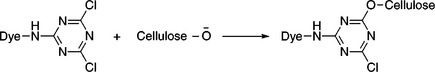Process control in dyeing of textiles
Abstract:
This chapter introduces the general technology for the dyeing of textile materials (yarn and fabric of cotton, polyester, nylon, and blends) with respect to dye applications (reactive, disperse, acid, etc.), dyeing methods (batchwise and continuous) and dyeing machines (package, overflow/jet and continuous). The corresponding dyeing processes and the process controls are discussed in detail.
13.1 Introduction
Dyeing and printing processes are value-added treatments for most textile materials. A dyeing process is the interaction between a dye and a fibre, as well as the movement of dye into the internal part of the fibre. Generally, a dyeing process involves adsorption (transfer of dyes from the aqueous solution onto the fibre surface) and diffusion (dyes diffused into the fibre). In addition to direct absorption, dyeing may also involve the precipitation of dyes inside the fibre (vat dyes), or chemical reaction with the fibre (reactive dyes). From the view of colouration, printing can be considered as partial dyeing with different colours on fabric to form an attractive pattern. Table 13.1 shows some typical dyes applied in the dyeing or printing of various textile materials. A dyeing or printing process is complicated, since it involves fibre kinds, yarn or fabric structures, dyes and chemical auxiliaries, as well as dyeing technology. In order to achieve the required dyeing or printing quality, all factors that may influence the dyeing or printing process must be precisely controlled (Table 13.2).
Table 13.1
Classification of dyes according to usage
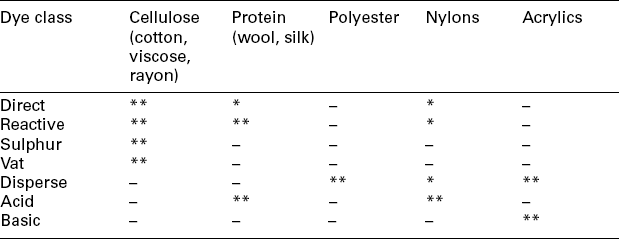
**The most important dye applied in dyeing and printing.
*Less important dye applied in dyeing and printing.
–No practical application.
Table 13.2
Influence factors in dyeing or printing process
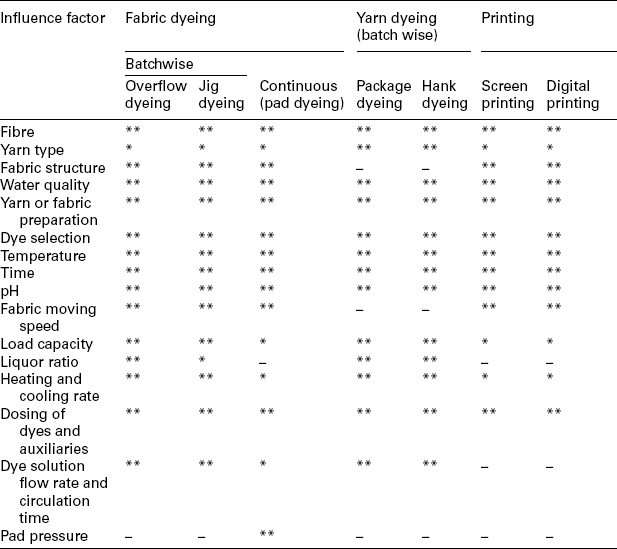
**The most important influence factor in dyeing or printing.
*Less important influence factor in dyeing or printing.
–No practical application.
The water quality for dyeing and printing is very important (Yeung and Shang, 1999), and it must meet the requirements as listed in Table 13.3. Generally, purification of the water is required to avoid such unpleasant dyeing defects as unevenness, dye precipitation, shade dulling, harsh handle or chalking. The preparation and pretreatment of yarns or fabrics also have significant effects on dyeing quality. For example, poor scouring and bleaching can lead to serious unevenness in dyeing, and the non-uniform winding of a yarn package can lead to colour differences in package dyeing. As a result, sufficient and uniform preparation and pretreatment can make the dyeing quality more controllable and predictable. Therefore, process control in dyeing and printing is of significance in achieving high-quality products and increasing dyeing production efficiency. Dyeing technology and dyeing process control will be discussed in detail in this chapter, and printing technology and printing process control will be discussed in next chapter.
Table 13.3
The basic requirements of water quality for dyeing and printing process
| Item | Requirement |
| Colour | Colourless (dilution factor < 10) and without turbid and suspended solid |
| pH | 6.5–7.5 |
| Total hardness (as CaCO3) | < 50 mg/L (for general use) |
| < 17.5 mg/L (for dyestuff dissolution) | |
| Iron | < 0.1 mg/L |
| Manganese | < 0.1 mg/L |
| Transparency | > 30 cm |
Note: The requirements were recommended by China Association of Dyeing and Finishing Industry (Xi, Chen and Ma, 2006).
13.2 Dyeing of cotton
Cotton is the most important nature textile fibre. Cotton fibres are composed of cellulose, and can be dyed with reactive dyes and direct dyes, as well as vat, sulphur and azoic dyes in proper processes to meet the diversified demands in end-use, such as shades and fastness standards.
13.2.1 Reactive dye and dyeing technology
Reactive dye is the dye that can react with a fibre to form a covalent link, that is forming a permanent attachment in the fibre and could not be removed by repeated treatment with boiling water under neutral conditions. Consequently, the dyes become parts of the fibre, leading to outstanding colour fastness to wash.
Due to the advantages of full colour ranges, brightness, high fastness, low cost, easy application, etc., reactive dye became the predominant dye for cotton dyeing and printing in textile industry since it was invented. Compared with direct dye, reactive dye is applied as easy as direct dye but has very high levels of fastness, especially for wet fastness.
Properties of reactive dye
The characteristic structure of a common reactive dye includes the following components:
1. chromogen, contributing to the colour display;
2. reactive group (s), enabling the chemical reactions between fibre and dye;
3. bridging link, linking the reactive group with chromogen;
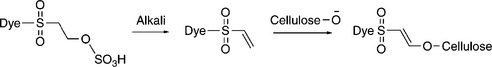
As the name implies, reactive dye has reactive group(s) to form covalent bonds chemically with cotton and become part of it, rather than as an independent coloured substance within the fibre. Under mild alkaline conditions, the reactive group(s) on the dye molecule can react with the oxygen atom in the cellulose hydroxyl group, either by nucleophilic substitution, or by addition, and sometimes by both mechanisms for a dye with two or more reactive groups (Broadbent, 2001). The representative substitution and addition reactions between dye and cellulose are shown in Fig. 13.1 and 13.2, respectively (Gordon and Hsieh, 2007).
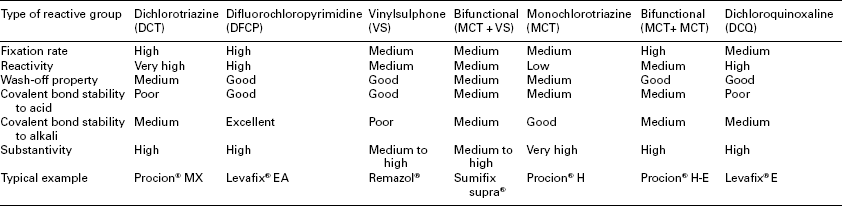
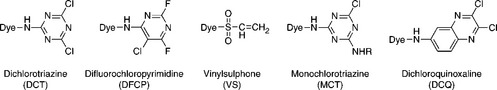
Both the chromophore and reactive group in a reactive dye govern its application characteristics, such as reactivity, substantivity/affinity, diffusion coefficient and solubility. Substantivity, that is the tendency of dye to transfer from dyebath to fibre substrate, is determined by both but the former has a more direct influence. Reactivity and the stability of the dye–fibre bond are determined by reactive group(s). Recently, reactive dyes with two, even three, reactive groups have been widely used for achieving a greater fastness and deeper shade. Based on the varied reactive groups, reactive dyes can typically be classified as dichlorotriazine (DCT), difluorochloropy-rimidine (DFCP), vinylsulphone (VS), monochlorotriazine (MCT), dichlo-roquinoxaline (DCQ), and the mixtures of MCT + VS, and MCT + MCT, etc. Typical structures of reactive dyes with one reactive group are shown in Fig. 13.3, and the major properties of the different types of reactive dyes are listed in Table 13.4.
A conventional dyeing process of reactive dyes contains three stages: (1) adsorption and diffusion, (2) fixation, and (3) wash-off. Reactive dyes can be used in both batchwise and continuous dyeing methods. Generally, dyes with relatively high substantivity and slow diffusion are more appropriate for exhaust dyeing in the batchwise method, such as yarn dyeing with a package dyeing machine or hank yarn dyeing machine, as well as fabric dyeing with an overflow dyeing machine or jig dyeing machine. Unlike exhaust dyeing, dyes with low substantivity and quick diffusion are more suitable for the continuous pad dyeing method. Generally, there are two types of the continuous pad dyeing process, the simpler single-pad in which dyes and alkali are padded in one dyebath, and the versatile double-pad in which dyes and alkali are padded separately and sequentially.
Although exhaust dyeing and continuous dyeing are quite different in terms of process control, as listed in Table 13.2, there are common fundamental technology parameters, apart from the machinery variables, which influence the dyeing procedure. These parameters include temperature, time, pH, liquor ratio and auxiliaries.
Temperature influence
In a reactive dyebath, temperature has profound effects on both dye and cotton in aqueous solution. The increased temperature may bring about better dye penetration, more rapid diffusion, better evenness, but may reduce dye substantivity and increase the risk of dye hydrolysis. Also, raised temperature leads to the opening-up of the cellulose structure, which activates the dye–fibre interaction (Ibrahim and Sayed, 1993). Therefore, the dyeing temperature is determined by both the substantivity and reactivity of the dye and the structure of fibre. For high twisted yarn or tight fabric, or for those dyes being not easily level dyed, temperature could be raised to 98 °C to promote migration and penetration in the adsorption phase with low reactivity dyes, and cooled to 80 °C for fixation by adding alkali. For dyeing loose fabric, such as knitgoods, generally a warm dyebath with temperature between 50 °C and 65 °C is preferred. Good results can be obtained by carefully controlling the temperature increase rate during heating-up.
The pH influence
The pH primarily influences the concentration of the cellusate anion (cellulose-O–) on the fibre, as well as the hydroxyl ion (-OH) concentration in the dyebath and on the fibre. Reactive dyes consume some alkali for both dye fixation and dye hydrolysis. The internal alkalinity of fibre also absorbs alkali. These lead to the dyebath pH at the dyeing end being always lower than its initial value. Generally increasing dyeing pH in the fixation stage can accelerate the reaction rate between dye and fibre. For batchwise dyeing method, the dyebath pH is recommended to be controlled ranging from 10.5 to 11. Even with the low reactivity dyes, a pH exceeding 11 is still not appropriate since an unduly high pH will enhance dye hydrolysis and reduce dyeing efficiency in terms of depth and fixation. NaOH, Na2CO3, NaHCO3 or a combination of these are the conventionally used alkalis, among which Na2CO3 is the most commonly applied. The selection of alkali is usually related to the dyes applied and the dyeing method adopted. The fixation rate of dyes and the dyeing evenness can be controlled by the dosing rate of alkali. Dyes with high reactivity are sensitive to alkali concentration in dyebath, and have an optimal temperature range of between 40 °C and 60 °C. Typical examples are DCT, DFCP, DCQ and VS.
Electrolyte effect
When cotton fibre is in an aqueous solution, the fibre surface presents negative charge, mainly due to the dissociation of accessible cellulose hydroxyl (Cell-OH) groups and the rearrangement of the charge groups at the interface between the fibre and the water. Reactive dyes, as well as other soluble dyes for cotton, carry negative charges because of sulphate group(s) on their molecules for solubility. Therefore, the electrostatic repulsive force between fibre surface and dyes has to be overcome in order to diffuse the dyes through the fibre-water interface. The most common method to overcome the electronic repulsion in exhaust dyeing is to add large quantity of electrolyte (so-called salt), sodium chloride (NaCl) or sodium sulphate (Na2SO4), in the dyebath. The presence of electrolyte in the dyebath reduces the extent of the surface charge, which leads to a reduction in repulsion between ionised dyes and fibre, thereby increasing the substantivity of dyes. Trichloropyrimidine and aminochlo-rotriazine dyes are typical dyes exerting high exhaustion without alkali, thus it is necessary to control the salt-dosing rate carefully with those dyes to ensure levelling. There are four methods of adding salt in general: (1) portion adding/dosing salt; (2) adding salt at the start of dyeing (especially for dyeing dark shade or nonsensitive colour); (3) adding salt and soda at the start of dyeing; and (4) all-in process. The first two methods are used widely in production.
Liquor ratio
Water is an essential medium for a dyeing process in most dyeing methods. The liquor ratio in an exhaust dyebath is the ratio of the weight of the dry material being dyed to the water weight of the dyebath. Example, a liquor ratio of 1:10 implies 1 kg fibre is dyed in 10 L water. A large liquor ratio benefits dyeing levelness, but has negative impacts on production costs and environment, and results in more carbon emission. Therefore, a low liquor-ratio dyeing machine, such as 1:5 for cotton, is being applied more broadly (Shang, 2002; Shang and Zhuo, 2003).
Auxiliaries
Suitable auxiliaries in the dyebath could enhance dyeing qualities. Both the anionic surfactant and the non-ionic surfactant can increase dyeing evenness. The former can improve dye uptake, and the latter may decrease the exhaustion, but both may slow down the hydrolysis rate of reactive dye. The presence of triethanolamine can improve the swellability of the cellulose structure, and thereby enhance colour strength. Urea could increase the solubility of reactive dye in water and enhance the swellability of cellulosic fibre, which is critical in continuous dyeing (Shamey and Hussein, 2005).
Reactive dye selection and dye compatibility
Dye selection is the first important issue in dyeing, considering both the dyeing behaviour and production cost. Table 13.5 lists the majority of considerations in dye selection.
Table 13.5
The key issues concerned for dye selection
| Issue | Requirement |
| Dyeing behaviour | Shade consistency |
| Evenness | |
| Exhaust and fixation rate | |
| Colour fastness | |
| Reproducibility | |
| Compatibility | |
| Production cost | Dye price |
| liquor ratio | |
| Auxiliary agents required | |
| Dyeing temperature and time | |
| Washing effectiveness | |
| Short dyeing process | |
| Right-first-time | |
| Others | Environmental friendly |
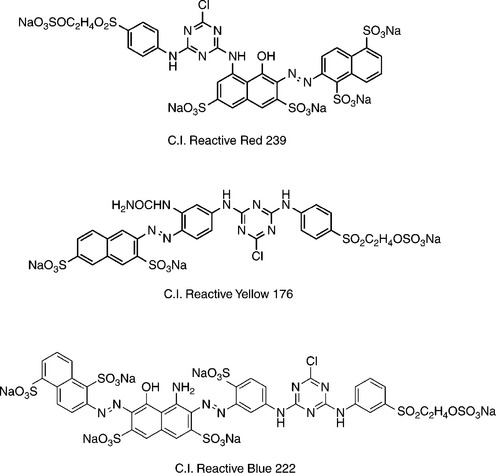
Nowadays, colour matching with three dyes, such as with the dyes of three primary colours in red, yellow, and blue, is very common in practice. Therefore the compatibility of the selected three dyes should be good, that is the dyes should have the similar dyeing behaviour, including tone-on-tone build-up properties, equal robustness to different dyeing conditions, and no blocking effect between them. Figure 13.4 shows a set of the typical commodity dyes of three primary colours in red, yellow, and blue applied for matching medium and deep shade. There are also sets of commodity dyes of three primary colours with higher colour fastness applied for matching light shade.
Reactive dyeing process control in the exhaust dyeing process
The exhaust dyeing method is mainly applied in the batchwise dyeing machines, such as jig or overflow dyeing machine for fabrics (Shang and Chong, 2002; Shang and Zhuo, 2003), and the package or hank dyeing machines for yarns.
In order to identify the tone-on-tone build-up properties of reactive dye in exhaust dyeing, the concept of SERF value is developed. As shown in Fig. 13.5, S is the exhaustion value before the addition of alkali, indicating the substantivity; E is the final exhaustion value, indicating the exhaustion ability; R is the fixation value when fixation lasts 10 min (another explanation is that R is the fixation rate when fixation lasts half of the total fixation time when fixation reaches the maximum value), indicating the fixation rate; and F is the final fixation value, indicating the reactivity.
Depending on the difference between E and S, the dyeing curve for exhaust dyeing can be classified into several categories.
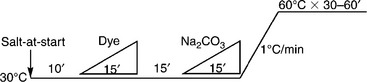
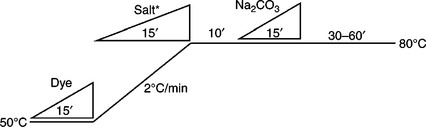
For dyes with a large (E–S) value, it means the substantivity of these dyes is low. Salt can be added at the beginning of dyeing to increase substantivity, which accelerates the dye adsorption rate. Fixation can be controlled by either the rate of alkali dosing or temperature increase.
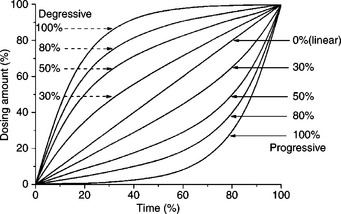
The dosing unit is widely equipped with dyeing equipment, and the dosing rate in some advanced dyeing machines can be controlled progressively or retrogressively (Fig. 13.6). The term ‘dosing’ here not only means simply the addition of chemicals by the dosing system, but also indicates that much more attention has to be paid to the ‘dosing’ stage, and a versatile dosing programme is necessary in order to obtain the best results.
Figure 13.7 shows the alkali-controllable process, in which alkali is added more carefully than with regular dosing, in a constant-temperature dyebath. Figure 13.8 shows the controlled temperature process in which alkali is regularly dosed at room temperature (assuming 30 °C) and the temperature rising is controlled more carefully. The other dyeing profiles are shown in Table 13.6. The symbol of the right-angled triangle ‘ ///inlineimage/// in all dyeing curves in this chapter denotes the linear dosing of chemicals and dyes. For alkali dosing, progressive dosing may be required for some dyeing processes in practice for achieving better dyeing results.
Table 13.6
The exhaust dyeing profiles of dyes with large (E–S) value
1Example of available dye: Remazol®.
2owf: on the weight of fabric.
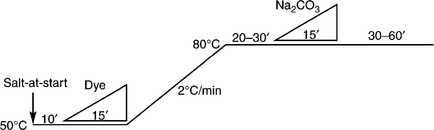
For dyes with low (E–S) value, indicating high substantivity, ‘salt-at-start’ is not recommended, because these dyes have the poor migration ability and unevenness is inevitable. As a result, controlled salt-dosing is necessary to control the substantivity. Salt-dosing combined with temperature increase is adopted to control the exhaustion rate (Fig. 13.9). Controlled dye-dosing can be adopted for dyes with moderate (E–S) value (Fig. 13.10). The other dyeing profiles are shown in Table 13.7.
Table 13.7
The exhaust dyeing profile of dyes with small to moderate (E-S) value
1Example of available dyes: Procion H-E for small (E–S) value; Procion XL + or Procion H-EXL for moderate (E–S) value.
13.9 Dyeing curve for dyes with small (E–S) value. * Linear dosing of salt during temperature rising period for time saving.
When the dyeing process is ended, a wash-off process is necessary to achieve optimal colour fastness. The unfixed dye on fibre will be effectively removed under hot soaping treatment.
Figure 13.11 shows a general batch wise wash-off process for products dyed with medium shade. More washing-off may be needed for deep shade, until the required colour fastness is achieved.
Reactive dyeing process control in continuous process
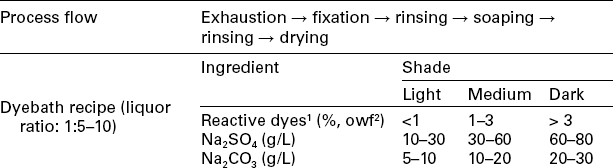
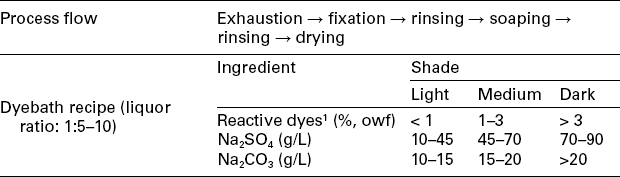
The continuous dyeing method for woven fabric, especially for cotton or cotton blend, is an important processing technology for reactive dyeing, especially when a large amount of fabric needs to be dyed. Unlike exhaust dyeing, in which dyes of different substantivity are properly all available, dyes with only low to moderate substantivity are preferable in continuous dyeing. The continuous dyeing process can generally be classified into four types: (1) pad-dry-pad-steam method (Table 13.8), (2) pad-steam method (Table 13.9), (3) Econtrol process (Table 13.10), and (4) pad-dry-cure method (Table 13.11).
Table 13.8
The continuous dyeing profile of the pad-dry-pad-steam method
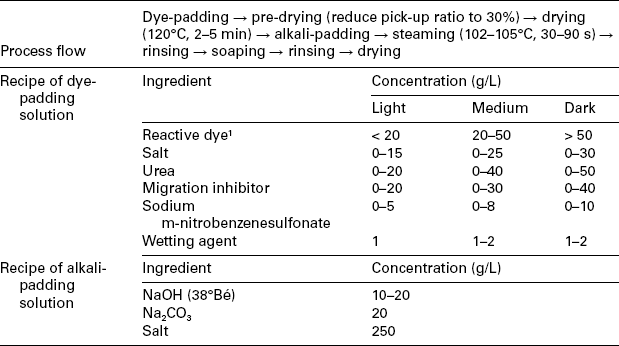
1Example of available dyes: Remazol®, Levafix® E.
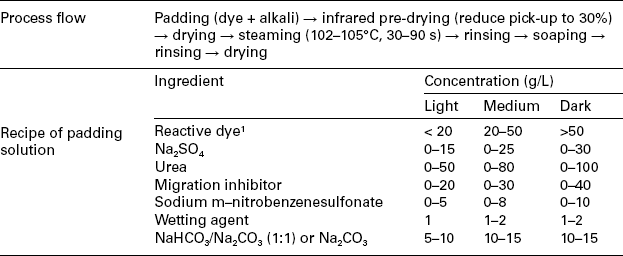
Table 13.9
The continuous dyeing profile of pad-steam method
1Example of available dyes: Remazol®, Procion® H-E.
Table 13.10
The continuous dyeing profile of Econtrol method
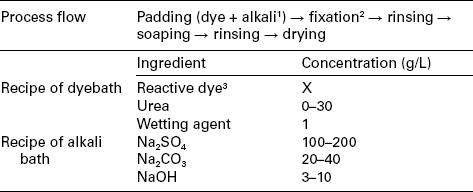
1The dye solution and alkali solution are mixed at the point when they were just introduced into pad-bath trough;
2Hot air with relative humidity of 25% (120 °C, 2–3 min);
3Example of available dyes: Remazol®.
Table 13.11
The continuous dyeing profile of pad-dry-cure method
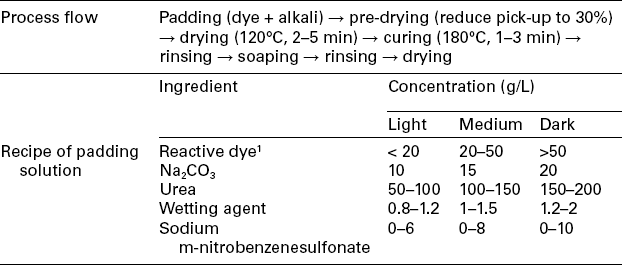
1Example of available dyes: Procion® H-E.
Note:
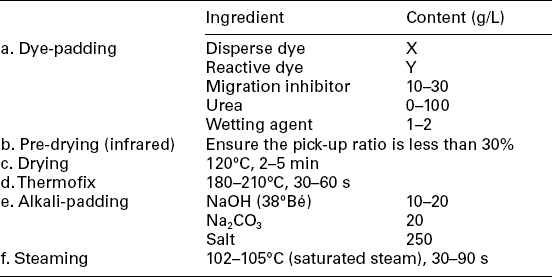
1. Pre-drying with an infrared dryer is recommended to reduce the pick-up ratio to 30% before drying in order to minimise unexpected dye liquor migration.
2. The addition of salt to the alkali bath is to minimise colour-bleeding. However for dyes with low solubility, a high concentration salt might cause dye precipitate, therefore a dispensing agent (10 g/L) in the pad-bath is needed to ensure stability.
3. Saturated steam is preferred for reactive dye fixing. The steaming time may vary depending on the dye applied.
4. In order to prevent the dye migration, a migration inhibitor, such as sodium alginate (4%), can be added into the dye solution (20–40 g/L).
The process parameters of pad-steam method are similar to those of the pad-dry-pad-steam process. But more attention has to be paid to the stability of the mixed dyebath. Generally Na2CO3 is more appropriate for dyes with relatively lower reactivity, such as MCT dyes; NaHCO3 is properly used with dyes of relatively high reactivity, for avoiding dye hydrolysis. It is recommended to use the continuous dyeing machine with the special chemical mixing equipment, which can mix dye and alkali at the point when they are just introduced into the pad-bath trough.
In the Econtrol process, recently developed to eliminate the drying process for energy saving and low carbon production, the padded fabric is directly steamed at 120 °C with hot air at a low relative humidity (25%). This process combines the advantages of short processing time and high fixation rate.
The pad-dry-cure process has the advantages of higher fixation rate, easy operation and good reproduction. The curing temperature is around 180 °C. Because little water is present on the fabric when curing, large amounts of urea must be added to the dyebath to help fibre swelling and dye fixation. One disadvantage of this process is the risk of urea decomposition during curing, especially when the temperature is extremely high. Recently, thermal-stable chemicals performing the same functions, such as dicyanoguanidine, are more appropriate (even though more costly) to replace urea to avoid harmful by-products generated during curing.
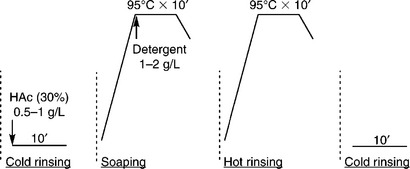
In the continuous dyeing range, the wash-off unit is usually installed at the end of the production line. The washing unit may include a set of washing boxes. The continuous washing process may involve three stages: (1) initial rinsing, (2) soaping, and (3) final rinsing. Initial rinsing employs either cold or warm water to remove superficial loose colour (sometimes HAc is required for neutralisation); soaping uses hot water (95 °C) containing detergent (1–3 g/L) to remove the unfixed dye inside the fabric; and final rinsing is to ensure that loose colour is cleaned thoroughly.
13.2.2 Other dyes available for cotton dyeing
In addition to reactive dyes, other available dyes for cotton include direct dyes, sulphur dyes, vat dyes and azoic dyes. The invention of direct dye, with substantivity for cellulosic fibres, greatly preceded the advent of reactive dyes. The weakness of direct dyes is poor colour fastness, especially the wet fastness. Although the wet fastness of direct dyes is generally not as good as vat dyes, due to easy application and a broader shade range direct dyes were the most important dye for cotton dyeing before the 1950s. Since then, the position of direct dyes in cotton dyeing has been replaced by reactive dyes. Direct dyes nowadays are only used in the applications where colour fastness is not important. An example of the direct dyeing process is shown in Fig. 13.12.
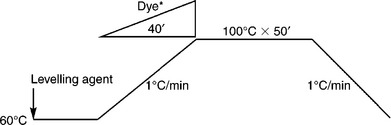
13.12 Direct dyeing curve for cotton. * Linear dosing of dyes during temperature rising period for time saving.
Sulphur, vat, and azoic dyes can be categorised together because they share the same dyeing mechanism, by which soluble forms of the dyes are first absorbed by cotton and then transferred into insoluble form in the fibre substrate. Vat dyes and sulphur dyes are still important for dyeing and printing (discharge and resistant style) of cotton. The dyed goods have very considerable fastness. Indigo dyes are the oldest vat dyes derived from natural sources. Indigo and sulphur dyes are the most important dyes applied in denim production. The dyeing application of vat dyes and sulphur dyes is similar, but the latter are particularly important for deep shades dyeing. The azoic dyes are of less importance in dyeing and printing because of their relatively low colour fastness.
13.3 Dyeing of synthetic materials
There are lots of synthetic textile fibres. However, the dyeing of polyester and nylon is discussed only below since polyester is the most popular synthetic textile fibre and nylon runs the second.
13.3.1 Polyester and its dyeing properties
Polyester (PET) is a synthetic fibre and has the properties of hydropho-bicity, crystallisation and thermoplasticity. It becomes softer when being heated over glass temperature Tg (the transfer temperature of polymer from glassy state to a state of higher elasticity) and melts at temperatures above melting point Tm. When polyester fibre was discovered in 1947, none of the water-soluble dyes used at that time, such as direct, acid and basic dyes, could be applied for polyester dyeing, since there is no dyeing site on this fibre. Later, disperse dye, which was originally developed for the dyeing of cellulose acetate fibre, was found to be practical for dyeing polyester.
The diffusion of disperse dyes into polyester, including all the thermoplastic fibres, is described by free volume theory (Vrentas and Duda, 1977). According to this theory, disperse dyes are adsorbed on the fibre surface and diffuse into the fibre through transient passages, resulting from the segmental motion of polymer chains in material substrate when sufficient thermal energy is provided. When polyester reaches Tg, the lowest temperature at which the polymer chains begin to vibrate and slide past each other when a force is applied, disperse dye begins to diffuse into fibre by ‘jumping’ from one site to another. With continuous thermal energy provided dyes are gradually embedded into the fibre substrate, until the distribution in fibre and dyebath reaches equilibrium. When the system temperature is decreased after equilibrium, the vibration and sliding of polymer chains is weakened, and finally the dyes in fibre are blocked inside the fibre. Based on the free volume theory, dyeing temperature must be beyond Tg to generate enough free volume in fibre to accommodate the dye.
13.3.2 Disperse dye and polyester dyeing
Disperse dye, as indicated in Table 13.1, is the only dyestuff being available for polyester dyeing.
Properties of disperse dye
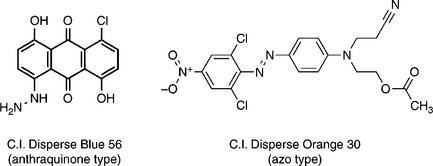
Unlike the anionic dyes for cotton, disperse dyes are relatively insoluble in cold water and have only limited solubility in boiling water. As their name implies, disperse dyes are present in the dyebath in a superfine aqueous suspension with dispersing agents. However, they possess substantivity for hydrophobic fibre, such as polyester and nylon, in which they are more ‘soluble’ than in aqueous. The slight water solubility of disperse dyes results from the presence of polar substituents in their molecular structures. It gives rise to the formation of dyes in their monomolecular forms in the true aqueous solution, which helps the disperse dye to penetrate into hydrophobic fibre during dyeing. Apart from a few bright pink and blue shades, anthraquinone disperse dyes (Fig. 13.13) are gradually being replaced by azo disperse dyes because of production costs and environmental threats in the synthesis process.
Disperse dyes are primarily marketed in the forms of powder or grain, and less frequently as paste or aqueous dispersion. All of these forms contain micro-fine dye particles and dispersants varying in dose and type. Dispersant is added, usually anionic surfactant, to clad each dye particle with a monomolecular layer of adsorbed dispersant. When the disperse dyes ‘resolve’ in the dyebath, hydrophobic chains in the dispersant structure embed into the hydrophobic dye particle, leaving the anionic groups exposed to the surrounding water. The overall negative charge on the surface of each particle prevents coalescence and aggregation. Consequently, the suspension of dye particles in the dyebath maintains stability and dyeing readiness (Broadbent, 2001).
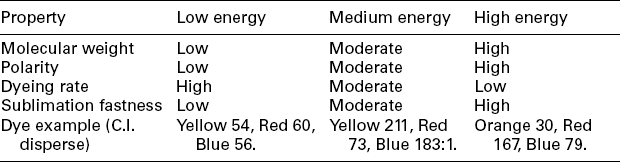
Disperse dyes have distinctive dyeing properties on polyester. They are often classified in accordance with their dyeing rate and sublimation fastness, which are closely related to the polar group number and molecular weight of dye molecule. Table 13.12 shows the most common classification of disperse dye in the exhaust dyeing of polyester, as well as a set of the typical commodity dyes of three primary colours, red, yellow, and blue, applied for relevant shade. Disperse dyes with low molecular weight, classified as low energy disperse dyes, present more levelling and faster dyeing rate in the dyeing progress but poor sublimation fastness for its final products. On the other hand, those dyes with higher molecular weight, which exhibit weak migration and low dyeing rate but good sublimation fastness, constitute the high-energy disperse dyes. Low energy dyes are more suitable in the exhaustion dyeing, while high-energy types are preferred in thermofix dyeing.
Factors influencing disperse dyeing
The major influencing factors on disperse dyeing process are temperature, time, pH and auxiliaries. Polyester, in common with other synthetic fibres, is quite crystalline and very hydrophobic. Even at 100 °C, which is greater than the Tg of polyester by about 20 °C, dye diffusion is so weak that no satisfactory exhaustion can be obtained for most of the disperse dyes. As dyeing temperature increases from 100 °C to 130 °C under pressure, dyeing rate accelerates considerably, giving a better coverage of filament irregularities due to improved dye migration. For each particular dyeing, there always exists an optimum temperature/time profile, relating to the type of goods, dyes and the dyeing equipment involved. Dyeing time can be minimised by controlling the temperature to offer a reasonable exhaustion rate giving absorption uniformity. Conventionally, the dyeing time ranges from 30 to 60 min at 130 °C to obtain sufficient equilibrium. The pH of dyebath is usually adjusted within 4.5–5.5 using either acetic acid alone or in combination with other acids. Acidic conditions could minimise reduction and hydrolysis of some disperse dyes with chemical groups that are sensitive to alkali at high temperature.
A carrier, an organic compound dissolved or emulsified in the dyebath, can be used to assist the dyeing of polyester around 100 °C. The typical method is also called carrier dyeing. These substances enable the deep shades can be obtained at the boil within a reasonable dyeing time. Nowadays, carrier dyeing is declining for environmental and other considerations (Broadbent, 2001).
Disperse dyeing process control
Dyeing of polyester with disperse dye can technically be launched in either batchwise or continuous processing.
Exhaust dyeing
Batchwise dyeing for polyester is for dyeing polyester with batchwise dyeing machines, such as package dyeing machines for yarn, and jet or overflow dyeing machines for fabric, at high temperature (usually around 130 °C).
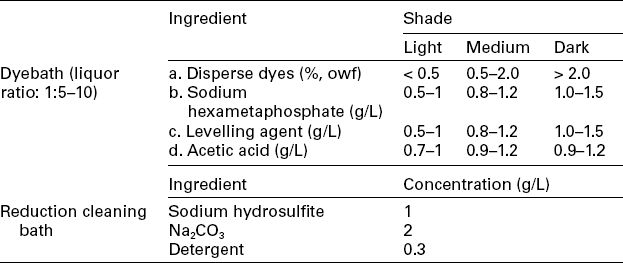
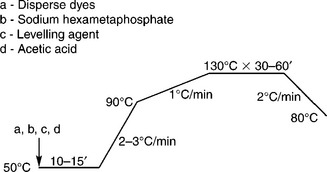
Figure 13.14 shows the high temperature dyeing profile for polyester. Dyes selected must be subject to the same energy class. The build-up of colour on shade requires that the compatibility of dyes should be good, that is having about the same dyeing rate, similar to reactive dyeing. When the dyebath temperature reaches 90 °C, at which dyeing initially begins, the rate of temperature increase should be slowed down to eliminate dye agglomeration. When dyeing equilibrium is obtained, slow cooling is much preferred to minimise the generation of oligomer, which would lead to deterioration in appearance of the dyed goods. Reduction cleaning is important to get rid of the loose colour in fabric, and colour fastness can be enhanced. Thorough cleaning is necessary to ensure the dyed goods possess satisfactory fastness. In practice, reduction cleaning at 80 °C for 15 min, followed by general cool rinsing, is usually enough to wash down the unfixed dye. To neutralise the dyed fabrics, acetic acid (60%) of 1–2 mL/L is required in the final rinse bath. The other related dyeing profiles are shown in Table 13.13.
Continuous dyeing
Continuous dyeing for polyester is usually carried out in the continuous Thermosol (thermofix) dyeing machine, which was invented by Du Pont in 1949. It involves first padding the disperse dyes into fabric, drying, and then fixing the dyes by dry heating at a temperature of about 190–205 °C. At this elevated temperature, the fibre molecular chains open up and the dispersed dyes vaporise and diffuse into the polymer. On cooling, the dyes are trapped within the fibre, yielding coloured fibres with good fastness. This process is rarely adopted for pure polyester dyeing, but is employed frequently in the dyeing of polyester/cellulose woven blends. The process control for the Thermosol process will be discussed later in Section 13.4.2.
13.3.3 Dyeing of nylon
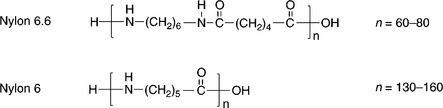
As the second important synthetic fibre, nylon is becoming more and more significant throughout the textile industry. Nylon, with a high strength but low density, is a polyamide fibre with two kinds of chemical structure, namely nylon 6 and nylon 6,6. Their structures are illustrated in Fig. 13.15.
Acid dyeing
Nylon fibre is a hydrophobic polymer with an α-terminal amino group, so it can be dyed with disperse dyes as well as other water-soluble dyes such as acid dye and reactive dye. The exhaust dyeing process with batchwise dyeing machine is the most common method for pure nylon dyeing.
Acid dyes for nylon are divided into three types depending on their dyeing properties. These dyeing properties are shown in Table 13.14.
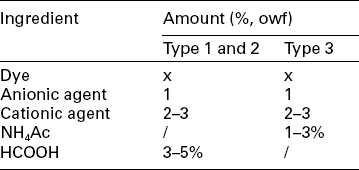
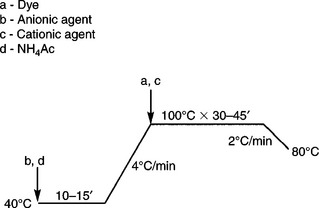
One of the most common defects of acid dyeing for nylon is the phenomenon of the barré result. Barré derives from chemical variation in manufacturing, oxidation of terminal amino in melt spinning and/or physical changes in spinning, drawing or heat-setting. It gives rise to the stripes on dyed goods. It is usual to add dyeing auxiliaries to control exhaustion to reduce the tendency of stripe formation and improve levelling. An anionic agent competes with an anionic acid dye for dyeing sites, while the cationic agent captures anionic acid dye first and then liberates it in proper condition, both of which decrease the dyeing rate to obtain levelness (Lewis and Marfell, 2004). A typical dyeing recipe is shown in Table 13.15, and the matched dyeing curves for different dye types are shown in Fig. 13.16 and 13.17.
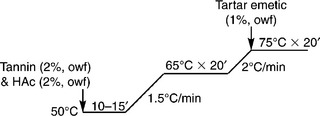
It is difficult to obtain satisfactory wet fastness for dyed nylon with acid dyes for medium and dark shades; dye fixation is therefore a necessity. For example, tannin and tartar emetic are applied for acid dye fixation, and the process details are shown in Fig. 13.18. It is important to note that excessive tannin and tartar emetic will cause deterioration in handle.
Disperse dyeing
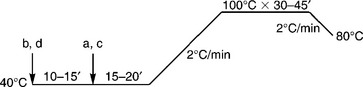
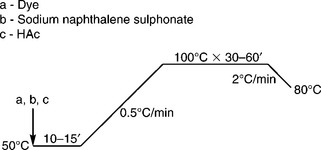
Disperse dye is another dye class that can be applied in nylon dyeing because of the hydrophobic property of nylon. The glass transition temperature (Tg) of nylon is 50–60 °C and it is easier to swell than polyester, thus dyeing at boil under atmospheric pressure can yield satisfactory exhaustion. Disperse dye has better coverage of barre than acid dye, but it is difficult to obtain dark shades, and some disperse dyes exert poor washing fastness, especially for relatively deep colour. Nowadays, with the increasing requirement of higher colour fastness, dyeing nylon with disperse dye is declining. The typical dyeing curve is shown in Fig. 13.19 and the dyeing recipe is shown in Table 13.16.
Table 13.16
Dyeing recipe for nylon with disperse dye
| Ingredient | Amount (%, owf) |
| a. Dye | × |
| b. Sodium naphthalene sulphonate | 1–2 |
| c. HAc (80%) | 1 |
In some cases, to obtain better levelling and dark shade, disperse dye and acid dye are combined in nylon dyeing. A cationic agent is not recommended in disperse dyeing, since it would be absorbed by disperse dye particles, decreasing the stability of dyebath. The non-ionic type is greatly preferred.
13.4 Dyeing of blends
Blends are composed of a mixture of two or more kinds of fibre materials into a single yarn or fabric. Polyester/cellulose blends occupy about 60% of the total production of all types of blends in textile industry, and are of more significant status than others.
When dyeing is involved in blend processing, the respective dyeing of each material in the blends is so different that solid dyeing mostly cannot be completed with a single class of dye and/or in a single stage like its pure components. The dyeing property of blends follows that of its component fibres when they are dyed alone, although there are slight differences when varied processing methods applied. These differences will be discussed in the following paragraphs, along with process control.
Dye selection is closely related to the nature of the composite of the blends. Polyester/cellulose blends are dyed mostly with disperse dye for polyester part, and with reactive dye for the cellulose component, either in exhaust or continuous process.
13.4.1 Exhaust dyeing
Exhaustion dyeing processes for blends with disperse/reactive dyes are of two kinds. These are (1) the two-bath method, in which the two fibre in blends are dyed in two independent dyebaths separately with different dye-stuffs, and (2) one-bath-two-stage, in which the dyestuffs are mixed together in a single bath, but the dyeing process is divided into separate stages for each fibre.
Two-bath method
In the two-bath method, usually the polyester is dyed first, followed by the cellulose part, which can avoid negative influences under the polyester dyeing conditions, such as high temperature, acidity and reduction cleaning on cellulose reactive dyeing. This process is similar to that of pure polyester and pure cotton dyeing. The two-bath process ensures the final goods have maximum brightness and colour fastness. Moreover, because of the independent dyebath for the different classes of dyes involved, there is considerable freedom in dye selection. There is no risk of interaction between reactive and disperse dyeing system. Nevertheless, the biggest shortcoming of such process is that the total dyeing time is greatly extended, which rapidly boosts production costs.
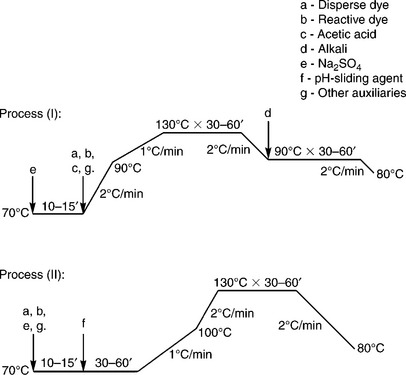
One-bath-two-stage method
The one-bath-two-stage method is an alternative method for blends dyeing. The term ‘two-stage’ means reactive dyeing for cotton is implemented in two steps. Exhaustion of reactive dye is carried out first and then fixation is activated by pH change (or pH-sliding) from acidic to alkaline. The dyeing curve for this process has some variation depending on the exhaustion sequence of each dyestuff. Fig. 13.20 shows two dyeing curves; in process (I) disperse dye and reactive dye are first exhausted by blends simultaneously, then the reactive dye is fixed by alkali, while in process (II) the pH adjustment is achieved by a pH-sliding agent. The pH-sliding agent is a specific auxiliary to gradually change the dyebath pH, either from acidic to alkaline, or from alkaline to acidic. In process (II), the pH-sliding agent presents alkaline character initially in the dyebath, and then it decomposes and liberates an acidic substance as the temperature increases. Reactive dye exhaustion is completed before the addition of the pH-sliding agent, and the dye fixation is carried out after the addition of the pH-sliding agent. When reactive fixation is accomplished eventually, the temperature is further raised and the pH-sliding agent releases acids to shift the dyebath from alkaline to acidic, meeting the requirements of disperse dyeing. In this process, the total processing time can be shortened to four hours. But there are further requirements for dye selection in this one-bath method.
In the above two methods, reactive dye and disperse dye are simultaneously present in one dyebath at the same time. As a result, each must be stable enough to withstand the dyeing condition for the other dyestuffs, that is reactive dye must withstand the acidic bath at a high temperature, and disperse dye must display sufficient stability to alkali as well as high electrolyte concentration. Furthermore, it is essential to choose reactive dyes and disperse dyes that do not react with each other. After dyeing, the reduction cleaning for unfixed disperse dye must be avoided otherwise there will be decomposition of reactive dye from the dyed cotton composite.
A more versatile dyeing process for blends is of dyeing in a neutral bath in a single stage with special reactive dye and disperse dye. The Kayacelon React CN® reactive dye, which can be fixed in a neutral condition as the temperature ranges from 110 °C to 130 °C, has admirable compatibility with disperse dye. In this dyeing process,
pH adjustment is no longer a necessity and the total dyeing time is only about four hours.
13.4.2 Continuous dyeing
Continuous dyeing for woven polyester/cellulose blends is another significant process throughout the dyeing industry because of having large production capacity and high efficiency. There are three process flows as follows:
1. Two-bath-two-stage: padding (disperse dye) → pre-drying → drying → thermofix → padding (reactive dye) → pre-drying → drying → padding (alkali) → steaming → soaping → drying.
2. One-bath-two-stage: which is similar to two-bath-two-stage but reactive dye is padded with disperse dye together in one bath.
3. One-bath-one-stage: padding (disperse and reactive dye) → pre-drying → drying → thermofix → soaping → drying.
The first process avoids mutual interference between the dyeing of two fibres and is free of dye selection, but is more time-consuming. The condition for each stage is similar to that for pure polyester and pure cotton. One of the distinct characteristics of the continuous process is that the staining of disperse dyeing on cellulose is more severe than that in exhaust dyeing. To overcome this, an effective dispersing agent is necessary to transfer disperse dye from cellulose to polyester. High-energy dye is always preferred for the thermofix process although a complicated control is needed to obtain optimum dyeing results. Before the second padding of reactive dye solution, reduction cleaning of unfixed disperse dye is essential if high fastness is required. If the former disperse dye used is alkali-sensitive, such as those with vulnerable heterocyclic rings or hydrolysable ester groups, reduction cleaning may not be necessary, because these loose colours would be destroyed after alkali-padding and cleaned in the final rinsing, which is more economical. Reactive dyeing for a cellulose component can use either high reactivity dyes or low reactivity dyes following their characteristic processes.
The second process is relatively shorter than the first one, but there is a problem in dye selection, as in batchwise dyeing. The dyeing condition for this process is listed in Table 13.17.
The one-bath-one-stage employs disperse dye, reactive dye and alkali in a single pad-bath. The padded two dyes are fixed on fabric simultaneously in the thermofix unit in a continuous production line. The dyeing condition is shown in Table 13.18. Suitable disperse dyes for this process are those with low alkali-sensitivity and less cotton-staining tendency. The reactive dye with low reactivity and good thermal stability, such as MCT type, is thus suitable.
13.5 Process control in batchwise dyeing machines
Dyeing machines are designed in different shapes and with different loading capacities to accommodate textile materials in varied forms and qualities. The materials being dyed can be fibre, yarn, fabric, or even garments (Broadbent, 2001). In an exhaust dyeing, dyes in the dyebath are gradually transferred onto material, which is thought to be exhausted from the dyebath to the substrate (Shamey and Hussein, 2005). Generally, dyeing equipment intended for exhaust dyeing is designed in the form of manageable batches (Ingamells, 1993). Therefore, exhaust dyeing is also called batchwise dyeing, in which textile material is dyed from batch to batch. The representative machines designed for batchwise processing include: (1) hanks and package dyeing machines for yarns, and (2) overflow, jet, jig and beam dyeing machines for fabrics. All of these are based on (1) circulation of the dye solution through the material, (2) circulation of the material through the dye solution, or (3) circulation of the material and dye solution simultaneously.
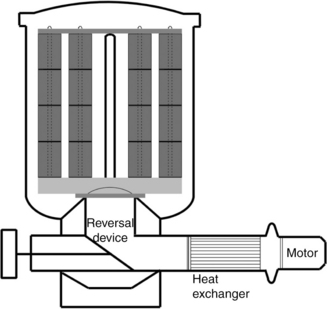
13.5.1 Package dyeing machines for yarn
In package dyeing, the most important dyeing method for yarn, yarns are wound on perforated cones. Before dyeing, yarn packages are first inserted onto vertical, perforated spindles in the dyeing machine. When dyeing begins, dye liquor can be pumped through the perforations in spindles and then forced to run into the package crosswise. Flow direction can be reversed from inside-to-outside to outside-to-inside automatically. A schematic of a package dyeing machine is shown in Fig. 13.21.
Evenness is always the predominant consideration in dyeing. For package dyeing, the evenness depends on good preparation of the yarn (proper package density, even winding), correct loading, right liquor ratio and appropriate liquor flow rate (good liquor circulation), and so on.
Package density
The porosity of the package must be uniform to prevent the uneven dyeing caused by the liquor short-cut. The evenness of winding has great influence on dyeing quality. Good winding requires the packages are constant in density, including in any single package from inner to outer, as well as that from package to package in the same batch. It is very important to find the proper package winding density. Too low a density would cause the collapse of yarn from the perforated tubes, while too high a density could result in difficulty for dye liquor penetration. The correct density of the yarn package is associated with the nature of material, yarn texture, yarn count, and the liquor circulation of package machine. The examples of package densities are listed in Table 13.19.
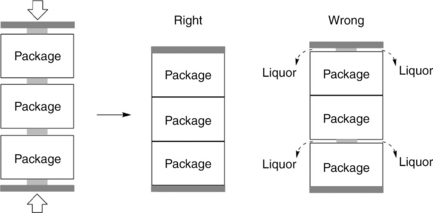
Mounting of package
After packages are prepared, they have to be mounted on a series of hollow, perforated, vertical spindles in the dyeing machine. It is critical to fix the packages firmly and compactly on spindles, without any slip space. Gaps between packages, top spacer and top package, as well as bottom spacer and bottom package, should be eliminated (Fig. 13.22). Otherwise a leakage may occur between these spaces when dye liquor flows pumped from either inside-to-outside or in reversed flow direction during the dyeing operation, which can lead to unevenness in dyeing.
Liquor circulation
Liquor circulation, as the most fundamental and critical requirement, must be sufficient and uniform (Shore, 1995). A better dyeing levelness can result by changing liquid flow direction from time to time. Table 13.20 gives the recommended flow parameters for package dyeing.
Table 13.20
Parameters of flow circulation
| Flow direction | Time (min) | Differential pressure (Kpa) |
| Inside-to-outside | 3–4 | 60–80 |
| Outside-to-inside | 4–6 | 40–60 |
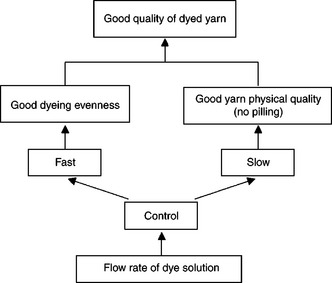
Dyeing quality of a dyed yarn depends on both having dyeing evenness and maintaining the yarn in good physical quality. Generally, the higher the liquor flow rate, the better the dyeing evenness that can be achieved (Shang, 2002). However, undue turbulence in the dyebath can cause pilling which declining in physical quality of the yarn (Shore, 1995). Therefore, the flow rate of dye liquor should be optimally controlled so as to balance the dyeing evenness and the physical quality of the dyed yarn (Fig. 13.23).
13.5.2 Overflow dyeing machines and jet dyeing machines for fabric
The principles of the overflow dyeing machine and the jet dyeing machine are same, that is fabric being dyed runs cyclically, and dye solution at the same time also flows cyclically. Fabric loaded in the dyeing machine is in rope form, and is moved by both the driving force from the lifting reel and the pushing force generated by nozzle liquor, which determines the fabric running rate. Dye solution is sprayed or jet-fed on to the fabric as the fabric passes through the nozzle, which is the main place that dye solution exchanges with fabric in terms of dye exhaustion. During repeated fabric circulation inside the machine, a dyeing process is carried out. Moreover, some machines are designed to operate above atmospheric pressure, which is particularly suitable for dyeing polyester at temperatures approaching 135 °C (Broadbent, 2001).
Machine classification
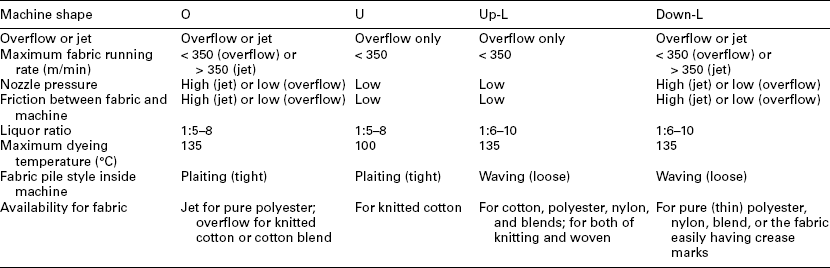
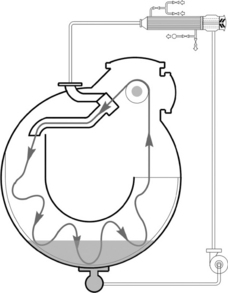
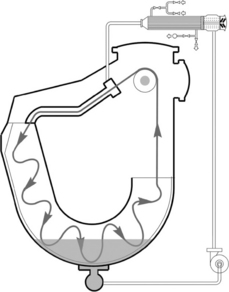
The overflow and the jet dyeing machine are distinguished on the basis of the fabric running rate inside the machine. Generally, a fabric running rate below 350 m/min is classified as an overflow dyeing machine, and that over 350 m/ min is called a jet dyeing machine. There are four main shapes of overflow or jet dyeing machines (Shang and Zhuo, 2003), that is O-shape (Fig. 13.24), U-shape (Fig. 13.25), up-L-shape (Fig. 13.26) and down-L-shape (Fig. 13.27). The comparison of these machines is listed in Table 13.21.
For the overflow dyeing machine, friction between fabric and machine, as well as that between fabric and liquor, is smaller than that of the jet dyeing machine, since the liquor pushing force generated by nozzle is gentle. The overflow dyeing machine is particularly suitable for those fabrics which cannot withstand high tension and high friction, such as cotton knits and their blends, as well as the elastic fabrics. Knitted fabrics should be dyed with overflow dyeing machines to reduce pilling and to retain its dimensional stability. This is because the nozzle liquor pushing force for fabric is moderate, and the slippage between fabric and lift reel is small. The jet dyeing machine is more suitable for pure synthetic fabrics, especially for thin fabrics and the easy crease-marked fabric.
Factors of dyeing process control
Overflow and jet dyeing machines are the most significant exhaust dyeing machines for fabric at present. Keeping the machines running under the optimal conditions has profound influences on the dyed goods. The important factors for dyeing process control include: (1) fabric loading weight, (2) liquor ratio, (3) fabric running rate in terms of the total actions of lifting reel speed and nozzle liquor pressure, (4) fabric cycle time, (5) chemical dosing rate, and (6) temperature/time profile.
Loading capacity
Generally, the maximum fabric loading capacity depends on the design capacity of the dyeing machine. However, the actual loading weight should be based on the fabric loading length. For a designed weight, the fabric length of thin fabric is longer, while the fabric length is shorter for thick fabrics. Since the requirement of shortening fabric cycle time for ensuring even dyeing, thick fabric could be loaded up to the designed capacity, while the loading of thin fabric should be discounted. The loading for easy crease marked fabric might also need to discount to avoid the un-removed crease marks happened. The actual loading capacity for a single chamber machine can be calculated as follows:
1. Determine the optimum fabric cycle time, usually no more than 3 min for cotton and its blends, and no more than 2.5 min for pure polyester.
2. Determine the optimum machine running rate, that is, no slippage between fabric and lift reel.
Fabric length = machine running rate × cycle time
The elongation of fabric and the slippage between fabric and lifting reel should be considered.
Actual fabric loading weight = fabric length × fabric weight per metre
For a machine with multi-chambers, its actual total loading capacity can be calculated as:
Total actual loading capacity = the actual loading weight of a single chamber x chamber number
The development trend of the overflow and jet dyeing machines is towards lower liquor ratio and shorter processing time for environmental and energy considerations, so-called low carbon dyeing. Right-first-time and reproducibility in dyeing for all batchwise dyeing machines are other important issues needed to be improved. Various modified overflow and jet dyeing machines approaching these targets are now appearing in the market.
13.7 References
Broadbent, A.D. Basic principles of textile coloration. Society of Dyers and Colourists: Bradford; 2001.
Gordon, S., Hsieh, Y. Cotton: science and technology. Cambridge: Woodhead; 2007.
Ibrahim, N.A., Sayed, W.A.E. Low-temperature dyeing of cotton fabrics with monochlorotriazine dyes. American Dyestuff Reporter. 1993; 82:44–49.
Ingamells, W. Colour for textiles: a user’s handbook. Society of Dyers and Colourists: Bradford; 1993.
Lewis, D.M., Marfell, D.J. Nylon dyeing. In: Hawkyard C., ed. Synthetic fibre dyeing. Cambridge: Society of Dyers and Colourists; 2004:82–121.
Shamey, R., Hussein, T. Critical solutions in the dyeing of cotton textile materials. Manchester: Woodhead Publishing Limited; 2005.
Shang, S.M. Package dyeing: Low Liquor Ratio and Short Cycle. International Dyer. 2002; 187:23–25.
Shang, S.M., Chong, C.L. Bleaching and dyeing cotton with a shorter process. AATCC Review. 2002; 2:31–33.
Shang, S.M., Zhuo, H.J. Breakthrough in Overflow Dyeing Machine Promotes the Development of Eco-friendly Dyeing Technology. China Textile Leader. 2003; 4:28–33.
Shore, J. Cellulosics dyeing. Society of Dyers and Colourists: Bradford; 1995.
Vrentas, J.S., Duda, J.L. Diffusion in polymer-solvent systems. I. Reexamination of the free-volume theory. Journal of Polymer Science: Polymer Physics Edition. 1977; 15:403–416.
Xi, D., Chen, J., Ma, C., The importance of water resource on development of dyeing and finishing industry. In: China Association of Dyeing and Finishing Industry, Annual symposium on environmental protection in dyeing and finishing industry, Qingdao, 2006, Beijing: China Textile & Apparel Press. 2006.
Yeung, K.W., Shang, S.M. The influence of metal ions on the aggregation and hydrophobicity of dyes in solutions. Journal of the Society of Dyers and Colourists. 1999; 115:228–232.
13.6 Process control in continuous dyeing machines
Continuous dyeing is mostly suitable for dyeing woven cotton and its blends in large amounts. Other advantages of the continuous process are the shorter dyeing process and the lower total water consumption than that of the batchwise method.
In continuous dyeing, the first piece of fabric to be dyed is fed into the dyeing range, and its end is stitched to the start of the next roll. Production goes at a constant rate without interruption until all the fabric is dyed (Broadbent, 2001). The dyeing range is assembled from pieces of basic equipment.
Figure 13.28 shows the complete continuous dyeing range for polyester/ cellulose blends by the pad-thermofix-pad-steam method. It contains a dye padder, pre-dryer, dryer, thermofix unit, second padder, steamer, washing boxes, and dryer, etc. For particular dyeing processes, some equipment may be omitted or bypassed.
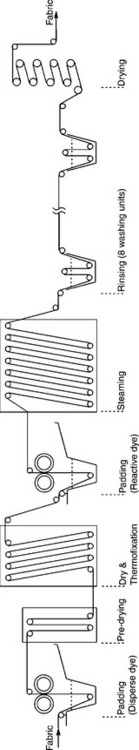
13.28 Schematic of continuous range for polyester/cellulosic blends dyeing with reactive and disperse dyes.
Factors of continuous dyeing process control
In continuous dyeing, the factors to be controlled include: (1) fabric moving speed, (2) dye liquor condition in padder trough, (3) padding pressure/ liquor pick-up ratio, (4) evenness of padding pressure, (5) pre-drying, (6) curing, and steaming temperature, (7) evenness of drying, curing and steaming, (8) retention time in each unit, (9) rinsing units and rinsing conditions.
Fabric moving rate in dyeing covers a broad range. It can be as low as 10 m/min but also could often exceed 100 m/min depending on the fabric dyed and the efficiency of each unit in the dyeing range. Higher speed always benefits productivity, but it is more important to consider the completion status of equipment involved. In the continuous range, fabric threads through the pieces of equipment at a constant speed without interruption. When it runs through certain units, it is necessary to make sure the retention time is sufficient to complete processing. If the retention time is less than 1 min in steamer, for instance, the movement has to slow down to meet the basic requirement.
With respect to dye liquor condition in the padder trough, there are factors to be considered, namely bath level and flow rate/dosing rate. The bath level must be kept constant to ensure the immersion time of fabric in the dyebath is without any variation, which would affect the consistency of shades. As fabric threads through dye liquor, a certain amount of dye liquor is absorbed by the fabric absorbency. Therefore the rate of padding solution complemented from a supply tank into the pad-bath should equal the rate of solution leaving the bath. The left solution from pad-bath is the fabric pick-up. If the processing loading is 15 kg/min, and the pick-up ratio is adjusted to 60%, the total flow supplied to pad-bath should be at a constant rate of 9 kg/min. In some cases the liquor would be complemented by two independent tanks, where for instance reactive dye solution and alkali solution are prepared separately. The complemented flow therefore equals the summation of the ingredients’ flow. Moreover, it is better to keep the pad-bath at a low volume, in which leaves less waste residual liquor when dyeing reaches the end. More importantly the rapid turnover of the dyebath keeps the pad-bath consistent and fresh. Rapid replacement of padding liquor can also minimise initial colour tailing. Generally, fibre tends to absorb dye more than water in the padding process. The dye liquor squeezed out at the nip flows back to the padder trough with a lower dye concentration due to the preferential dye absorption by the fabric dyed. Consequently, while the dyeing lasts, the dye liquor in the padder trough is gradually diluted, and the drop in colour depth increases. This would be much more serious at the end of the process, causing the defect known as ‘tailing’ or ‘ending’. To overcome this, rapid turnover of dyebath is effective, and selection of dyes with low substantivity is necessary.
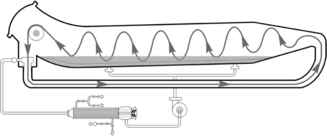
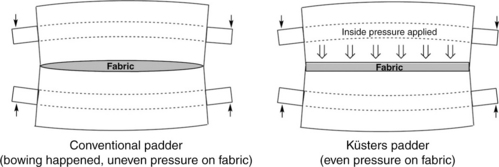
Before padding, the fabric must be well prepared for good absorbency since the retention time of fabric in padding liquor might be as short as 0.5 s. Moreover, flat selvedges and crease-free surfaces are basic requirements for level dyeing. During padding, fabric goes into the padding solution under a roller submerged in liquor and then passes out of the bath towards squeeze rollers. Excess solution on fabric will be removed by pressured squeeze rollers, which are horizontal or vertical, the former being more common. This process impregnates dry fabric with dye solution mechanically. Impregnation must be as uniform as possible. The uniformity of the distribution is closely related to constant immersion time in the bath and a constantly even squeeze force, which gives constant pick-up on fabric. Constant immersion time depends on the fixed liquor level of pad-bath, as well as the constant moving speed of fabric as mentioned above. Even pick-up depends on the uniform pressure loaded by squeeze rollers. Generally about 1.6–3.6 m long and 35–40 cm in diameter. They can squeeze a fabric with a linear pressure up to about 50 kg/cm of its length by a pneumatic system applying pressure at the two ends of one of the rollers for horizontal form, or the upper roller for vertical form. The higher the pressure exerted, the lower the pick-up. In practical application, the pressure at the two ends of the mandrel is usually higher than that in the centre due to the method of applying pressure. Such a non-uniform squeeze results in more pick-up in the centre, leading to paler depth at fabric selvedges known as ‘listing’. To counteract this defect, modification of the pressure applying system is necessary to give level pick-up across the nip width under constant and even pressure. The swimming roller developed by Kusters is well known in that its linear pressure at the nip is more uniform across the width than that of other conventional equipment (Fig. 13.29).
Before going through any units in which the dye on fabric is being fixed, fabric must be dried completely to eliminate migration during fixation. The infrared heater, as a uniform drying unit, is used for pre-drying to reduce the pick-up, and migration inhibitor is added to the padding solution to increase viscosity, both of which are effective in minimising dye migration in fixation and for obtaining even dyeing.
Nowadays, thermofix dyeing is mainly for dyeing polyester blends, and very rarely for pure polyester. In the thermofix unit, polyester fabric is heated by air or metal rollers to 190–220 °C for 30–120 s depends on the particular situation. When polyester/cellulose blends are padded in a single dyebath, the fixation of disperse dye on polyester and reactive dyeing on cellulosic fibre can be simultaneously completed in the thermofix unit. In an alternative process, fixation for reactive dyes can be achieved by steaming the padded fabric with saturated and slightly superheated (105 °C) steam for a reasonable time. Good roller alignment in the steamer is necessary to get rid of lengthways creases. The steamer roof must be well insulated to prevent vapour condensing into liquid drops, which may fall onto fabric causing drop marks. It is better to keep the steamer free of air by cold water exit seal. No matter what kind of heating methods are applied in the fixation stage, uniformity is still fundamental, and much more crucial than any other process stages, because mistakes occurring in this step cannot be repaired.
The fixation ratio is never 100%, thus any unfixed dye must be cleaned from the fabric by rinsing and washing to give optimum fastness. Removal of unfixed dye is carried out on continuous washer assembled in the continuous dyeing range. In the continuous process, coloured fabric passes into washing boxes with the counter-current flow of the washing solution. A typical number of wash boxes is eight. The first three may be used for initial rinsing to remove loose colour; the next three are settled for hot soaping to clean embedded colour; the last two boxes are for the final rinse. The removal of unfixed dye should be as thorough as possible. After that, the wet fabric is dried by heated cylinders and the process then totally completed.