Chapter 7
Green Chemistry
7.1 Green Chemistry
Chemical products can be manufactured using a wide variety of synthesis routes. The designer of a chemical process must choose from alternative raw materials, solvents, reaction pathways, and reaction conditions, and these design choices can have a significant impact on the overall environmental performance of a chemical process. Ideal chemical reactions would have attributes such as
• simplicity
• safety
• high yield and selectivity
• energy efficiency
• use of renewable and recyclable reagents and raw materials
In general, chemical reactions cannot achieve all of these goals simultaneously and it is the task of chemists and chemical engineers to identify pathways that optimize the balance of desirable attributes.
Identification of environmentally preferable pathways requires creative advances in chemistry as well as process design. Because the number of choices in selecting reaction pathways is so large and the implications of those choices are so complex, systematic, quantitative design tools for identifying green chemistries are not available. Nevertheless, an extensive body of knowledge concerning green chemistry exists and some qualitative and quantitative design tools are emerging.
Green chemistry, defined as the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances, is presented in two basic parts—one qualitative and the other quantitative. The first part describes qualitative principles to be used in developing alternatives—alternative solvents, alternative reactants, alternative chemistries—that may lead to environmental improvements. Section 7.2 provides this overview of qualitative principles that can be used to identify green chemistry alternatives. Section 7.3 describes quantitative, optimization-based approaches that have been used to identify environmentally preferable reaction pathways. Finally, Section 7.4 briefly describes the US Environmental Protection Agency’s Green Chemistry Expert System, which provides case studies of many of the principles described in this chapter.
7.2 Green Chemistry Methodologies
The design of a chemical manufacturing process involves feedstock selection, selection of solvents, catalysts and other materials, and selection of reaction pathways. This section describes some of the alternatives that are available in making these design decisions and suggests a set of principles that process designers can use to identify alternatives. Specifically, the following issues are addressed:
• alternative feedstocks
• green solvents
• synthesis pathways
• inherently safer chemistry
7.2.1 Feedstocks
The synthesis and manufacture of any chemical substance begins with the selection of a starting material from which the final product will be synthesized. In many cases, the selection of a starting material can be the most significant factor in determining the impact of a chemical manufacturing process on the environment. There are a number of criteria that can be used in evaluating the potential environmental impacts of materials and these will be discussed at length in Chapter 8. For now, note that criteria that may be important in evaluating the environmental performance of a material include its persistence in the environment, its bioaccumulation, potential, its ecotoxicity and human toxicity. The scarcity of the material and whether it is a renewable or non-renewable resource may also be considered. In addition to considering the persistence, bioaccumulation, and toxicity of materials, the designer should be cognizant of the environmental impacts associated with creating the feedstock material. If a material does not pose any hazard to human health or the environment, for example, but the retrieval or isolation of the substance causes significant risk, then this should be taken into account in the selection. These issues will be addressed more completely in Chapters 13 and 14.
Setting aside, for the moment, the issue of what criteria will be used to evaluate environmental performance of materials, the question remains—How do we identify alternative raw materials that might improve environmental performance? Case studies can illustrate a variety of approaches to answering this question.
Consider the case of adipic acid manufacturing shown in Figure 7.2-1. The traditional method for adipic acid manufacture uses benzene, a fossilfuel based, carcinogenic feedstock. The designer may wish to consider feedstocks which are renewable or less toxic. One potentially environmentally preferable alternative uses glucose, a renewable feedstock which is innocuous. Thus, the adipic acid pathway using glucose (Draths and Frost, 1998), shown in Figure 7.2-1, has some advantages. A complete evaluation, however, would need to consider the environmental issues associated with glucose and benzene production and purification (for a more detailed discussion of these types of issues, see Chapters 13 and 14).
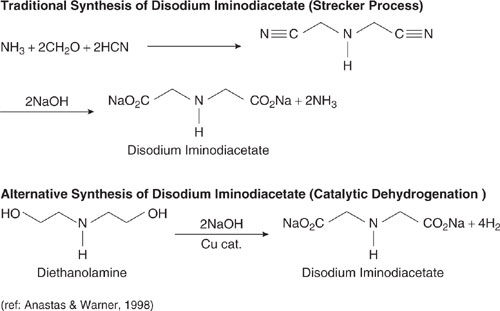
A second example of the use of less hazardous materials is provided by the synthesis of disodium iminodiacetate as shown in Figure 7.2-2. The traditional synthesis uses hydrogen cynanide, while an alternative route using diethanolamine avoids these substances.
These few examples can be expanded into a set of more general principles and guidelines, which are described below.
Innocuous
The selection of starting materials should start with an evaluation of the materials themselves, using the methods described in Chapters 5 and 6, to determine if they possess any hazardous properties. Inherent to this analysis is determining whether the process or reaction step requiring the hazardous material is necessary, or whether the final target compound could be obtained from an alternative pathway that uses a less hazardous material.
Generates Less Waste
An important consideration associated with the use of a particular raw material is whether it is responsible for the generation of more or less waste than other materials. The amount of waste either generated or eliminated, however, cannot be the only consideration. The type of waste generated must also be assessed. Just as all chemical products are not equal in terms of their hazard, neither are chemical waste streams. Waste streams therefore must also be assessed for any hazardous properties that they possess.
Selective
Utilizing a raw material or reaction pathway that is more selective means that more of the starting material will be converted into the desired product. High product selectivity does not always translate into high product yield (and less waste generated), however. Both high selectivity and high conversion must be achieved for a synthetic transformation to generate little or no waste. Using highly selective reagents can mean, however, that separation, isolation, and purification of the product will be significantly less difficult. Since a substantial portion of the burden to the environment that chemical manufacturing processes incur result from sepa-ration and purification processes, highly selective materials and reaction pathways are very desirable.
Figure 7.2-1 Traditional and alternative synthesis pathways for adipic acid; the traditional pathway uses benzene, a fossil-fuel-based, carcinogenic feedstock. The alternative uses glucose, a renewable feedstock which is innocuous. (Draths and Frost, 1998.)
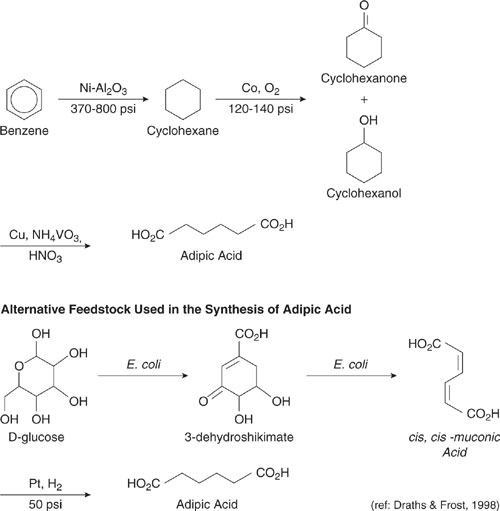
Figure 7.2-2 Traditional and alternative synthesis pathways for disodium iminodiacetate. The traditional synthesis uses hydrogen cynanide, while an alternative route using diethanolamine avoids these substances. (Anastas and Warner, 1998.)
Efficient
Reaction efficiency, much like product selectivity, has long been a goal of synthetic design, and even prior to the advent of green chemistry principles, has offered benefits. When the overall yield of a process is increased by 10 or 20 percent, less material ends up in waste streams and more is converted into product. However, yield and selectivity are not entirely adequate as a measure of reaction efficiency. As Trost (1991) has outlined, a synthetic transformation can achieve 100 percent selectivity to product and still generate a substantial amount of waste if the transformation is not “atom economical.” Atom economy, a ratio of the molecular weight of the starting materials and reagents and the molecular weight of the target molecule, provides a measure of the intrinsic efficiency of the transformation; its use is described in an example later in this chapter.
7.2.2 Solvents
The use of solvents in the chemical industry and the chemical-related industries is ubiquitous. In 1991, the production of the 25 most commonly used solvents was more than 26 million tons per year. According to Toxic Release Inventory (TRI) data, of the chemicals and chemical categories tracked by the program in 1994, 5 of the top 10 chemicals released or disposed were solvents and include methanol, toluene, xylene, methyl ethyl ketone, and dichloromethane. The total quantity of these chemicals released or disposed was over 687 million pounds, which accounts for 27 percent of the total quantity of TRI chemicals released and disposed in that year. With increasing regulatory pressure focusing on solvents, there is significant attention being paid to the use of alternatives to traditional solvents. General guiding principles in the selection of solvents are given below.
Less Hazardous
Solvents have been developed with an eye toward safety since they are used in such large volumes. The earliest and most obvious hazards that were addressed in the design of solvent molecules were their ability to explode or ignite. With the greater understanding of the health and environmental effects that could be caused by a large number of solvents, new solvents are being scrutinized for other hazards as well.
Human Health
Solvents are of particular concern because the likelihood of significant levels of exposure is high. Many solvents, by their nature, have high vapor pressure and, in combination with the volumes that are often used, can result in significant exposures. Halogenated solvents such as carbon tetrachloride, perchloroethylene, and chloroform have been implicated as potential and/or suspect carcinogens while other classes of solvents have demonstrated neurotoxicological effects. However, the direct toxicity to humans is only one aspect of the total hazards that solvents possess. There are a number of environmental implications of the use of large volumes of solvents.
Environment (Local and Global)
The use of solvents has caused both global and local environmental concerns. At the global level, the role of chlorofluorocarbons (CFCs) in stratospheric ozone depletion has led to a global phase-out of the substances from virtually all uses. Other solvents have been found to possess significant global warming potential and are thought to contribute to the overall greenhouse gas loading in the environment. At a more local level, the use of certain volatile organic compounds (VOCs) as sol-vents and in other applications has generated concern about their ability to elevate air pollution levels.
Case Studies
Some of the main alternatives to traditional solvents include supercritical fluids, aqueous applications, polymerized/immobilized solvents, ionic liquids, solvent-less systems and reduced hazard organic solvents. Some examples are provided below.
Consider first the use of supercritical carbon dioxide as a reaction medium. Supercritical CO2 is non-toxic, non-flammable, renewable, and inexpensive. Further, because solubility of most solutes in supercritical fluids changes dramatically around the critical point, it is often possible to recover materials from supercritical CO2 merely by reducing pressure below the critical point. Thus, a range of applications of supercritical CO2, such as decaffeinating coffee (where supercritical CO2 replaced methylene chloride), rely strictly on the physical, solvating properties of this solvent. Not all materials are soluble in supercritical CO2, however. For materials such as high molecular weight hydrocarbons, which are not highly soluble in supercritical CO2, the advantages of supercritical fluids can be obtained by adding a surfactant to the supercritical CO2. Adding the right surfactant creates a micelle phase in which materials not normally soluble in supercritical CO2 can be suspended. Figure 7.2-3 shows an example of this concept. In this case, a polymerization reaction, which produces high molecular weight materials, is conducted in a surfactant-supercritical CO2 system, replacing the use of conventional solvents.
While in many cases solvents are used strictly for their physical, solvating properties, in some situations, such as chemical reactions occurring in solvents, the solvents play a role in the chemical synthesis. In cases where reactions occur in a solvent, the use of a supercritical fluid may enhance or inhibit the desired reaction. The effect of supercritical fluids on reaction chemistry is an active area of research, and Figure 7.2-3 shows examples of recent progress. As shown in the figure, a class of reactions, referred to as asymmetric catalytic reductions, have been conducted in supercritical CO2. For this class of reactions, selectivity in supercritical CO2 are comparable or superior to those achieved in conventional solvents (Burk, 1991; Burk, et al., 1993, 1995; U.S. EPA, 1996).
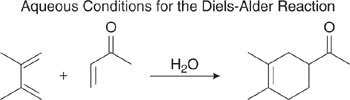
Consider next the use of water as an alternative solvent. As in the case of supercritical CO2, water is non-toxic, non-flammable, renewable, and inexpensive. The limited solubility of many hydrocarbon reactants in water has often limited its utility, however. A number of case studies of innovative use of water as a reaction medium have been reported by Anastas and Williamson (1998) and by Li and Chan (1997). One example is shown in Figure 7.2-4 (Breslow and Zhu, 1995; Breslow, et al., 1996), where water with an alcohol co-solvent is used in Diels Alder reactions. Some Diels Alder reactions, such as the dimerization of 1,3 cyclopentadiene, are accelerated in water. This is due to favorable packing of hydrophobic surfaces in the reaction’s transition state (Breslow, et al., 1995), a generally unanticipated result.
Many other organic reactions that have traditionally been carried out in organic solvents have now been carried out in aqueous media. Examples include the Barbier-Grignard reaction, pericyclic reactions, and transition metal catalyzed reactions (for a thorough discussion, see Li, 1998).
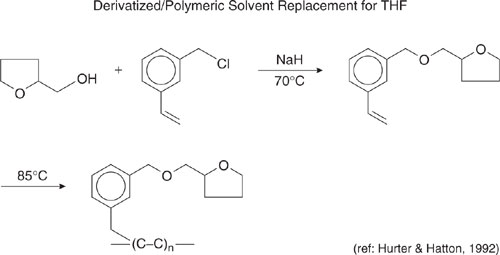
As a final case study, consider the use of derivatized, immobilized solvent materials. The concept behind this solvent replacement is to reduce the emissions and promote the recovery of hazardous solvents by attaching the solvent to a hydrocarbon backbone. The use of this concept in the replacement of tetrahydrofuran (THF) is shown in Figure 7.2-5 (Hurter and Hatton, 1992; U.S. EPA, 1996). In this case, the hazardous substance, THF, is attached to a polymeric backbone using a chlorinated styrene derivative. The THF remains relatively mobile, but because it is attached to a polymeric backbone, it is less likely to volatilize and is easily recoverable using ultrafiltration or other methods.
Figure 7.2-3 Examples of the use of supercritical CO2 to replace conventional solvents; in the first example, a co-solvent is used along with the supercritical CO2 to allow a polymerization reaction to take place; in the second example, the use of supercritical CO2 enhances the selectivity of a catalytic reaction (Buelow, et al., 1998).
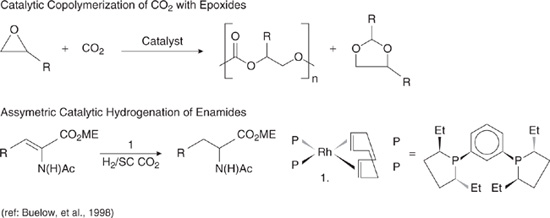
Figure 7.2-4 When water is used as a solvent for certain Diels Alder reactions, reaction rates can be accelerated.
These limited examples provide some indication of the variety of alternatives available for conventional solvents. More examples are described in the volume edited by Anastas and Williamson (1998) and in the Green Chemistry Expert System available from the US EPA.
7.2.3 Synthesis Pathways
Identifying chemical synthesis pathways that may lead to superior environmental performance is complex and relies on extensive knowledge of synthetic organic chemistry. It is beyond the scope of this chapter to provide a detailed review of this rapidly advancing field, but it is important for a process engineer to be able to identify classes of chemical reactions that have the potential for improvement. Addition reactions (A + B → AB), substitution reactions (A + B C → AC + B), and elimination reactions (AB → A + B), for example, can have different degrees of impact on human health and the environment. Addition reactions incorporate the starting materials into the final product and, therefore, do not produce waste that needs to be treated, disposed of, or otherwise dealt with. Substitution reactions, on the other hand, necessarily generate stoichiometric quantities of substances as byproducts and waste. Elimination reactions do not require input of materials during the course of the reaction other than the initial input of a starting material, but they do generate stoichiometric quantities of substances that are not part of the final target molecule. This guidance is qualitative. A semi-quantitative tool that a process engineer or chemist can use in evaluating synthetic pathways is the concept of atom efficiency. The atom efficiency characterizes the fraction of starting materials that are incorporated into desired products and is best illustrated through an example.
Figure 7.2-5 Use of a solvent functionality (tetrahydrofuran), attached to a large polymer backbone, to replace a volatile solvent (tetrahydrofuran) (Hurter and Hatton, 1992).
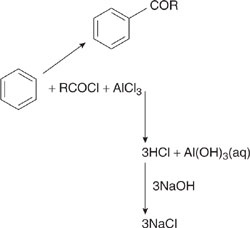
Friedel Crafts acylations have atom efficiencies that are relatively low (Clark, 1999). The process typically involves the substitution reaction of an acid chloride with an aromatic substrate. The reaction is frequently accomplished using an aluminum chloride catalyst. The product forms a complex with the catalyst, requiring a water wash, resulting in the formation of hydrochloric acid and salt wastes. The overall reaction is shown in Figure 7.2-6.
A simplistic overall atom and mass balance, outlined in Example 7.2-1 (Clark, 1999), suggests that only about 30% of the starting material ends up in the product. Thus, a simple calculation of atom efficiency identifies Friedel Crafts reactions as a potential target for environmental improvements. One type of improvement would be to retain the chemical pathway, but to regenerate and reuse the aluminum chloride catalyst. In the synthesis of ethylbenzene via the alkylation of benzene with ethylene, for example, approximately 1 ton of AlCl3 waste is generated per 100 tons of product (Davis, 1994), a significant reduction in waste generation relative to the case of no catalyst recovery. Another alternative for improving environmental performance would be to identify an alternative catalyst, such as highly acidic zeolites (Davis, 1994).
Figure 7.2-6 Friedel Crafts acylation generates a relatively large amount of waste material, even if the reaction is carried out at 100% yield and 100% selectivity, because of the dissipative use of the aluminum chloride catalyst.
Calculate atom and mass efficiencies for the Friedel Crafts reaction shown in Figure 7.2-5. Assume that the substituent R on the organic chloride is a methyl group.
Solution: To calculate the atom efficiencies, determine the fraction of the carbon, hydrogen, aluminum, chlorine, sodium, and oxygen atoms that emerge as product and the fraction that emerges from the reaction as waste.
• Virtually all of carbon (100%) becomes product.
• Most of hydrogen (excluding water in the water wash) becomes product; however, if hydrogen used in the water wash is included, virtually all of the hydrogen becomes waste.
• All of the aluminum becomes waste (0% efficiency).
• All of the chlorine becomes waste (0% efficiency).
• All of the sodium becomes waste (0% efficiency).
• One mole of oxygen in the organic chloride is incorporated into the product. Three moles of oxygen in the sodium hydroxide becomes waste. Therefore, excluding oxygen in water, the atom efficiency is 25%.
The mass efficiency can be calculated using atomic weights (ignoring water use).
Mass in product = 8 moles carbon * 12 + 10 moles hydrogen * 1 + 1 mole oxygen * 16 = 122
Mass input = 8 moles carbon * 12 + 16 moles H * 1 + 4 moles oxygen * 16 + 1 mole aluminum * 27 + 3 moles chlorine * 35.5 + 3 moles sodium * 23 = 378
Approximate mass efficiency = 122/378 = 0.32
Partial oxidations provide additional examples of industrially important reactions where atom utilization can be low. Figure 7.2-7 shows a number of commercially important partial oxidation reactions.
In the case of partial oxidations the poor atom utilization can be due to the oxidizing agent. If molecular oxygen is used in the partial oxidation, then atom utilization may be high if selectivity is high. If, however, oxidizing agents such as dichromate or permanganate are used, atom efficiencies can be low, as shown in Table 7.2-1. Environmental performance might be improved in these cases by carefully selecting the oxidizing agents.
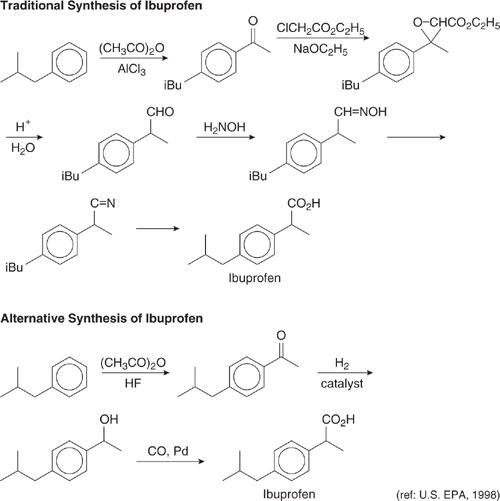
Wastes can sometimes be reduced by simplifying synthesis pathways. Consider the synthesis of Ibuprofen, an over-the-counter pain reliever. The traditional synthesis method (note that it involves Friedel-Crafts chemistry in the first step) is shown in Figure 7.2-8. An alternative synthesis, replacing the AlCl3 acid catalyst with HF and reducing the number of subsequent transformations and solvent usage, is also shown in the figure. Atom utilization increased from less than 40% in the traditional synthesis to approximately 80% using the new pathway (US EPA, 1998).
Figure 7.2-7 Commercially important partial oxidation reactions (Clark, 1999).
These few brief examples—Friedel Crafts reactions, partial oxidations, and reducing the number of steps in synthesis pathways—only scratch the surface of the rich variety of work in synthetic organic chemistry that can improve the performance of chemical manufacturing. In examining reaction pathways, the process engineer should employ tools, such as calculating atom and mass efficiencies, that help determine the magnitude of environmental improvements that may be possible. Additional methods for assessing reaction pathways that may lead to critical environmental improvements are described in Chapter 8. Later sections in this chapter describe some tools and analysis methods that can help to identify alternative chemical reagents and pathways.
Table 7.2-1 Atom Utilization in Oxidizing Reagents (from Davis, 1994, adapted from Sheldon, 1993).
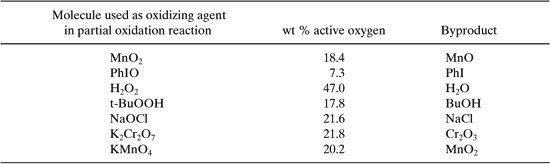
Figure 7.2-8 The traditional synthesis of Ibuprofen involved a large number of steps, including a Friedel Crafts reaction that generated byproducts of the type shown in Figure 7.2-8. The new route is simpler and employs a recoverable strong acid as the catalyst. (U.S. EPA, 1998.)
7.2.4 Functional Group Approaches to Green Chemistry
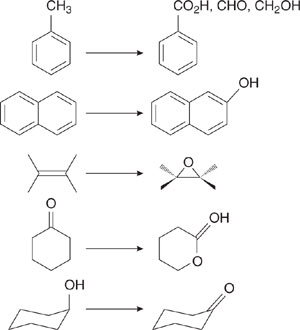
A number of tools can be used in the design of more environmentally benign chemistries, including structure-activity relationships, identification and avoidance of toxic functional groups, reducing bioavailability, and designing chemicals for innocuous fate. These concepts rely on the same functional group principles introduced in Chapter 5, and are described qualitatively below.
Structure-Activity Relationship
Many times the mechanism of action may not be known, but structure-activity relationships can be used to identify structural modifications that may improve a chemical’s safety. As an example, if the methyl-substituted analog of a substance has very high toxicity, and the toxicity decreases as the substitution moves from ethyl to propyl, it might be reasonable to increase the alkyl chain length to design a safer chemical. Even in cases where the reason for the effect the alkyl chain length has on decreasing toxicity is not known, if the results can ultimately be borne out empirically, then the structure-activity relationship is certainly a powerful design tool. Chapters 5 and 6 describe in detail the use of structure-activity relationships in evaluating a chemical’s environmental fate, bioaccumulation, and toxicity.
Elimination of Toxic Functional Group
A class of chemicals is often defined by certain structural features, such as aldehyde, ketone, nitrile, or isocyante functional groups. If information is not available about the specific chemical’s toxicity or the mechanism by which it produces that toxicity, the assumption that certain reactive functional groups will react similarly within the body or in the environment is often a good one. The assumption is especially good if there are data on other compounds in the chemical class that demonstrate a common toxic effect.
In cases such as this, the design of a safer chemical could proceed by removing the toxic functionality, which defines the class. In some cases this is not possible because the functionality is what gives the molecule the properties that are required for the chemical to perform in the desired way. In these cases, there are still options such as masking the functional group to a non-toxic derivative form and only releasing the parent functionality when necessary.
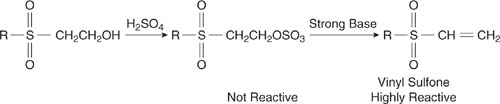
The masking of vinyl sulfones provides an interesting example of this technique. The vinyl sulfone functionality is highly electrophilic and reacts with cellulosic fibers, making it an effective component of dyes. There are, however, a variety of toxic effects associated with this functionality (DeVito, 1996). The sulfones can be made safer by masking the functional group. Rather than manufacturing, storing, and transporting the relatively hazardous sulfone, the sulfone can be generated when and as needed by converting a hydroxyethylsulfone into a vinyl sulfone, using the chemistry shown in Figure 7.2-9 (DeVito, 1996).
Figure 7.2-9 Masking of vinyl sulfones as hydroxyethylsulfones allows these relatively hazardous materials to be used more safely.
Reduce Bioavailability
If it is not known what structural features of the molecule need to be modified in order to make it less hazardous, then there is still the option of making the substance less bioavailable. If the substance is unable, due to structural design, to reach the target of toxicity, then it is in effect, innocuous. This can be done through a manipulation of the water-solubility/lipophilicity relationships that often control the ability of a substance to pass through biological membranes such as skin, lungs, or the gastrointestinal tract (see Chapter 6). The same principle applies to designing safer chemicals for the environment such as ozone depleting substances. For a substance to have a significant ozone depleting potential, it must be able to both reach the altitudes and have a sufficient lifetime in those altitudes in order to cause damage. Many substances are now being designed which have the same properties as substances which are known ozone depleters but without the ability to be available to the target of the hazard, in this case the stratospheric ozone layer.
Design for Innocuous Fate
It was often the goal of the chemist to design substances which were robust and could last as long as possible. This philosophy has resulted in persistent, and at times bioaccumulative and toxic substances. It is now known that it is more desirable to not have substances persist in the environment or a landfill forever, but that they should be designed to degrade after their useful life is over. Therefore, the design of safer chemicals cannot be limited to only hazards associated with the manufacture and use of the chemical but also that of its disposal and ultimate end of life cycle.
7.3 Quantitative/Optimization-Based Frameworks for the Design of Green Chemical Synthesis Pathways
One of the challenges associated with the design of green chemical synthesis pathways is identifying alternatives. Section 7.2 provided general guidelines and suggestions for improving the environmental performance of raw materials, reagents, and synthesis pathways. While useful, these guidelines still rely on the knowledge and creativity of chemists and chemical engineers in identifying specific alternatives. Identifying all possible alternatives is beyond the knowledge or experience of any single individual, so an increasingly popular method for rapidly searching for possible alternative materials or chemical synthesis routes relies on combinatorial approaches.
In a combinatorial approach to identifying green chemistry alternatives, the first step is to select a set of molecular or functional group building blocks from which a target molecule can be constructed. Next, a series of stoichiometric, thermodynamic, economic, and other constraints can be identified. These constraints serve to reduce the number of possibilities that might be considered. Finally, a set of criteria can be used to identify reaction pathways that deserve further examination.
The first step in the systematic construction of alternative chemical pathways is to select a set of functional group building blocks. Because the number and variety of pathways that are generated is a strong function of the starting materials that are used, this is a critical step. To keep the alternatives as varied as possible, it is desirable to include as many functional group building blocks as possible, yet to keep the search focused and tractable, the number of groups should be limited. Buxton, et al. (1997) have reported a number of rules and guidelines that can be effective in selecting a group of starting materials. They are:
• Include the groups present in the product.
• Include groups present in any existing industrial raw materials, coproducts or byproducts.
• Include groups which provide the basic building blocks for the functionalities of the product or of similar functionalities.
• Select sets of groups associated with the general chemical pathway employed (cyclic, acyclic, or aromatic).
• Reject groups that violate property restrictions.
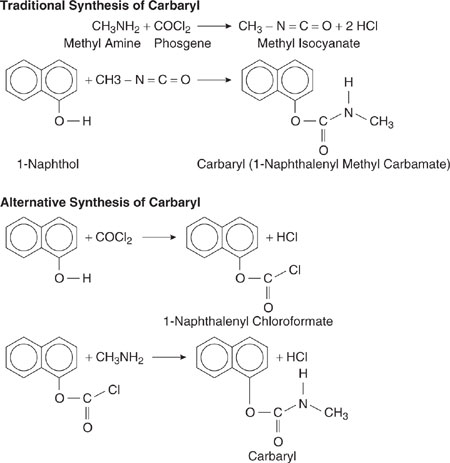
These rules can be clarified through an example. Consider the synthesis of 1-naphthyl-methylcarbamate (Crabtree and El-Halwagi, 1995), manufactured by Union Carbide and known as carbaryl. In 1984, a catastrophic release of methylisocyanate, a reactant used in the synthesis of carbaryl, occurred at a carbaryl manufacturing facility in Bhopal, India, killing thousands. This incident, and other less catastrophic events, demonstrate the importance of identifying reaction pathways that minimize the use of hazardous materials. For the carbaryl synthesis used in 1984 in Bhopal, α-naphthol and methylisocyanate were used as reactants, as shown in Figure 7.3-1. An alternative chemistry for carbaryl is also shown.
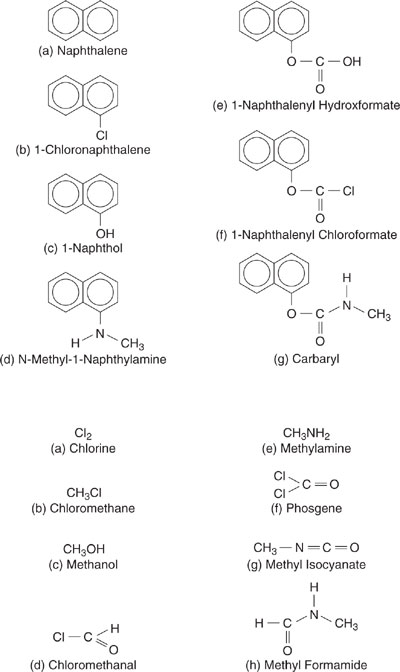
Are other chemistries for the synthesis of carbaryl possible or desirable? The first step in identifying alternative pathways is to select a set of functional group building blocks that will be included in the analysis. Since the product molecule contains aromatic groups, it will be necessary to include a range of aromatic functionalities such as aromatic carbon bound to hydrogen (ACH in the notation of Chapter 5), aromatic carbon bound to other aromatic carbon (AC –), aromatic carbon bound to chlorine, and aromatic carbon bound to a hydroxl group (ACOH). More aromatic functionalities could be chosen, if desired. Other groups appearing in the product molecule, or related to the groups appearing in the product molecule, are (using the notation of Chapter 5) –CH3, CH3NH<, CH3NH2–, –COO–, –CHO–, –CO2H, –OH, –Cl.
Figure 7.3-1 The synthesis of carbaryl can be accomplished with a methylisocyanate intermediate. An alternative route, not involving methylisocyanate, is also shown.
These functional group building blocks can be used to identify a set of potential molecular reactants. Going from a set of functional group building blocks to potential molecular starting materials can generate very large numbers of potential reactants, so constraints, based on chemical intuition, are generally imposed. For example, in identifying alternatives to the carbaryl synthesis, Buxton, et al. (1997) assumed that only monosubstituted aromatic molecules would be used, since the product is monosubstituted; they also assumed that reactants for which the carbon skeleton would need to be altered would not be used (for example, benzene would not be used as a reactant since forming the product would require a ring condensation reaction). Using these and other assumptions, a limited set of reactants can be identified. Potential reactants identified by Buxton, et al. (1997) are shown in Figure 7.3-2.
Figure 7.3-2 Potential reactants in carbaryl synthesis identified by Buxton, et al. (1997).
Once a set of potential reactants has been selected, a set of rules and constraints must be applied to describe how the reactants can interact to form molecules. The most obvious of these constraints are stoichiometric. For example, the product molecule contains 7 aromatic carbons bound to hydrogen and two aromatic carbons bound to other aromatic carbon. Thus, the reactants must provide sufficient aromatic carbons, of various types, to generate the product molecule. Similar stoichiometric constraints could be written for the other types of groups in the molecule. Some reaction pathway analysis methods assume that reactions, appropriately balanced for stoichiometry, can proceed with 100% selectivity and yield. Other methods include thermodynamic constraints on selectivity (see, for example, Crabtree and El-Halwagi, 1995).
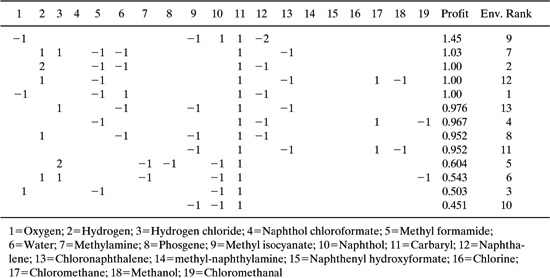
Once constraints are established, pathways can be identified and ranked. Ranking schemes might include cost and environmental performance metrics. Buxton, et al. (1997) identified and ranked 13 different reaction pathways for the synthesis of carbaryl. The results are shown in Table 7.3-1. The economic ranking is based on the price differential between product and reactants. The environmental ranking is based on the assumption that a fixed percentage of the materials used is released to the environment.
While the results of Table 7.3-1 are intriguing, it would be inappropriate to suggest that this type of analysis will yield the optimal reaction pathway. Rather, the point of these analysis methods is to inject systematic decision rules into the search for alternative pathways. Sets of starting materials are identified based on stoichiometry and chemical intuition. Then, pathways can be identified and potential upper bounds for selectivity can be estimated using thermodynamics. Finally, alternatives can be quickly ranked using economic and environmental criteria. These systematic procedures may lead to a desirable alternative pathway, or they may merely lead to a clear definition of the constraints that should be considered in evaluating alternative pathways.
7.4 Green Chemistry Expert System Case Studies
The concepts and examples described in this chapter provide only an introduction to the concepts of green chemistry. A mechanism for further exploring this area is the Green Chemistry Expert System (GCES), which is downloadable from the US EPA website (http://www.epa.gov/greenchemistry/tools.htm). This software provides more depth on many of the concepts and tools presented in this chapter; it also provides a searchable literature database on green chemistry.
Table 7.3-1 Alternative Pathways for the Synthesis of Carbaryl (Buxton, et al., 1997). The species numbers are listed below the Table and refer to the compounds shown in Figure 7.3-2; the profit is the difference in value between reactants and products and the environmental ranking is determined by assuming that a fixed fraction of the reactants and product are released to the environment.
If, for example, the concept of supercritical solvents were to be explored in more detail, a search of the GCES online database would provide literature citations for a number of case studies. More detail on alternative pathways for partial oxidation reactions or Friedel Crafts reactions could be found. A database of sol-vents could be searched, or the design of inherently safer chemicals could be explored. Some of the problems at the end of this chapter involve using the GCES to explore green chemistry alternatives.
Questions for Discussion
1. Suggest quantitative metrics that could be used to rank the impact that a synthesis method would have on the environment. How do your suggestions compare to those in Chapter 8?
2. What are the strengths and weaknesses of combinatorical approaches to identifying reaction pathways?
References
Anastas, P.T. and Williamson, T.C., eds., Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, Oxford University Press, New York, 1998.
Anastas, P.T. and Warner, J.C., Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998.
Breslow, R., Connors, R. and Zhu, Z., “Mechanistic studies using antihydrophobic enantioselective hydrogenation reactions,” Pure Appl. Chem., 68, 1527–33.
Breslow, R. and Zhu, Z., “Quantitative antihydrophobic effects as probes for transition state structures. 2. Diels Alder reactions,” Journal of American Chemical Society, 117, 9923–4 (1995).
Burk, M.J., “C2-symmetric bis(phospholanes) and their use in highly enantioselective hydrogenation reactions,” Journal of American Chemical Society, 113, 8518–8519 (1991).
Burk, M.J., Feaster, J.E., Nugent, W.A. and Harlow, R.L., “Preparation and use of C2-symmetric bis(phospholanes): Production of -amino acid derivatives,” Journal of American Chemical Society, 115, 10125–10138 (1993).
Burk, M.J., Gregory, T., Harper, P., and Kalberg, C.S., “Highly enantioselective hydrogenation of β-keto esters under mild conditions,” Journal of American Chemical Society, 117, 4423–4424 (1995).
Buelow, S., Dell-Orco, P., Morita, D., Pesiri, D., Birnbaum, E., Borkowsky, S., Brown, G., Feng, S., Luan, L., Morganstern, D., and Tumas, W., “Recent advances in chemistry and chemical processing in dense phase carbon dioxide at Los Alamos,” in Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, P.T. Anastas and T.C Williamson, eds., Oxford University Press, New York, 1998 (pp. 265–285).
Buxton, A., Livingston, A.G., and Pistikopoulos, E.N. “Reaction Path Synthesis for Environmental Impact Minimization,” Computers in Chemical Engineering, 21, S959–964 (1997).
Clark, J.H., “Green Chemistry: challenges and opportunities,” Green Chemistry, 1 (1), 1–11, 1999.
Crabtree, E.W. and El-Halwagi, M.M., “Synthesis of Environmentally Acceptable Reactions,” in Pollution Prevention via Process and Product Modifications, M.M. El-Halwagi and D.P. Petrides, eds., AIChE Symposium Series, 90(303), 117–127, 1995.
Dartt, C.B. and Davis, M.E., “Catalysis for Environmentally Benign Processing,”Industrial and Engineering Chemistry Research, 33, 2887–2899 (1994).
DeVito, S.C., “General Principles for the Design of Safer Chemicals: Toxicological Considerations for Chemists,” in Designing Safer Chemicals, S.C. DeVito and R.L. Garrett, eds., ACS Symposium Series 640, American Chemical Society, Washington, D.C. (1996).
Draths, K.M. and Frost, J.W., “Improving the environment through process changes and product substitutions,”Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, P.T. Anastas and T.C Williamson, eds., Oxford University Press, New York, 1998 (pp. 150–165).
Hurter, P.N. and Hatton, T.A., Langmuir, 8, 1291–9 (1992).
Li, C.-J., “Water as a benign solvent for chemical synthesis,” Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, P.T. Anastas and T.C Williamson, eds., Oxford University Press, New York, 1998 (pp. 234–249).
Li, C.-J. and Chan, T.H., Organic Reactions in Aqueous Media, Wiley, New York, 1997.
US Environmental Protection Agency, The Presidential Green Chemistry Challenge Awards, Summary of 1996 Award Entries and Recipients. EPA 744-K-96-001 (July, 1996).
US Environmental Protection Agency, The Presidential Green Chemistry Challenge Awards, Summary of 1997 Award Entries and Recipients. EPA 744-S-97-001 (April, 1998).
Trost, B.M., “The Atom Economy—A Search for Synthetic Efficiency,”Science, 254, 1471–7 (1991).
Problems
1. The text noted that the atom and mass efficiencies for addition reactions are generally higher than for substitution or elimination reactions. To illustrate this concept, calculate the mass and atom efficiencies for the following reactions:
(a) Addition reaction
Isobutylene + methanol → methyl, tert-butyl ether
C4H8 + CH3OH → (C4H9)–O–CH3
Calculate mass, carbon, hydrogen and oxygen efficiencies.
(b) Substitution reaction
Phenol + ammonia → aniline + water
C6H5–OH + NH3 → C6H5–NH2 + H2O
Calculate mass, carbon, hydrogen, nitrogen, and oxygen efficiencies.
(c) Elimination reaction
Ethylbenzene → styrene + hydrogen
C6H5–C2H5 → C6H5–C2H3 + H2
Calculate mass, carbon, and hydrogen efficiencies.
(d) Identify additional industrially significant examples of addition, substitution, and elimination reactions; calculate atom and mass efficiencies for these reactions.
2. In Table 7.2-1, atom economies are presented for a variety of oxidation agents. Confirm these calculations.
3. Use the US EPA’s Green Chemistry Expert System to identify a new solvent replacement technology. Review the original scientific literature on the technique and write a one-page summary of the new technology and the solvent it replaces.
4. Use the US EPA’s Green Chemistry Expert System to identify a new chemical synthesis method. Review the original scientific literature on the chemistry and write a one-page summary of the new reaction pathway and the pathway it replaces.
