2000; Vol. 27(3):307–308.
demir, O. Beneficial effect of BioGlue® surgical adhesive in repair of iatrogenic aortic dissection. Texas Heart Institute Journal. 2000; Vol. 27(3):307–308.
Lim, K., Tan, L. Interaction force measurements for the design of tissue adhesives. Acta Biomaterialia. 2009; Vol. 5(1):84–92.
Lowinger, J., Lowinger, B., DeLustro, F., Cox, D., Browdie, D.A. Tissue adhesive sealant, 2009. [US 2009/0287313 A1].
Lu, H.S.M. Dextran-based polymer tissue adhesive for medical use, 2010. [US 2010/0255101 A1].
Lundblad, R.L.Application of Solution Protein Chemistry to Biotechnology. Boca Raton, FL: CRC Press/Taylor & Francis, 2009.
Lutolf, M.P., Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005; Vol. 23(1):47–55.
Mahdavi, A., Ferreira, L., Sundback, C., Nichol, J.W., Chan, E.P., Carter, D.J.D., Bettinger, C.J., Patanavanich, S., Chignozha, L., Ben-Joseph, E., others. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proceedings of the National Academy of Sciences. 2008; Vol. 105(7):2307.
Malapert, G., Hanna, H.A., Pages, P.B., Bernard, A. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. The Annals of Thoracic Surgery. 2010; Vol. 90(6):1779–1785.
Miller, S.H., Price, G., Buck, D., Neeley, J., Kennedy, T.J., Graham, W.P., 3rd., Davis, T.S. Effects of tourniquet ischemia and postischemic edema on muscle metabolism. The Journal of Hand Surgery. 1979; Vol. 4(6):547–555.
Mo, X., Iwata, H., Ikada, Y. A tissue adhesives evaluated in vitro and in vivo analysis. Journal of Biomedical Materials Research Part A. 2010; Vol. 94A(1):326–332.
Mortenson, M.M., Xing, Y., Weaver, S., Lee, J.E., Gershenwald, J.E., Lucci, A., Mansfield, P.F., Ross, M.I., Cormier, J.N., others. Fibrin sealant does not decrease seroma output or time to drain removal following inguino-femoral lymph node dissection in melanoma patients: a randomized controlled trial (NCT00506311). World Journal of Surgical Oncology. 2008; Vol. 6:63.
Oelker, A.M., Berlin, J.A., Wathier, M., Grinstaff, M.W. Synthesis and characterization of dendron cross-linked PEG hydrogels as corneal adhesives. Biomacromolecules. 2011; Vol. 12(5):1658–1665.
Osbun, J.W., Ellenbogen, R.G., Chesnut, R.M., Chin, L.S., Connolly, P.J., Cosgrove, G.R., Delashaw Jr., J.B., Golfinos, J.G., Greenlee, J.D.W., Haines, S.J., Jallo, J., Muizelaar, J.P, Nanda, A., Shaffrey, M., Shah, M.V., Tew Jr., J.M., van Loveren, H.R., Weinand, M.E., White, J.A. and Wilberger, J.E. n.d., ‘A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (duraseal dural sealant system) as a dural sealant in cranial surgery’, World Neurosurgery, published online 10 December 2011, viewed 25 March 2012, www.sciencedirect.com/science/article/pii/S1878875011014872.
Panda, A., Kumar, S., Kumar, A., Bansal, R., Bhartiya, S. Fibrin glue in ophthalmology. Indian Journal of Ophthalmology. 2009; Vol. 57(5):371.
Pathak, C.P., Barman, S.P., Philbrook, C.M., Sawhney, A.S., Coury, A.J., Avila, L.Z., Kieras, M.T. Multiblock biodegradable hydrogels for drug delivery and tissue treatment, 2001. [US 6201065].
Peng, H.T., Shek, P.N. Novel wound sealants: biomaterials and applications. Expert Review of Medical Devices. 2010; Vol. 7(5):639–659.
Preul, M.C., Campbell, P.K., Bennett, S.L., Muench, T.R., A unique dual-function device – a dural sealant with adhesion-prevention properties. European Musculoskeletal Review, 2006:41–44.
Redl, H., Khakpour, Z., Ariagno, S.R., Kellner, A., Zakarija, L.G. Hand triggered tissue sealant spray apparatus and system, 2009. [US 7537174].
Redl, H., Schlag, G., Eibl, J. Fibrinogen-based tissue adhesive containing an elastase inhibitor, 2011. [US 7892802].
Rehor, A. and Cerritelli, S. n.d., ‘Polymeric tissue sealant’, US 2008/0253987 A1.
Reynolds, M.W., Clark, J., Crean, S., Samudrala, S., others. Risk of bleeding in surgical patients treated with topical bovine thrombin sealants: a review of the literature. Patient Safety in Surgery. 2008; Vol. 2(1):5.
Sawhney, A.S., Lyman, M.D., Jarrett, P.K., Rudowsky, R.S. Compliant tissue sealants, 1999. [US 6177095].
Schwabbauer, M.L. Use of the latent image technique to develop and evaluate problem-solving skills. American Journal of Medical Technology. 1975; Vol. 41(12):457–462.
Sehl, L.C., Trollsas, O.M., Wallace, D.G., Toman, D., DeLustro, F.A., Schroeder, J.A. and Chu, G.H n.d., ‘Adhesive tissue repair patch and collagen sheets’, US 2010/0233246 A1.
Serra-Mitjans, M., Belda-Sanchis, J., Rami-Porta, R., Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database of Systematic Reviews (Online), 2005;(3):CD003051.
Shalaby, S.W., Vaughn, M.A. Polyester/cyanoacrylate tissue adhesive compositions, 2008. [US 7351426].
Shalaby, S.W., Vaughn, M.A., Lindsey, J.M. and III n.d., ‘Cyanoacrylate tissue adhesive formulations with multipurpose rheology modifiers’, US 2009/0264555 A1.
Shammas, N.W., Rajendran, V.R., Alldredge, S.G., Witcik, W.J., Robken, J.A., Lewis, J.R., McKinney, D., Hansen, C.A., Kabel, M.E., Harris, M., Jerin, M.J., Bontu, P.R., Dippel, E.J., Labroo, A. Randomized comparison of vasoseal and angioseal closure devices in patients undergoing coronary angiography and angioplasty. Catheterization and Cardiovascular Interventions. 2002; Vol. 55(4):421–425.
Shinya, N., Oka, S., Miyabashira, S., Kaetsu, H., Uchida, T., Sueyoshi, M., Takase, K., Akuzawa, M., Miyamoto, A., Shigaki, T. Improvement of the tissue-adhesive and sealing effect of fibrin sealant using polyglycolic acid felt. Journal of Investigative Surgery. 2009; Vol. 22(5):383–389.
Silverman, H.G., Roberto, F.F. Understanding marine mussel adhesion. Marine Biotechnology. 2007; Vol. 9(6):661–681.
Spotnitz, W.D., Burks, S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008; Vol. 48(7):1502–1516.
Spotnitz, W.D., Burks, S.G., Prabhu, R. Fibrin-based adhesives and hemostatic agents. In: Quinn J.V., ed. Tissue Adhesives in Clinical Medicine. Hamilton, Ontario: BC Decker; 2005:77–112.
Sternberg, K., Rohm, H.W., Lurtz, C., Wegmann, J., Odermatt, E.K., Behrend, D., Michalik, D., Schmitz, K.-P. Development of a biodegradable tissue adhesive based on functionalized 1,2-ethylene glycol bis(dilactic acid). I. Journal of Biomedical Materials Research Part B: Applied Biomaterials. Vol. 94b(2), 2010. [August 2010].
Suzuki, Y., Ujiie, H., Takahashi, N., Iwaki, M. Biological repair material compatible with biological tissue adhesive, 2010. [US 7722934].
Tredree, R., Beierlein, W., Debrix, I., Eisert, A., Goffredo, F., Gómez de Salazar, E., Rambourg, P., Saint-Remy, J.M., Sturm, C., Watson, N. Evaluating the differences between fibrin sealants: recommendations from an international advisory panel of hospital pharmacists. European Journal of Hospital Pharmacy Science. 2006; Vol. 12(1):3–9.
Vera, L., Benzerroug, M., Gueudry, J., Varin, R., Haghighat, S., Gérard, G., Muraine, M. Mise au point sur l’utilisation des colles tissulaires en ophtalmologie. Journal Français d’Ophtalmologie. 2009; Vol. 32(4):290–305.
Vollenweider, L., Murphy, J.L., Xu, F., Brodie, M., Lew, W.L., Dalsin, J.L., Lee, B.P. Biomimetic Adhesive Coatings for Soft Tissue Repair. Nerites Corp., 15th International Coating Science and Technology Symposium, September 13–15, 2010:20.
von der Brelie, C., Soehle, M., Burkett, C.J., Patel, S., Tabor, M.H., Padhya, T., Vale, F.L. Polyethylene glycol (PEG) hydrogel dural sealant and collagen dural graft matrix in transsphenoidal pituitary surgery for prevention of postoperative cerebrospinal fluid leaks. Journal of Clinical Neuroscience. 2011; Vol. 18(11):1513–1517.
Wagman, M.E., Qi, K., Kunitsky, K., Yim, H.W. Hydrogel tissue adhesive having increased degradation time, 2009. [US 2010/0160960 A1].
Weinstein, J.S., Liu, K.C., Delashaw, J.B., Burchiel, K.J., van Loveren, H.R., Vale, F.L., Agazzi, S., Greenberg, M.S., Smith, D.A., Tew, J. The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. Journal of Neurosurgery. 2010; Vol. 112(2):428–433.
Weisel, J., Cederholm-Williams, S. Fibrinogen and fibrin: characterization, processing and medical applications. In: Domb A.J., Kost J., Wiseman D.M., eds. Handbook of Biodegradable Polymers. Australia; United States: Harwood Academic Publishers; 1997:347–366.
Xie, H. Tissue sealant made from whole blood, 2009. [US 2009/0011043 A1].
Zawaneh, P.N., Singh, S.P., Padera, R.F., Henderson, P.W., Spector, J.A., Putnam, D. Design of an injectable synthetic and biodegradable surgical biomaterial. Proceedings of the National Academy of Sciences. 2010; Vol. 107(24):11014.
Zhang, S. and Ruiz, R. n.d., ‘Stable and sterile tissue adhesive composition with a controlled high viscosity’, US 2009/0318583 A1.
Zhang, S., Ruiz, R. and Sr. n.d., ‘Cyanoacrylate tissue adhesives with desirable permeability and tensile strength’, US 2011/0117047 A1.
16.13 References
Abando, A., Hood, D., Weaver, F., Katz, S. The use of the Angioseal device for femoral artery closure. Journal of Vascular Surgery. 2004; Vol. 40(2):287–290.
AccessClosure IncMynx Cadence Instructions for Use. AccessClosure, Inc, 2010.
Alleyne, C.H., Cawley, C.M., Barrow, D.L., Poff, B.C., Powell, M.D., Sawhney, A.S., Dillehay, D.L. Efficacy and biocompatibility of a photopolymerized, synthetic, absorbable hydrogel as a dural sealant in a canine craniotomy model. Journal of Neurosurgery. 1998; Vol. 88(2):308–313.
Anderson, N.J., Hardten, D.R. Fibrin glue for the prevention of epithelial ingrowth after laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2003; Vol. 29(7):1425–1429.
Baxter Healthcare Corp. SSED – CoSeal™ Surgical Sealant – P030039, 2003.
Baxter Healthcare CorpCoSeal Surgical Sealant Instructions for Use. Baxter Healthcare Corp, 2009.
Baxter Healthcare CorpTISSEEL [Fibrin Sealant] Instructions for Use. Baxter Healthcare Corporation, 2009.
Bayfield, M.S., Spotnitz, W.D. Fibrin sealant in thoracic surgery: pulmonary applications, including management of bronchopleural fistula. Chest Surgery Clinics of North America. 1996; Vol. 6:576–583.
Bennett, S.L., Melanson, D.A., Torchiana, D.F., Wiseman, D.M., Sawhney, A.S. Next-generation hydrogel films as tissue sealants and adhesion barriers. Journal of Cardiac Surgery. 2003; Vol. 18(6):494–499.
Berrevoet, F., de Hemptinne, B. Clinical application of topical sealants in liver surgery: does it work? Acta chirurgica Belgica. 2007; Vol. 107(5):504.
Boogaarts, J.D., Grotenhuis, J.A., Bartels, R.H.M.A., Beems, T. Use of a novel absorbable hydrogel for augmentation of dural repair: Results of a preliminary clinical study. Neurosurgery. 2005; Vol. 57(1 Suppl.):146–151.
Browdie, D.A., Cox, D. Tests of experimental tissue adhesive sealants: analysis of strength effects in relation to tissue adhesive sealant standards. Texas Heart Institute Journal. 2007; Vol. 34(3):313.
Brubaker, C.E., Kissler, H., Wang, L.J., Kaufman, D.B., Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials. 2010; Vol. 31(3):420–427.
Capello, J. Genetically engineered protein polymers. In: Domb A.J., Kost J., Wiseman D.M., eds. Handbook of Biodegradable Polymers. Australia; United States: Harwood Academic Publishers; 1997:387–416.
Carnahan, M.A., Butlin, J.D.G. Crosslinked polyalkyleneimine hydrogels with tunable degradation rates, 2011. [US 2011/0044932 A1].
Chan, S.M., Boisjoly, H. Advances in the use of adhesives in ophthalmology. Current Opinion in Ophthalmology. 2004; Vol. 15(4):305–310.
Chao, H.-H., Torchiana, D.F. BioGlue: albumin/glutaraldehyde sealant in cardiac surgery. Journal of Cardiac Surgery. 2003; Vol. 18(6):500–503.
Chauvet, D., Tran, V., Mutlu, G., George, B., Allain, J.-M. Study of dural suture watertightness: an in vitro comparison of different sealants. Acta Neurochirurgica. 2011; Vol. 153(12):2465–2472.
Cooper, C.W., Falb, R.D. Surgical adhesives. Annals of the New York Academy of Sciences. 1968; Vol. 146(1):214–224.
Cosgrove, G.R., Delashaw, J.B., Grotenhuis, J.A., Tew, J.M., Van Loveren, H., Spetzler, R.F., Payner, T., Rosseau, G., Shaffrey, M.E., Hopkins, L.N., others. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. Journal of Neurosurgery. 2007; Vol. 106(1):52–58.
Coury, A. Chemical and biochemical degradation of polymers. In: Ratner B., Schoen F., Hoffman A., Lemons J., eds. Biomaterials Science: An Introduction to Materials in Medicine. 2nd. Amsterdam; Boston: Elsevier Academic Press; 2004:696–706.
CryoLife, Inc. SSED – CryoLife BioGlue Surgical Adhesive – P010003, 2001.
CryoLife, Inc.BioGlue Surgical Adhesive Instructions for Use. CryoLife, Inc, 2010.
CryoLife, Inc. CryoLife: BioGlue® Surgical Adhesive, 2012. www.cryolife.com/international/products/bioglue-surgical-adhesive [viewed online 26 March 2012].
Dang, W., Daviau, T., Brem, H. Morphological characterization of polyanhydride biodegradable implant Gliadel® during in vitro and in vivo erosion using scanning electron microscopy. Pharmaceutical Research. 1996; Vol. 13(5):683–691.
Epstein, N.E. Dural repair with four spinal sealants: focused review of the manufacturers’ inserts and the current literature. The Spine Journal. 2010; Vol. 10(12):1065–1068.
Ferroli, P., Acerbi, F., Broggi, M., Schiariti, M., Albanese, E., Tringali, G., Franzini, A., Broggi, G., A novel impermeable adhesive membrane to reinforce dural closure: a preliminary retrospective study on 119 consecutive high-risk patients. World Neurosurgery, 2011 http://linkinghub.elsevier.com/retrieve/pii/S1878875011011016 [Available online 1 November 2011, viewed 26 March 2012].
Focal Inc. SSED – FocalSeal-Synthetic Absorbable Sealant P990028, 2000.
Friedman, M., Schalch, P. Middle turbinate medialization with bovine serum albumin tissue adhesive (bioglue). The Laryngoscope. 2008; Vol. 118(2):335–338.
Glickman, M., Gheissari, A., Money, S., Martin, J., Ballard, J.L., others. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Archives of Surgery. 2002; Vol. 137(3):326.
Grinstaff, M.W. Designing hydrogel adhesives for corneal wound repair. Biomaterials. 2007; Vol. 28(35):5205–5214.
Harris, J.M., Zalipski, S.Poly(ethylene Glycol): Chemistry and Biological Applications. Washington, DC: American Chemical Society, 1997.
Hill, A., Estridge, T.D., Maroney, M., Monnet, E., Egbert, B., Cruise, G., Coker, G.T. Treatment of suture line bleeding with a novel synthetic surgical sealant in a canine iliac PTFE graft model. Journal of Biomedical Materials Research. 2001; Vol. 58(3):308–312.
HyperbranchSafety and Effectiveness of the Adherus Dural Sealant System When Used as a Dural Sealant in Cranial Procedures. HyperBranch Medical Technology, Inc, 2010. [ClinicalTrials.gov identifier NCT01158378].
Johnson, C.S., Wathier, M., Grinstaff, M., Kim, T. In vitro sealing of clear corneal cataract incisions with a novel biodendrimer adhesive. Archives of Ophthalmology. 2009; Vol. 127(4):430–434.
Kalayci, D., Fukuchi, T., Edelman, P.G., Sawhney, A.S., Mehta, M.C., Hirose, T. Hydrogel tissue adhesive for sealing corneal incisions. Ophthalmic Research. 2003; Vol. 35(3):173–176.
Lagoutte, F., Gauthier, L., Comte, P. A fibrin sealant for perforated and preperforated corneal ulcers. British Journal of Ophthalmology. 1989; Vol. 73(9):757–761.
Lee, B.P., Messersmith, P.B., Israelachvili, J.N., Waite, J.H. Mussel-inspired adhesives and coatings. Annual Review of Materials Research. 2011; Vol. 41(1):99–132.
Mai-ngam, K., Prahsarn, C., Seetapan, N., Chumningan, P. Thermo-responsive chitosan-g-poly-N-isopropylacrylamide for tissue adhesives. Proceedings of the 8th Polymers for Advanced Technologies International Symposium, Budapest, Hungary, 2005.
McCrea, K.R., Ward, R.S., Tian, Y. Silicone hydrogels for tissue adhesives and tissue dressing applications, 2011. [US 2011/0086077 A1].
McKerracher, L. Methods for making and delivering Rho-antagonist tissue adhesive, 2011. [US 2011/0086077 A1].
Metters, A., Hubbell, J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2004; Vol. 6(1):290–301.
Mulder, M., Crosier, J., Dunn, R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine. 2009; Vol. 34(4):E144–E148.
Murakami, Y., Yokoyama, M., Nishida, H., Tomizawa, Y., Kurosawa, H. In vivo and in vitro evaluation of gelation and hemostatic properties of a novel tissue-adhesive hydrogel containing a cross-linkable polymeric micelle. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009; Vol. 91B(1):102–108.
Narváez, J., Chakrabarty, A., Chang, K. Treatment of epithelial ingrowth after LASIK enhancement with a combined technique of mechanical debridement, flap suturing, and fibrin glue application. Cornea. 2006; Vol. 25(9):1115–1117.
Neomend Inc. SSED – ProGEL™ Pleural Air Leak Sealant – P010047, 2010.
NeoMend Inc. ProGELTM Surgical Sealant Instructions for Use, 2012.
Park, J.B., Lakes, R.S.Biomaterials. New York: Springer Science + Business Media, LLC, 2007.
Patel, M.R., Louie, W., Rachlin, J. Postoperative cerebrospinal fluid leaks of the lumbosacral spine: management with percutaneous fibrin glue. American Journal of Neuroradiology. 1996; Vol. 17(3):495–500.
Puppa, A.D., Rossetto, M., Scienza, R. Use of a new absorbable sealing film for preventing postoperative cerebrospinal fluid leaks: remarks on a new approach. British Journal of Neurosurgery. 2010; Vol. 24(5):609–611.
Rabotyagova, O.S., Cebe, P., Kaplan, D.L. Protein-based block copolymers. Biomacromolecules. 2011; Vol. 12(2):269–289.
Rathinam, S., Naidu, B.V., Nanjaiah, P., Loubani, M., Kalkat, M.S., Rajesh, P.B., others. BioGlue and Peri-strips in lung volume reduction surgery: pilot randomised controlled trial. Journal of Cardiothoracic Surgery. 2009; Vol. 4:37.
Reul, R., Walsh, G. Tissue adhesives in thoracic and cardiovascular surgery. In: Franco K.L., Putnam J.B., eds. Advanced Therapy in Thoracic Surgery. Hamilton, Ont.; Lewiston, NY: B.C. Decker; 2005:47–60.
Sawhney, A.S., Jarrett, P.K., Coury, A.J., Rudowsky, R.S., Powell, M.D., Avila, L.Z., Enscore, D.J., Goodrich, S.D., Nason, W.C., Yao, F., Weaver, D., Barman, S.P. Polymerizable biodegradable polymers including carbonate or dioxanone linkages, 2001. [US 5900245].
Sawhney, A.S., Pathak, C.P., van Rensburg, J.J., Dunn, R.C., Hubbell, J.A. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. Journal of Biomedical Materials Research. 1994; Vol. 28(7):831–838.
Scheinert, D., Sievert, H., Turco, M.A., Schmidt, A., Hauptmann, K.E., Mueller, R., Dadourian, D., Krankenberg, H., Grube, E. The safety and efficacy of an extravascular, water-soluble sealant for vascular closure: initial clinical results for MynxTM. Catheterization and Cardiovascular Interventions. 2007; Vol. 70(5):627–633.
Schenk, W.G., Burks, S.G., Gagne, P.J., Kagan, S.A., Lawson, J.H., Spotnitz, W.D. Fibrin sealant improves hemostasis in peripheral vascular surgery: a randomized prospective trial. Annals of Surgery. 2003; Vol. 237(6):871–876.
Schneider, G. Tissue adhesives in otorhinolaryngology. GMS Current Topics in Otorhinolaryngology – Head and Neck Surgery. 2009; Vol. 8:1–10.
Shaffrey, C.I., Spotnitz, W.D., Shaffrey, M.E., Jane, J.A. Neurosurgical applications of fibrin glue: augmentation of dural closure in 134 patients. Neurosurgery. 1990; Vol. 26(2):207–210.
Sharma, A., Kaur, R., Kumar, S., Gupta, P., Pandav, S., Patnaik, B., Gupta, A. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003; Vol. 110(2):291–298.
Singh, A., Hosseini, M., Hariprasad, S.M. Polyethylene glycol hydrogel polymer sealant for closure of sutureless sclerotomies: a histologic study. American Journal of Ophthalmology. Vol. 150(3), 2010. [346–351.e2].
Smith, D. Adhesives and sealants, in biomaterials science. In: Ratner B., Schoen F., Hoffman A., Lemons J., eds. Biomaterials Science:An Introduction to Materials in Medicine. Amsterdam; Boston: Elsevier Academic Press, 2004.
Spotnitz, W.D. History of tissue adhesives, in surgical adhesives and sealants. In: Sierra D.H., Saltz R., eds. Surgical Adhesives and Sealants: Current Technology and Applications. Lancaster, PA: Technomic Pub, 1996.
Spotnitz, W.D., Burks, S. State-of-the-art review: hemostats, sealants, and adhesives II: update as well as how and when to use the components of the surgical toolbox. Clinical and Applied Thrombosis/Hemostasis. 2010; Vol. 16(5):497–514.
Thavarajah, D., De Lacy, P., Hussain, R., Redfern, R.M. Postoperative cervical cord compression induced by hydrogel (DuraSeal): q possible complication. Spine. 2010; Vol. 35(1):E25–E26.
TissuemedTissue Patch 3 Instructions for Use. Tissuemed Limited, 2012.
Wain, J.C., Kaiser, L.R., Johnstone, D.W., Yang, S.C., Wright, C.D., Friedberg, J.S., Feins, R.H., Heitmiller, R.F., Mathisen, D.J., Selwyn, M.R. Trial of a novel synthetic sealant in preventing air leaks after lung resection. The Annals of Thoracic Surgery. 2001; Vol. 71(5):1623–1628. [discussion 1628–1629].
Wang, M., Mattson, M.S., Tirrell, D.A., Kornfield, J.A. Tissue adhesive using engineered proteins, 2010. [US 2010/0160960 A1].
Wathier, M.C., Carnahan, M.A., Jung, P.J., Kim, T., Grinstaff, M.W. Dendrimer hydrogels as new alternative for the repair of clear corneal incisions. Investigative Ophthalmology & Visual Science. 2005; Vol. 46(5):4993.
Wathier, M., Grinstaff, M. Hydrogel sealants for wound repair in ophthalmic surgery. In: Chirila T., ed. Biomaterials and Regenerative Medicine in Ophthalmology. Woodhead, Boca Raton, FL; Oxford: CRC Press, 2010.
Webster, I., West, P. Adhesives for medical applications. In Dumitriu S., ed.: Polymeric Biomaterials, 2nd, New York: Marcel Dekker, Inc., 2002.
Wheat, J.C., Wolf, J.S. Advances in bioadhesives, tissue sealants, and hemostatic agents. Urologic Clinics of North America. 2009; Vol. 36(2):265–275.
Wong, S.C., Bachinsky, W., Cambier, P., Stoler, R., Aji, J., Rogers, J.H., Hermiller, J., Nair, R., Hutman, H., Wang, H. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC: Cardiovascular Interventions. 2009; Vol. 2(8):785–793.
Wright, N.M., Kim, K.D., Cosgrove, R., Tew, J.M., Kaiser, M.G., McCormick, P.C., Chestnut, R., Ellenbogen, R.G., Van Loveren, H.R., Vale, F.L., Shaffrey, M.E., Chen, T.C., Wang, M.Y., Mayberg, M.R., Sharan, A.D., Gerszten, P.C., Welch, W.C., Graham, R.S., Brown, F.D., Fessler, R.G., Deutsch, H., Shah, M.V., McCutcheon, I.E., Karahalios, D.G., Long, D.M., Hopkins, L.N., Jallo, J., Delashaw, J.B., Ondra, S.L., Gross, R.E. DuraSeal spinal sealant as an adjunct to sutured dural repair in the spine. Neurosurgery. 2009; Vol. 65(2):410.