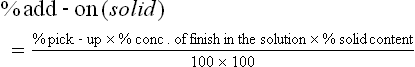Process control in finishing of textiles
Abstract:
The chapter discusses the importance of process control in textile finishing operations. Controlling parameters for basic finishing machines as well as for various mechanical and chemical finishing processes are discussed. Low liquor foam finishing and eco-friendly finishing processes such as enzyme and plasma finishing are explained.
15.1 Introduction
The textile industry is one of the oldest industries in the world. The earliest known textiles include scraps of linen cloth found in Egyptian caves dating from about 5000 BC. In the western world, textile manufacturing remained a family business until the early 1500 s, when the first factories were built. In Asia, especially China, centralising and standardising textile production occurred as early as the Zhou Dynasty (eleventh to eighth centuries BC).
In the West, an early regulation for quality assurance in the textile trade reported during the fourteenth century in Germany named ‘Tuchshau’ that is showing of cloth, which involved expert inspectors along with an equal number of city council members. As in most other manufacturing industries, for many centuries quality in the textile industry was achieved through inspection of finished goods. This final inspection was often used to create different grades of quality, products of which were then sold at various prices. Manufacturers slowly began to add inspection and control to the quality of raw materials and the production processes.
Intense competition, both nationally and globally, has resulted in a new focus on quality management in textile companies. The first reports on statistical quality control of yarn-manufacturing products appeared during the late 1940s and 1950s. Dyeing a textile yarn or fabric is one of the most difficult, monitored and controlled processes in the textile manufacturing chain. The finishing process, by contrast, still has few controls and relies heavily on inspection and testing. The two main methods used in finishing are chemical and mechanical. Evaluating the results of both these methods is still highly subjective, and very little statistical process control has been applied in these areas (Clapp et al., 2001).
15.1.1 Approach to process control
The choice of process condition for the given product is taken by the previous history and forming new norms without affecting quality. The optimum norms may vary from unit to unit and machine to machine. This is for various reasons, such as working condition, type of machine, layout of machine, provision of utilities and variations in the quality of the fabric. Therefore every process house has to carry out their own experiments to identify their own optimum processing levels. Once the processing conditions are standardised, the implementation of these conditions during the normal course of production is carried out by maintaining required documents. However it is important to make regular inspection checks to ensure that the particular process is going on according to the norms fixed.
For quality optimisation it is necessary to know about causes for quality defects. Based on the cause and effect diagrams published by K. Ishikawa (1985) there are four critical causes for quality defects:
With machines or equipment, quality deviations may occur because of inadequate maintenance or servicing. In the textile industry, a breakdown in quality is said to have occurred if, for example, the required washing effect, degree of whiteness or absorbency of the fabrics being finished is not achieved. These are defects in the specification of the system because the expectations of the customer are not fulfilled.
A known problem in wet finishing ranges is the wear of the roller rubber, even if this happens to be outside the influence of the machine supplier. This is a potential source of risk of a reduction in quality. Two questions must be asked about a potential source of risk to quality:
1. Is this component really necessary?
2. Does the component have to be modified to suit the operating conditions, or can the conditions be improved to suit the critical component?
Rubberised rollers are critical components in the textile finishing industry. High temperatures and large amounts of chemicals lead to rapid and uncontrollable wear. It is well known that washing efficiency at boiling temperature is considerably higher than in cold washing water. Washing processes at boiling temperature create unfavourable conditions for rubber rollers. Cold washing would improve the operating conditions but make the textile results worse. This means that the critical component, in this case the first washing unit in a washing process, will have to be modified to suit the ambient conditions. The simplest way to do this would be to avoid using rubber rollers in such situations (Ströhle, 2009).
15.2 Instrumental process control
The increased demands of the textile finishing industry on the machinery with regards to quality of the products, productivity, cost reduction and reproducibility are not satisfied by the conventional machines. The cost-effectiveness of textile machinery as a result of automation systems has been widely discussed in several publications. The purpose and the benefits of such systems have never been questioned. Considerable differences of opinion exist, however, about the design of automation equipment, about strategies, versatility, systems and functions with or without computer, whether with integrated organisational hierarchy or as a straightforward automation of machine functions (Schreiber and Maschen, 1992).
Automation and control can be used in dyeing, printing and finishing for the following processes:
• Temperature–time programmes,
• running partial or fully automatic processes,
Automation of regulation and control processes in production equipment requires a programme that allows analogue and digital techniques to be mixed on the screen without any problems, and the microprocessors that are introduced to be designed so that they can be integrated easily into the bus system. The introduction of Expert systems as the process control technology (e.g. for stenters or continuous open-width washing ranges) has been especially relevant to automation in textile dyeing and finishing (Rouette, 2000)
Any control system may be divided into three components (Duckworth, 1983):
1. The sensor detects a change in a process variable and produces a proportional output signal.
2. The controller receives the signal, compares it with the desired set value and emits a correction signal proportional to the deviation from the set value.
3. An actuator and servo device – commonly a power-operated valve – which receives the correction signal and mechanically adjusts the process variable.
A process control system should be applicable indiscriminately to all finishing machines. Identical hardware and software should be capable of controlling all continuous textile finishing processes.
Five steps to automate a continuous textile finishing machine (International Textile Reports, 1991) are as follows:
1. Division of the machine into a number of functional zones mostly based on drive sections of the machine to make controls flexible.
2. Defining various control parameters (e.g. temperature, flow, etc.) for each zone.
3. Calculation of the number of controllers required in order to control each zone and thereby for the whole machine. Analogue inputs and outputs are used to control temperature, flow, pH, etc., whereas digital inputs and outputs are used for level control, drain activation, end switches, etc.
4. Connection of multiplexer to the system in order to achieve a correct communication in two directions between the computer and the controllers.
5. Connection of a standard personal computer or preferably its industrial version that presides over each machine and is connected to the multiplexer via an interface. Generally the computer is situated near the operating panel of the machine.
The software system should take care of the following points:
1. Parameter definition – each control parameter should be properly defined. A series of standard control algorithms should be available. The possibility of relating control parameters should exist. For simple definitions, the computer system should be menu-controlled.
2. Process library – a library of machine processes can be defined with chosen set points for each process parameter. When a process is chosen or changed on the status screen, the machine sets itself to the desired set points. Intervention in the process is possible on the computer and local levels.
3. Status screen – while the machine is running, an on-line status screen should be available. The status screen should display the actual status of the machine, set points, actual values as well as high and low alarms. In addition, the software should offer a number of user-selectable reports and graphs.
The standard control functions in a continuous textile finishing machine are as follows:
1. Bath temperature is measured by a PT 100 element and is controlled by a proportional valve to assure processing at the optimum temperature.
2. Cloth temperature is measured by pyrometer and is controlled to avoid over-drying.
3. Steam pressure may be controlled as an alternative to temperature control in the steamer.
4. Flow of chemicals and water is measured by an inductive flow-meter and is controlled by a proportional valve or through a measuring cylinder dosing system, in order to assure a constant flow, depending on machine production.
5. The pH is measured by a pH sensor and mainly controlled in washing of processes.
6. Washing efficiency is assessed by measuring and controlling conductivity of bath and/or cloth by a conductivity sensor.
7. Density (concentration) control is mostly used in mercerising range to achieve a constant mercerising concentration.
8. Compensator pressure control in order to run the cloth with correct cloth tension.
9. Squeezing unit pressure control, especially for heavy-duty squeezing units to assure reproducible liquor pick-up.
10. Level control in washing units and chemical tanks used for safety interlocks (temperature control), as a safety level and to fill the machine automatically.
11. Speed measurement and control as an indication for the machine efficiency in order to make production-dependent dosing of chemicals and water into the machine.
12. Control of consumption of steam, water, electricity and chemicals to maintain quality and cost-effectiveness.
15.2.1 Flow-meters
These are used to measure the flow rate of a fluid in a pipe, duct or open channel. Flow-meters are of two types – quantity and inferential. While the former measures fluid flow rate directly, the latter measures fluid velocity, and thereby fluid rate. The most commonly used inferential flow-meters are:
Valves are devices used to control the flow of a fluid by placing an obstruction in the fluid's path. There are various types of valves; the selection for a specific function depends on whether it will be used as a stop valve (only ‘off’ or ‘on’ service) or a throttling (regulating) valve. The later again may be of various types namely cocks, ball, globe, gate, diaphragm, pinch, etc. A typical automatic process control valve (which may be globe or diaphragm types) consists of four separate but integrated components – actuator, packing gland, body and valve plug. The sensing element of the valve measures a particular process parameter (flow, temperature, pressure, level, pH) and if the value deviates from the desired value, the gas (air) pressure on the diaphragm in the pressure casing is altered automatically to deflect the diaphragm to move the value stem and plug, thereby altering the flow area in the valve to change the fluid flow rate (Duckworth, 1983).
The logical objective of a process control system is to assure repeatable process quality and efficiency by controlling process variables to predetermined and preset values and to provide management information assuring proper equipment operation. To fulfil these objectives, the system should have the following characteristics (Fulmer, 1983):
1. Communication with plant personnel through CRTs, printers or alarm systems (light and/or horns).
2. Input to the system by plant personnel may involve card readers, push buttons, keyboards, etc.
3. Reliability of the system in the industrial environment involving heat, humidity, dirt, etc. should be assured.
4. Ease of use and maintenance are desirable. Standard instructions for each type of fabrics and finishes are to be set-up.
5. Back-up control is necessary to avoid loss of production due to system breakdown.
6. The system should be flexible to cope with new fabrics and finishes.
The important parameters in textile processing are the quantities of chemicals, pH value, water quantity, temperature and reaction time. For many products, especially knitwear, the fabric tension also plays a decisive role. For reputed textile machine manufacturers it is necessary to accurately measure the parameters and to record these values in order to ensure long term both internal (cost reductions, quality assurance) as well as external (reliability for customers, handling of complaints) process reliability.
The chemical metering system offers the greatest potential for savings. Inductive flow rate meters ensure that expensive additives are dosed with millilitre accuracy on their way into the impregnating bath. Reproducibility can also be increased with the aid of impregnation compartments with low liquor content and a rapid rate of liquor exchange.
Modern databases for recipes or formulas also make the day-to-day work of machine operators easier. Not only the corresponding recipes for all product groups are saved, but also these recipes can be extrapolated for the specific fabric weight resulting reproducibility and quality of the highest order. The water quantity, temperature, fabric tension and pH value remain a key factor in these recipes.
One of the key factors for data management is the sensor systems, which are integrated in the machine. The process can only be started and finished correctly if the produced data are totally reliable. If a temperature sensor indicates an incorrect value then no data management system in the world will be able to save the overall result. Therefore continuous monitoring of the ‘eyes and ears’ of a system is still the best insurance policy for ensuring that, once selected, the parameters are maintained in the long term. Temperature monitoring with an external thermometer (or alternatively a second sensor), regular determination of the pH value via titration or via the Morapex process, checking the flow-meters through capacity gauging and constant checking of bearings and drives, are all important factors for guaranteeing constant fabric tension. Consequently, once modern technology is in place and the monitoring capabilities and process reliability of a state-of-the-art data management system is correctly set up, it is possible to deliver top quality in a cost-optimised framework (Kehry and Uhl, 2010).
15.3 Textile finishing processes and process control in finishing
Any operation (other than preparation and colouring) that improves the appearance and/or usefulness of fabric after it leaves the loom or knitting machine is called ‘textile finishing’. Finishing is the final series of operations that produces marketable textile fabric from grey goods.
15.3.1 Textile finishing processes and their classification
The word ‘finish’ means all the different treatments applied to a fabric to change one or more of the following:
Finishing improves aesthetic aspects or serviceability of the fabric or imparts certain desirable characteristics.
Textile finishes are classified in several ways. According to function, these can be classified into:
1. Aesthetic finishes, which modify the appearance and /or hand or drape of the fabrics.
2. Functional finishes, which improve the performance properties of the fabric such as durability, strength, etc. Property-changing functional finishes provide the added qualities desired for a particular fabric or they may be used to change an undesirable property to a more desirable one. Many such finishes add more than one property to a fabric.
Examples of aesthetic finishes are:
Examples of functional finishes are:
• crease resistant/durable press,
• shrinkage control, Sanforizing,
Some of the finishes could result in both aesthetic and functional effects. Mercerisation is considered as an aesthetic finish, as it increases lustre. It may also be considered as functional, as it increases strength. Sometimes it is even considered as a pre-treatment process, as it improves dyeability. The classification of finishes and class-wise discussion of finishing processes are, therefore, quite difficult.
Depending on the machines or chemicals used, both aesthetic and functional finishes can be further classified into:
1. Mechanical finishes – these usually involve specific physical treatment to a fabric surface causing a change in fabric appearance. These are also known as dry finishes.
2. Chemical finishes – chemicals are usually applied to fabric by padding followed by curing and drying. These are also called as wet finishes.
Mechanical finishing typically refers to finishing effects ‘done by machine’ as opposed to finishes achieved through chemicals. Mechanical finishes have some advantages over chemical finishes. Mechanical finishing can process the fabric at very high speeds. The repeatability is better over time, since there are no formulations to change, and changing chemical restrictions and prices are not an issue. The mechanical finishes are now more acceptable because they are environmental friendly.
Except shrinkage control or Sanforizing process, all functional finishes mentioned above are basically chemical processes.
According to the quality, finishing may be classified into:
1. Temporary: a finish which is not stable and goes off after the first wash is known as temporary finish.
2. Semi-permanent: if the finish is stable for ten washes but not beyond.
3. Permanent: if the finishing effect in the fabric does not disappear and remains unaffected through all the conditions of wear and washing treatments, then the finish is said to be permanent.
The types of finishing agents or finishing processes applied to various textile materials are indeed very large in number and they can be classified in various ways. It is, therefore, difficult to describe each of them systematically.
15.3.2 General process control in finishing
The number of finishes applied to various textile materials is very large and are selected as per properties desired for the end products. One or more properties are modified by each finishing agent and most of such properties can only be assessed subjectively. The situation is further complicated by the fact that many finishes are mixed or combined. Quality control during finishing is, therefore, very challenging and subjective.
Compatibility
It is necessary to check various properties of the finish mixture before and during application. There are quite a number of factors making the chemicals used in a recipe mutually incompatible – the ionic characteristics, solubility, emulsion stability, pH conditions, etc. Stabilisers used in an emulsion may be disturbed by new or alternates additive in a recipe, thus causing breakup of the emulsion.
When two or more finishing chemicals in a recipe are contemplated or when the recipe is changed for reasons of non-availability of one of the components, it is desirable to check their mutual compatibility in the laboratory before administering in bulk.
Strength of the finishing agent
It is desirable to apply the recommended level of the finishing agents. Commercial finishing agents come in different strengths and it is the active matter that is important. In some cases, when dilution needs to be done to a working concentration level from concentrated pastes/emulsions with the application level fixed, the new consignments should be evaluated for the active content and accordingly the recipe should be adjusted to match the standard recipe quantity.
Moisture regain of substrate
The moisture regain of cotton is around 8%. This plays an important role in the feel of the fabric. Over-dried fabrics tend to feel relatively harsh despite application of softeners. It is also necessary for the fabrics to have satisfactory moisture regain before finishing by padding methods, particularly in the case of resin finishing, facilitating better and even diffusion and penetration of the finish.
It is desirable to control the moisture regain level of the fabrics during drying using control instruments like ‘Textometers’ in a stenter operation or ‘cool tumbler’ in the drying of the garments. There are conditioning cycles in the drying operations (e.g. in yarn drying) in the modern batchwise machinery.
The following process technologies take place in textile finishing (Rouette, 2000):
• monitoring and control of work processes across all areas by process computer (process data registration),
• shortening of work processes,
• reduction of reaction temperatures,
• recovery of chemicals and energy,
• increase of passage or turnaround speeds (e.g. continuous systems at 300–500 m/min),
• use of chemicals having no adverse effect on the environment such as formaldehyde-free finishing,
• products that dye and finish simultaneously,
• use of electrical power as a finishing agent,
• complete recovery of products that are not fully exhausted or fixed,
15.4 Process control in basic finishing machines
Two types of machines are used in finishing of textile materials. Some basic machines are used in all or most of the finishing processes while some other machines are used for specific finishing processes. The former type includes padding mangles, drying and curing machines, etc. The process control in some of the basic finishing machines is discussed below.
15.4.1 Padding mangle
Traditionally, padders are used to apply chemical finishes in low wet pick-up techniques. In recent years such techniques have become more cost effective as the cost of energy has escalated significantly in comparison with the cost of finishing agents.
A padder consists of a trough and a pair of squeeze rolls (mangle). In the padding process, the fabric passes under a submerged roll in a trough filled with a treatment bath and then through squeeze rolls. In the simplest arrangement, a single dip is followed by a single nip. Alternately, three mangles can be used and the fabric is impregnated twice, double dipped – double nipped.
The amount of finishing solution or emulsion applied is referred to as the ‘%wet pick-up’ which is usually expressed as a percentage on the weight (wt) of the dry untreated fabric at room temperature and humidity.
where % conc. is the concentration of the finishing chemical in the applied solution or emulsion expressed as percentage by weight (wt/wt).
To determine the amount of supplied chemical or finish added to the fabric, the ‘% add-on’ is given by Equation [15.2]
Since most finishing formulas are given in terms of grams per litre (g/L), Equation [15.3] can be used to convert the g/L concentration to percentage by weight.
When the actual solid level added to the fabric is desired, the percentage of solids add-on can be calculated from % solid content in finishing chemical using Equation [15.4] (Schindler and Hauser, 2004).
Typically, for uniform application the wet pick-ups are maintained in the range of 70–100% during chemical finishing by padding. Subsequently it is necessary to remove of large amounts of water during drying. The evaporation of this water can lead to uneven finish distribution in the dried textile owing to migration of the finish to the fabric surface during drying. The high rate of evaporation at the fabric surface leads to movement of the finish solution from the wet fabric interior to the dried fabric exterior resulting in a higher concentration of the finish at the fabric surfaces with a corresponding lower concentration in the fabric interior regions.
The migration of finish may be minimised by reducing the amount of water applied initially. However, too low a wet pick-up can be equally problematic and also lead to uneven finish distribution. ‘Critical application value’ (CAV) is defined as the minimum amount of durable press finish liquid that can be applied to a given cotton fabric without producing a non-uniform distribution of cross-links after drying and curing. Cellulosic fibres, because of their inherent hydrophilicity, have CAVs in the range of 35–40% wet pick-up. Hydrophobic fibres like polyester can have CAVs of less than 5%, allowing much lower wet pick-ups than hydrophilic fibres.
There are two main types of low wet pick-up applicators. The first is the saturation–removal type where the fabric is completely saturated with the finish liquid and then the excess liquid is removed mechanically or with a vacuum before drying. With the second type, a precise amount of finish liquid is uniformly applied to the fabric using transfer roll, spray or foam techniques.
The factors controlling the amount of solution remaining on the fabric (i.e. %pick-up) after padding (Schindler and Hauser, 2004; Tomasino, 1992) and the relevant conditions for higher liquor pick-up (under bracket) are:
1. Fibre type (hydrophilic fibres).
2. Yarn construction (low twist yarn and open end yarn).
3. Fabric construction (loosely constructed fabrics).
4. Wettability (easily wettable fabrics).
5. Squeeze pressure (lower pressure).
6. Hardness of roll covering (softer covering).
7. Immersion time (longer immersion time).
8. Viscosity of the padding liquor (higher viscosity).
9. Surface tension of the solution or emulsion (faster wetting solutions).
10. Temperature of the solution (viscosity and surface tension change with temperature thereby changing pickup-values).
All fabrics have upper and lower wet pick-up limits. Within these limits, adjustments in wet pick-up can be made by increasing or decreasing the squeeze pressure.
15.4.2 Drying and curing
Drying is a process of evaporation of the embedded liquid from the fabric. While the concept of drying is simple, in practice it can be the source of unsuspected problems. For drying to take place, the liquid must be converted to vapour and the vapour must be moved away from the fabric surface. Some of the factors affecting drying rate are air temperature, relative humidity of drying air, and volume of air passing over fabric (air flow). The following factors are to be considered in controlling the cost of drying processes (Heywood, 2003):
1. Mechanical removal of water must be maximised and achieved as uniformly as possible.
2. Drying should not proceed beyond the equilibrium regain of the fabric.
3. Thermal efficiency of convection drying requires that optimum humidity of the drying medium be maintained.
4. For speedy drying, air temperature should be high, but the cost increases if the temperature is increased above 150 °C.
5. Air velocity at the fabric surface should be maximised – through-flow drying shows a clear advantage.
6. Losses by radiation and in the exhaust should be minimised – steam supply losses should be eliminated by direct heating within the drying chamber.
Generally speaking, the machinery used to dry fabrics can be used for curing, provided the fabrics are capable of reaching curing temperature. In many finishing procedures, drying and curing are divided into two steps. Each step will have its own individual specified conditions. Sometimes however, no delineation between the two is made. Wet fabric enters the oven and cured fabric exits. It is well to remember that fabric temperature will not approach air temperature until all the moisture is gone. In many chemical finishing procedures, the fabric’s actual time/temperature relationship is critical in order to activate the chemical reactions. Therefore, in one-step drying and curing operations, it is important to know when the fabric is completely dry so that the residual time matches the required curing time at that particular temperature.
The thermometer probes generally indicate the temperatures. However it would be prudent to counter check the temperatures with thermal papers periodically to confirm that the temperatures shown by the probes are accurate particularly where temperature parameters are critical like in curing machines. All instruments can go wrong and there must be some verification procedure to ensure that such failures do not affect performance. Similar checks on the temperature of stenter chambers need to be monitored particularly during heat setting and thermosoling. Such precautions are necessary for all finishes involving chemical bonding or thermo-fixations at elevated temperatures.
15.5 Process control in stenter machines
A stenter is the most universal and most expensive fabric drying and finishing machine, but is indispensable for a modern process house. It is also known as a ‘tenter’ in the woollen industry. The stenter consists of two endless auto-lubricated driven chains, typically 40 to 60 m in length carrying pins or clips to hold the fabric edges while passing through a number of hot-air chambers (3–5, each of about 3 m). Hot air is directed onto the fabric equally from above and below. A stenter has the provision for overfeeding the fabric to allow required shrinkage during heat setting of fabric while the width is increased to the precisely specified value by the chains. The use of clip stenter has declined because of the difficulty of applying overfeed. The stenter speed ranges from 10 m/min for heavyweight furnishing fabrics to 100 m/min for lightweight dress-goods. The speed also depends on the processes carried out in stenter namely:
The controllers fitted in a modern stenter may monitor and control the following parameters:
Efficiency and uniformity of drying demand attention to the airflow. Powerful fans (one or two) fitted on one side of each chamber pushes air into two tapered (sideways) ducts fitted at the top and bottom of the chamber. The ducts possess precisely designed nozzles through which air is injected at high velocity on both sides of the fabrics passing between the ducts. The slightly cooler air passes through the filter and heating section before returning to the fans.
For optimal thermal efficiency, it is essential to monitor and control the humidity of the chambers. The energy cost increases dramatically when humidity falls below 10% (0.1 kg water per kg of air) (Heywood, 2003). It is possible to control humidity with a fluidic oscillator developed by Mahlo (Merritt, 1982) or by adjusting exhaust dampers. Energy costs can be reduced by 50% by using various heat recovery systems.
Skewing of the product during manufacture alters the structure of textile materials. A distorted product depreciates in useful value. The Orthopac RVMC (Mahlo) is just such a modular process control system for use on stenters. It is a modular system for both fully automatic correction of bow- and-skew and process control. A number of scanners, sensors and lamps are spaced evenly across the passage of the fabric. The structure of the passing weft and courses modulates the light intensity measured by the scanners. The repetitive nature of the passing picks or courses creates a regular light–dark pattern. This in turn generates a modulated signal in the scanner's receiver system. A centrally-pivoted, cylindrical lens in the scanner oscillates to a specific angle in relation to the passing picks. When the lens is parallel with the pick or course, signal modulation is at its maximum. It then decreases as the oscillating lens progressively cuts across the weft line. Unwanted signals that differ from the frequency of the picks, knitted courses or rows of tufts are filtered out digitally, so that the system can analyse the pure bow-and-skew related signals and, from those, automatically compute the weft or course configuration (www.mahlo.com,RVMC-12_84-010242-002_en.pdf). Mahlo also developed OPTIPAC® VMC-12, a modular system for measuring, logging and controlling critical process parameters such as dwell time, thread density, residual moisture, weight/m2, etc. across the full width of the product, and exhaust air humidity (www.mahlo.com,VMC-12_84-010243-003_en.pdf).
Controlling parameters for the stenter (Shah, www.sulphurdyes.com) are as follows.
15.5.1 Nip pressure
It is examined by checking liquor pick-up. It should be uniform throughout the width and length. To obtain uniformity the necessary action is to check the surface of padding mangle and adjust the pressure (pneumatically or hydrolytically).
15.5.2 Bow and heading (skew) controllers
During processing visual checking is necessary. There should be no bow or heading in the fabric. The synchronisation of photo cell, heading and bowing rollers is to be checked. The hardness and alignment of bowing rollers are to be checked.
15.5.3 Chamber temperature
Checking is by dial or digital thermometer. It is to be set as per the process and quality. The necessary action is to regulate oil supply in the radiator. Proper functioning of solenoid valve and digital controls are to be checked and confirmed.
15.5.4 Dwell time
This may be checked during the finishing process with a stopwatch. Dwell time depends on the material quality and time recommended for the particular process and is to be regulated accordingly.
15.5.5 Overfeeding
Overfeeding is allowed during heat setting so that the fabric can recover from the stress applied on it at various stages of its production by shrinking and becoming dimensionally stable. During overfeeding fabric is fed to the stenter faster than the running speed of the fabric. If the shrinking is to be allowed in width-wise direction then the chains are kept closer and are not stretched.
Method of checking: to check % overfeed a certain length of cloth is marked before heat setting and the length between the marks is measured again after the process.
Action: the required optimum overfeed of synthetic and blended fabric of a particular variety during heat setting is to be assessed beforehand.
15.5.6 Underfeeding
During underfeeding speed of the fabric feed to the stenter machine should be less than the output. This is done to stretch the length of the fabric.
15.5.7 Expanders and uncurlers
During this process, usually the working of uncurlers is to be checked. Pneumatic uncurlers and mechanical uncurlers are generally used.
Standard: there should be no crease on the fabric.
Necessary action: the smooth working condition of the uncurlers and smooth revolution of the expanders are to be checked.
15.5.8 Blower
Proper functioning of the blower during the finishing process is to be checked.
Standard: proper air circulation.
Necessary action: for proper air circulation, the air filters are to be cleaned. The fan direction is to be checked – air is to be taken from out to in.
15.5.9 Width of the fabric
Width is decided by the distance between the chains and it is to be checked at the delivery end.
15.5.10 Leakages of thermic fluid
There should be no leakages. If many small brown spots are seen on the fabric it means that there are small leakages where the fluid falls as a spray. Larger brown spots on the fabric may indicate bigger leakages.
15.5.11 Concentration of the chemicals
The chemicals and their respective concentrations are to be listed.
Necessary action: the required optimum concentrations are to be maintained. Higher concentrations of the chemicals will lead to white patches, called chalk marks, when scratched with nail. Chalk marks may be due to higher concentration of chemicals.
15.5.12 Temperature and viscosity of the finishing bath
Temperature and viscosity of the finishing liquor are to be kept constant throughout the process.
15.5.13 Drying efficiency
It is checked during and after the drying process with a conductometer that is with the help of transducers.
Standard: no over-drying of the fabric, which will lead to high-energy consumption and strength loss. Drying efficiency of 95–98% is expected.
15.6 Calendering process
Calendering may be defined as the modification of the surface of a fabric by the action of heat and pressure. It is mainly done to impart lustre and smoothness to the fabric. The finish is obtained by passing the fabric between heated rotating rollers when both speed of rotation and pressure applied are variable. The surface of rollers can be either smooth or engraved to provide the appropriate finish to fabric. The rollers may be made of various materials, from hardened steel to elastic thermoplastic.
A calender is a multi-roll machine (2–16 rollers or bowls). The bowls rotate under adjustable mechanical, pneumatic or hydraulic pressure. The principle of operation is based on at least two adjacent calender rolls, one or more being a:
1. Metal roller (usually steel or chilled iron), the surface of which may be ground, polished, chromium-plated or engraved and can be heated (by steam, gas, electricity) if required, with.
To prevent damage to the surface of the resilient rolls when particularly thick seams pass through, ‘seam detectors’ are provided, which cause instantaneous pressure relief. Calendering effects are varied by various factors such as (Rouette, 2000; Heywood, 2003):
3. bowl composition (e.g. metal or resilient),
4. bowl pressure (50–100 tonnes, varying according to fabric type and width),
5. metal bowl temperature (up to 300 °C depending on fabric and effect),
7. the degree of friction (e.g. up to 300% friction by having an inclined fabric-filled bowl or faster running metal bowl),
9. fabric construction (weave),
• To change the fabric handle – softening or stiffening effect.
• To impart a smooth silky touch to the fabric.
• To compress the fabric and reduce its thickness and porosity.
• To reduce the air permeability by closing the threads.
• To change appearance – increase lustre of the fabric.
Various types of calendering are discussed below.
15.6.1 Swissing, normal gloss or simple calendering
In this case, the fabric runs through the nips of several bowls having the same surface speed. As a result, the fabric achieves a lustrous paper-like finish depending on the number and composition of the bowls.
15.6.2 Chasing finish
The fabric is passed through the nips of the calender, over external rollers and back into the bottom nip of the calender. Multiple layers of fabric run through the nip resulting in a thready appearance with soft handle. This is mostly done for linen fabric.
15.6.3 Chintz, glazing or friction calendering
The main difference between a swissing calender and a glazing calender is the use of a gear system to drive the smooth metal roller faster than the softer resilient roller – the peripheral speed may be up to three times higher. The latter machine is also comparatively heavier. Apart from the lustrous effect, the cloth handle can become quite papery and thin. Incorrect fabric conditions (especially incorrect moisture content) can lead to unacceptable handle, which cannot be corrected.
15.6.4 Schreiner calendering
The problem of producing lustrous but paper thin handle by friction calender can be solved by using a ‘Schreiner’ calender. Schreiner finish, though not fast to washing, produces a fabric more attractive from marketing point of view, often called ‘silk’ finish. Extremely lustrous fabrics can be obtained with the correct type of cloth and engraving (V-shape or U-shape) of 500 lines per inch at an angle of 20° to the axis. The angle of engraving should follow the approximate angle of the line of the twist of the yarn (left or right inclination at angles of 15–25° from horizontal and 15° from vertical for weft-faced and warp-faced fabrics respectively). Imitation Schreiner finish can also be obtained using bowls with 150 to 200 lines per inch only. A usual production problem is the picking-up of lint and dirt by the fine engraving spoiling the optical effects. Schreiner finishes reduce tensile strength especially with deep V-type engravings. The darker the colour of a fabric, the better is the lustre of the fabric after Schreiner finish.
15.6.5 Embossed calendering
This usually consists of two bowls. The top metal roller is engraved with a pattern and the softer composition roller has a surface that will accept the embossing pattern. The embossed rollers are quite expensive to produce. Originally these calenders were used to produce imitation leather cloths and book cloths. Embossed crepe design rollers easily deformed viscose fabrics to give a family of creping effects.
15.6.6 Moiré calendering
The moiré effect is an optical effect produced when a tightly woven fabric with very fine yarn is subjected to surface pressure that distorts the weave structure by yarn movement or yarn self-compression. The moiré effect can be produced only when the fibres being treated are deformable – wool fibres are not suitable for the purpose due to their high resilience.
Some of the process controlling parameters in calendering are as follows (Shah, www.sulphurdyes.com).
15.6.7 Nip pressure
The traditional method of applying pressure to the bowl set-up was by a system of levers or direct screw loading. The modern method of pneumatic or hydraulic pressure systems has altered the calender set-up. Calendering effect depends on the nip pressure, which is generally 7–9 tons on the rollers. The pressure is checked prior and during calender. The method of checking is nip pressure reading. The compressor valve and pneumatic control valve are to be regularly checked.
15.6.8 Threading
The threading through various calendering rolls depends on the finish required. It is to be checked visually before running of the machine. Proper threading of the fabric is to be ensured.
15.6.9 Damping
Controlled damping of the fabric is done before calendering. It is checked with the feel of the fabric, or with the conductivity meter. Follow-up action: adjust water spray intensity.
15.6.10 Speed of the roll
Speed is checked during the process with speedometer.
Standard: there is no standard speed, but 40–80 m/min is usually maintained.
15.6.11 Width of fabric
This is checked during the process by measuring with tape as per the sort or quality of the fabric. Follow-up action: ensure desired width at the delivery end.
15.6.12 Bowl surface
Prior to running it is checked visually.
Standard: there should be no crack, hole or rough surface or deposition on the roller.
Follow-up action: proper cleaning of calendering rollers is to be carried at the end of each process.
The following parameters are to be accurately controlled for consistency of the calendering process on a day-to-day basis:
1. Pressure and distribution of pressure across the nip – the traditional method of applying pressure to the bowl by a system of levers or screw loading has been largely replaced by pneumatic or hydrostatic pressure. The former gives a more resilient system, while hydrostatic systems are very close to the dead-set screw method. The effect of loading at the ends of the bowls causes the bowls to deflect, resulting in the nip pressure in the middle of the nip decreasing. The remedy is to have a bowl whose diameter varies along its length so that the lifting of the middle is compensated by its greater diameter there.
2. Temperature of the bowls – accurate control of the metal bowl temperature is vital for consistent results and this is achieved by gas, electric, thermal fluid or steam heating. The temperature can vary between ambient for a light smoothing to 190 °C for full lustrous calender finish. Modern hand-held infrared pyrometers can assess temperature more accurately as compared with conventional surface probes.
3. Speed and relative speed of the bowls – modern thyristor motor controls give highly accurate speed control as compared to conventional large DC motors or expensive AC motor controllers. The production speed of calenders depends on many factors and varies from a very slow speed of 5 m/min to a high speed of 75 m/min.
Quality control test: shine or lustre may be measured by lustre meter.
15.7 Surface raising and pre-shrinking finishes
Raising, emerising and pre-shrinking are three important machines widely used in textile finishing. They are mechanical finishes and hence, more eco-friendly. Moreover, they are economic as no chemicals are used. The process control for the above mechanical finishing machines are discussed below.
15.7.1 Raised surface finishes
Raised surface finishes are mostly mechanical finishes. Raised fabrics are mainly of two types, namely:
1. Raised from staple-fibre spun yarn fabrics such as woollens, worsteds, or cotton winceyette and velours – it consists of pulling out a layer of fibres from the structure of a fabric to form a pile resulting lofty handle, subdued weave/ pattern and blending of colours.
2. Raising from continuous filament synthetic yarns to form loop fabrics used for nightwear or bed-sheets – in this case loops in the fabric structure are stretched, but are not usually broken.
3. The machine consists of a drum or cylinder, round the surface of which are mounted a number of wire-covered rollers. Most modern raising machines are double-acting having both pile and counter-pile rollers (generally 12 each), depending on the direction of the point of the bent wires. The fabric is transported over the wire points, which penetrate the cloth surface to a depth depending on the relative speeds of cloth and rollers.
The causes of defects in raised knitted fabric as described by Pehl (1991) are as follows:
1. Variation in temperature and humidity condition of the fabric. Cotton fabric processed in warm and dry conditions may be badly creased. Such fabric may be pre-wetted and redried.
2. The cloth tends to cling to the pile rollers if the pile action is much greater than the counter-pile rollers. The cloth becomes very tight on the feed side and slack on the backside resulting creasing. The machine should be reset to a more balanced action.
3. Bad setting of the cleaning brushes can do a lot of damage – uneven raising should be corrected by regrinding or replacing the wire.
4. Changing fabric width may produce lines due to wire damage at the selvedge of the previous cloth.
5. Lateral stripping can be caused by yarn variation, which may not show up before raising.
6. Streaky or patchy raising may be due to traces of finishing agents.
The construction of knitted fabrics has an important bearing on the effectiveness of the raising process. The loops should stand erect without twisting, so twist factor (English) must not be higher than about 3.8 mm. Loop height should be 3.0–3.8 mm unlike normal terry customarily having a loop height of less than 2.5 mm. Usually raising is carried out after dyeing and drying.
Emerising
Emerising, sueding or sanding is a raising process in which the fabric in open-width is passed over one or more rotating emery-covered rollers to produce a suede-like finish. The major effect of emerising the fabric is the production of a very low pile – that is, short fibres protruding from the fabric surface. The handle after emerising depends on the fibre(s) present, linear density of the fibre and the intensity of the emerising. The handle becomes much softer, especially when micro fibres and chemical softeners are used and to provide a peach-skin finish. Emerising of microfibre fabrics should be carried out prior to dyeing.
The most versatile emerising machine is multi-roller type having 4–8 rollers covered with emery papers and driven independently in clockwise or anticlockwise directions. Surface character of the rollers can be varied widely.
Ideally, the emery-covered rollers function as a cutting tool, severing the protruding fibres surface to a velvet-like, very short pile or nap. The effect on the fabric may be fine or severe depending on the emery grade (or grain) size. Microfibre fabrics are usually emerised with fine grade emery paper, followed by emerising with coarse grade emery paper, while for many fabrics, the opposite order gives more satisfactory results.
In a multi-roller machine, an emerised effect depends on the following parameters (Heywood, 2003):
1. Number of rollers in operation.
2. Direction of rotation of the rollers (i.e. with or against the fabric) – generally first and third roller run in the opposite direction to fabric passage, while second and other even rollers run in the same direction.
3. Fabric construction and tension – a tight fabric construction in plain will be more difficult to suede or emerise than 2/1 or 3/1 twill where the long weft float can be used to enhance surface fibre development. The tension must be adjusted to suit the particular fabric being emerised or sueded.
4. Fabric wrapping angle on the rollers.
5. Fabric speed (12–15 m/min for micro fibre fabric, 15–25 m/min for woven fabric using spun yarn and 10–20 m/min for similar knitted fabric).
6. Grade of abrasive grit used in emery paper covered rollers – a relatively coarse grade of 80–100 allows the weft threads to be caught and lifted by the rollers producing a dense, long pile. The grains having size of 280–320 produces short and dense naps on lightweight ladies’ outerwear fabrics having weight of 100–180 g/m2 The grain size is increased to 400–600 for emerising finer micro fibres of polyester and nylon. Still higher grain sizes of 600–800 are rarely used as they exert polishing rather than emerising action.
7. Additional device that may be fitted for sueding back side of fabric.
8. Single-roller emerising or sueding machines generally consist of one abrasive-covered metal roller (optionally water-cooled) and one rubber-covered compression or pressure roller. The single-roller emerising machine is less productive – typical operating speed for microfibre fabrics is about 7.5 m/min. The single-roll machine, however, is used especially on fabrics with terry loops on the face that must be broken and also on difficult styles where the fabric surface must be effectively shaved or polished.
Multi-roller emerising machines may be cylindrical or of slatted design.
15.7.2 Pre-shrinking finish
In the past relaxation drying was the only way in which fabric shrinkage could be reduced. In the so-called London shrinkage the fabric is placed in contact with a dampened wrapping material under tensionless condition for a few days followed by drying by hanging. For virtual elimination of length shrinkage, however, it is necessary to resort to the technique called ‘compressive shrinkage’.
Sanforizing and Rigmel are two methods of producing anti-shrink cellulosic materials developed by Sanford Cluett (USA) and Wrigley and Melville (UK) respectively. The methods are based on the fact that in an elastic material sinuous waveform, the convex surface is extended and concave surface is contracted. If cotton material is placed on the extended crest of the wave formed by the elastic material and moves with it into the contracted portion, being held firmly in contact with the elastic material during the movement, then the cotton material will be contracted by compression. Both methods very judiciously utilise a thick blanket for a closing up of the warp. They both allow the weft closing up to take care of itself. The fabric is not stretched weft-wise or set to an unstretched width in the stentering operation as a final stage in fabric finishing. Both processes pay particular attention to warp shrinkage, as most fabrics shrink more in the warp than in the weft and in garment warp shrinkage is usually more important (Marsh, 1979). The ‘Sanforized’ mark indicates that a fabric has been treated so that it will not shrink or stretch more than 1% or 2% on wetting.
Some process controlling parameters in a Sanforizer as described by Shah (www.sulphurdyes.com) are as follows.
Damping
Damping is done by sprinkling water over the fabric prior to compress it. It is checked by the conductometer.
Standard: it should be optimum. The fabric should not be too wet, it should be just damped. The spray intensity is to be adjusted.
Temperature
Shrinking is induced at the higher temperature of a Palmer drier. The temperature is checked by thermometer. The standard range is 140–160 °C. The steam supply is regulated to get required temperature.
Width of the fabric
After shrinking the width of the fabric is to be checked at the delivery end manually. Dwell time and % shrinkage are to be adjusted.
Speed
During pre-shrinking the speed is generally kept in range of 20–40 m/min. The speed should be uniform throughout the batch.
Belts and blankets
The Sanforizer belt is made up of rubber and the blanket, which is a part of the Palmer unit is made up of wool. The surface of belt is to be checked before and during finishing.
Standard: it should have smooth surface. Correct surface of the belts and blankets is be maintained by regular grinding.
Cooling of belt
Water is sprayed for cooling and protecting the belt. As the belt is in constant contact with the heated roller, it must be cooled. During running, the temperature of the belt should not be more that 15–20 °C above room temperature and the belt should be cooled to room temperature after completion of the process.
Standard: it must be always cool. Chocking of sprayer nozzle heads is to be checked from time to time.
15.8 Finishing with alkali
Strong caustic soda solution is used for mercerisation of cellulosic materials as well as for weight reduction of polyester to impart silk-like softness.
15.8.1 Mercerisation
Mercerisation is a treatment that gives lustrous appearance to a cotton fabric or a cotton thread. The process is applied to materials like cotton and hemp. The process was devised in 1844 by John Mercer of Great Harwood, Lancashire, England, who treated cotton fibres with sodium hydroxide. Mercerisation alters the chemical structure of the cotton fibre. It results in the swelling of the cell wall of the cotton fibre, increase in the surface area and reflectance, and gives the fibre a softer feel.
The cellulosic materials in yarn or fabric form are treated with a concentrated solution of caustic alkali whereby the fibres are swollen and the following properties are modified:
1. The strength and dye affinity of the materials increase.
3. If the material is stretched during or after the treatment, the lustre of the material is enhanced.
Some controlling factors in mercerisation are described below:
Moisture control
Drying cylinders are kept before the mercerisation tank to have the same moisture content in the fabric throughout for uniform results. Anther technique is wet on wet mercerisation, in which fabric is pre-wet but it requires high precaution.
Caustic soda in padding solution
Method of checking: Twaddle meter or titration.
Necessary action: Adjust the concentration according to the requirement.
At a later stage the concentration in the padding trough may increase due to the impurities from the fabric such as thickening agent, etc.
Temperature of padding solution
Ideally it is carried at room temperature. If the temperature is higher, then it is because the moisture in the fabric is more. Water and caustic soda lead to an exothermic reaction that will increase the temperature. So dry the fabric properly. If the temperature still increases then check the water cooling line.
Wet pick-up
Wet pick-up is generally 120–125% but it should be perfectly uniform throughout the width and length. Pick-up is examined by comparing the original weight of fabric with fabric weight after padding. Pick-up is checked randomly.
Washing
The first compartment after mercerisation tank is the recuperator. Here the concentration of caustic soda should not be more than 10°Tw. If the concentration is very low then take less water (because if more water is present then caustic soda associates itself more with water molecule and its size becomes bigger which is very difficult to remove from the core of the fibre while washing and the washing may not be successful).
Temperature of recuperator
Here the hot washing is carried out. Live steam is blown in water. The temperature should not be less than 90 °C.
Souring
This treatment is carried out when bleaching is performed on the fabric. In grey mercerisation souring is not done. Removal of caustic soda is very difficult and hence acid neutralisation is very cheap and easy.
Removal of alkali by washing consumes a large amount of water.
Standard: Extract of fabric must be neutral after the souring treatment. Fabric after washing: Check the extract of fabric by pH indicator or pH paper
Standard: pH must be neutral. If fabric is acidic then adjust flow of water, if alkaline in nature than adjust souring percentage.
Residual caustic soda
Removal of all caustic soda is very difficult and uneconomical. It should not be more than 1% on fabric and if the quantity is more than 1% then check the washing efficiency, efficiency of recuperator and adjust the flow of water.
The most important controlling factor is the concentration of sodium hydroxide solution, which is monitored with a hydrometer. Several hydrometer scales are in use. Mercer used the Twaddell hydrometer scale (°Tw), which is still used in England, USA and elsewhere, especially in the mercerisation of woven fabrics. The mercerisation of circular knits developed in continental Europe, where Baume scale (B) has been traditionally used and most knit-good mercerisers are familiar with the system. The concentration may also be described in terms of specific gravity (S).
The relationship between the specific gravity (S) and Baumé (°Bé) (B) may be expressed as shown in Equation [15.6].
The relationship between °Tw and °Bé is shown in Equation [15.7].
The most important quality control parameters during mercerisation are:
15.8.2 Weight reduction of polyester
The weight reduction of polyester by chemical method confers special aesthetic properties on polyester (drape, suppleness etc.). Modification of polyester fibre has been necessitated to overcome several inherent shortcomings in the polyester fibre properties.
The drawbacks of polyester include very low moisture absorption (0.4% moisture regain) and poor dyeability due to high degree of ‘hydrophobicity’ of the fibre molecular structure and compactness of polymer structure.
The weight reduction of polyester is done by saponification of terephthallic ester by sodium hydroxide in presence of a suitable wetting agent. The partial alkaline hydrolysis causes progressive ‘weight reduction’ of polyester fibre in the surface, resulting in a small weight loss, whereas its other favourable properties remain almost unchanged or changed for the better. The weight reduction treatment is thus a major breakthrough to obtain ‘Skin care’ polyester fabric.
Important variables for weight reduction method of polyester are as follows:
• temperature – most critical,
• caustic soda concentration – amount of chemical and volume of bath are important,
• time – governed by temperature and concentration of caustic and weight loss desired,
To achieve 15% weight reduction, at the optimum conditions the treatment was carried with 5% caustic soda and 0.5% wetting agent at 90 °C for 60 min (Satees, www.scribd.com/). It is reported (Vigneswaran and Anbumani, 2007) that the treatment of 100% polyester rotor spun yarn (twist multiplier 3.0–4.0) with 5–15% concentrated caustic soda at 60 °C resulted in 3–12% weight loss and 7–19% strength loss and treatment at 100 °C registered 6–53% weight loss and 17–72% strength loss.
The following points are to be managed (Hayakashi, 2008) during dew-eighting of polyester:
1. There are many variations in the processes, such as atmospheric pressure method, high pressure method, continuous process, hanging method – the most suitable method should be followed.
2. Caustic soda concentration should be around 4–20%.
3. Use of accelerator (quaternary ammonium salts) – control to be exerted on the type and amount of accelerator.
4. Treatment temperature should be around 95–130 °C, and liquor ratio between 1:20 and 1:60.
5. Weight reduction rate is generally restricted between 10% and 30% and precautions should be taken to prevent excess weight reduction.
6. Uneven weight reduction at different parts of the fabric is to be prevented which may otherwise cause uneven dyeing.
7. The deterioration of strength of fabric should be within the tolerance limit.
8. Removal of decomposed polyester oligomers from the deweighted materials which may otherwise cause clogging during beam dyeing, staining on dyeing machine and breaking of knitting needle.
9. No yellowing of fabrics – yellowing may occur due to the presence of residual accelerator.
10. Troubles caused by accelerator such as interaction with disperse dyes causing poor shade reproducibility.
11. Waste-water treatment problems, especially caused by accelerators such as increased BOD, and the effect on activated sludge.
15.9 Softeners
A survey conducted by the Marimount University concluded that 56% of the polled people believed that a soft hand feel relates directly to quality and would be helpful in making their purchasing decision (Biancalani, 2009).
Textile softeners are used to vary the handle of fabrics (similar to filling, stiffening and weighting finishes). They should demonstrate a positive effect on the handle of treated textiles; many textiles require softer, smoother, more supple handle for the best sales potential. They may also serve to improve the processability and wear characteristics of the textiles. Most textile processes, according to their intensity, will more or less lead to the removal of oils and the embitterment of textiles. The softeners must restore the natural softness and suppleness. Often, a softener must also reduce the tendency of textiles to build electrostatic charge. A wide variety of chemical compounds of differing constitutions are used for formulating textile softeners. They often contain a hydrophobic molecular component. The hydrophobic part is usually an alkyl chain of 16–18 carbon atoms length, if it is to contribute a softening effect. The varied application possibilities for softeners are further complicated by the variety of subjective assessments possible with so many different chemical types.
The requirements profile for textile softeners are as follows (Rouette, 2000):
15.9.1 Influence on textile properties
• Textile characteristics: handle, volume, softness, fall, odour.
• Mechanical properties: stretch, elasticity, abrasion resistance, tensile strength, tear strength, smoothness, pilling tendency, sewability.
• Functional properties: moisture management (hydrophilic/hydrophobic), antistatic, flame retardant, dirt resistant, sewability, rope crease prevention, antimicrobial.
• Aesthetic properties: colour nuance, fastness, permanence, whiteness, thermal migration.
15.9.2 Influence on production parameters
• Environmental acceptability (manufacture and use):biological breakdown, toxicity, irritant potential, corrosiveness, bath exhaustion, transport.
• Resistant to: electrolytes, water softeners, acids, alkalis, suitability for jet dyeing (foam, shear forces), storage stability (heat, frost), drying and fixation processes.
• Handling: viscosity (suitable for metering), concentrate, stock emulsion, solubility.
• compatibility: bleach liquor, dye liquor, reductive post-cleaning, optical brighteners, synthetic resins, catalysts, chemical finishes.
15.9.4 Cationic softeners
From an exhaust bath, the speed of exhaustion of the cationic finishing agent on to cotton fabric depends on the strength of the positive charge it carries. This, in turn, depends on the pH. At lower pH, cationic softeners carry relatively higher positive charge and therefore are exhausted more rapidly even in cold. At pH 4–5 the exhaustion is almost total. This high rate of exhaustion on cotton is also very undesirable, since it tends to develop uneven spots/stains on the fabric surface. This is due to rushing and exhaustion of the softeners into sites that are easily penetrated and relatively less or none is available for sites that are more difficult to penetrate.
Therefore for different softeners the optimum pH conditions are to be established considering temperature of application and M:L ratio. Fabric construction and geometry also will influence ease/difficulty to penetrate. Generally, weakly acidic conditions are recommended by the manufacturers/suppliers of the softeners to achieve uniform and even exhaustion. The exhaust time should normally be 20–30 min.
Secondly, the variation in pH (particularly falling on the alkaline side) on the fabric across the width/length can cause differential exhaustion on the fabric surface. Accordingly the performance in terms of the actual ‘finish’ characteristics such as softness, lubricity, feel, drape, etc. and wash fastness also will vary. It is recommended that both the fabric substrate and the bath are maintained slightly acidic with safe organic acids.
Incomplete removal of anionic soaps and detergents normally used in the earlier soaping operations results in the cationic finishing agent forming a complex with the anionic soap/detergent and causing precipitation and thus diminishing the softening effect. This point is often neglected. A proper rinsing cycle after soaping is required to minimise this problem.
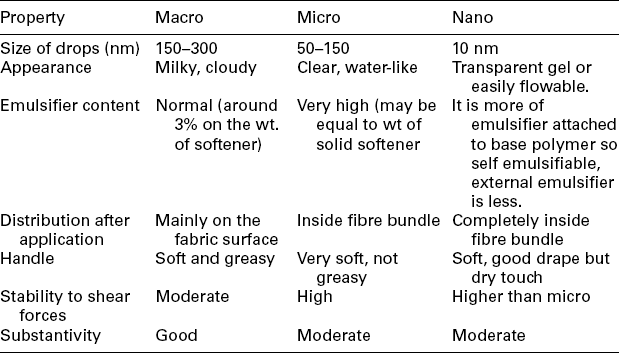
Softeners are water insoluble and are marketed in the form of emulsions. Microemulsions of softeners, mainly amino-modified silicones, give special softening affects. Due to their high emulsion stability, they are very suitable for applications with high shear as in jet or package dyeing machines. Unstable emulsion deposits on equipment and fabrics are very difficult to remove. The properties of various types of emulsions are shown in Table 15.1. The macro softener has made the fabric less flexible and less hydrophilic in nature than the same with the micro. The micro softener has imparted maximum flexibility and the nano softener the best hydrophilicity (Roy Choudhury, 2012).
Some properties of softener emulsions are as follows (Schindler and Hauser, 2004):
1. Emulsion stability – highly stable normal (not micro or nano) softener emulsions cannot provide a high degree of softness. The emulsions of moderate stability give better softness probably because small softener droplets deposit on the fibre surface. Unstable emulsion causes stains.
2. Reactive softeners – some softeners have functional groups (e.g. N-methylolated amines) that can react with the corresponding groups of textile fibres (e.g. hydroxyl group of cellulose). They provide a highly durable finish combined with typical merits–demerits of cross-linking chemistry.
3. Thermomigration of dyes – some hydrophobic softeners are solvents for disperse dyes and thereby increase thermomigration causing poor wash, crock fastness and staining of adjacent yarns.
4. Yellowing – the oxidation of cationic softeners or amino-modified silicones may cause yellowing of undyed finished fabrics. Yellowing may also arise due to interaction of cationic softeners and anionic fluorescent brightening agents. Careful selection of softeners and use of dispersing agents are essential for finishing of undyed fabrics.
5. Effect on dyeing shade: the use of silicone results in deeper shades as in the case of wet textiles due to lower refractive index of silicone (1.43) as compared to those of cotton (1.56) and nylon (1.57). Due to high reflection from a polyester surface arising from smooth surface and high refractive index (1.63), a higher amount of dye is required for obtaining deep black shade. The problem is more critical with microfibres having larger surface (about double that of normal). Deeper black and other shades may be obtained on polyester micro fibres by applying silicone, preferably amino-modified finish. The increased thermomigration by silicones may be controlled by avoiding overdosing and drying over 120 °C.
15.10 Resin finishes
For the formation of wrinkles/creases in fabrics, the forces distorting the fabric must be transmitted to the individual fibres. The forces must then place a strain on the individual fibres and distort them. Some of the physical factors for wrinkling are set out below (Tomasino, 1992).
15.10.1 Fibre properties
Fabrics made from fine cotton fibre do not wrinkle as badly as fabrics from coarse fibre. This is because the bending radius of curvature is greater for a thick fibre than for a thin one. The greater the radius of fibre, the greater is the stress on the polymer chains. Fabrics made from fine Egyptian cotton wrinkle less than those made from coarse fibres.
15.10.2 Yarn properties
Fabrics made from high twist yarns wrinkle more than those made from low twist yarns. For low twist yarns, the distortion stresses are dissipated by the physical rearrangement that takes place as adjacent fibres slip by each other. The stress is dissipated before it can affect the individual fibres.
15.10.3 Fabric properties
Tightly woven fabrics wrinkle more than loose structured fabrics. In a loosely constructed fabric, the yarns can move as they respond to the wrinkling forces. The individual fibre is spared. Woven fabrics wrinkle more than knits. The knit loops allow an even greater freedom of yarn movement again sparing the individual fibre.
Resin finishing improves wet and dry crease recovery and crease retention. The chemicals used are thermosetting resins and catalysts. The resins may be nitrogenous, such as dimethylol urea, melamine formaldehyde, DMEU, DMDHEU, etc., or non-nitrogenous, such as dichloropropanol. The catalysts used are mineral and organic acids, metal salts, etc.
Crease resistant finishes are applied to cellulose fibres (cotton, linen and rayon) that wrinkle easily. ‘Permanent Press’ fabrics have crease resistant finishes that resist wrinkling and also help to maintain creases and pleats throughout wearing and cleaning. Wrinkle recovery is dependent on the presence of cross-links that hold adjacent molecules together and pull them back into shape when they are distorted.
Resin finish improves the following properties of fabrics:
The desirable properties of easy-care finishing are as follows:
1. high ‘durable press’ rating, high dry and wet crease recovery angles,
2. minimum shrinkage and minimum loss of abrasion, tensile and tear strength,
3. negligible effect on shade and fastness of dyed materials,
4. no yellowing of white materials,
5. negligible effect on absorbency of the textile materials,
6. low or zero formaldehyde release during application and storage,
The disadvantages of resin finishing are:
3. lowering of abrasion resistance,
4. retention of chlorine as chloro-nitrogen compounds resulting in the damage of fabrics during subsequent ironing. The problem can be overcome by using nitrogen-free cross-linking agents,
6. increase in soiling and affinity for soil,
7. loss in moisture absorption,
8. Release of formaldehyde during application and use of finished fabric.
The release of carcinogenic formaldehyde during application and fabric use is highly objectionable. The liberation of formaldehyde depends on the following factors (Lewin and Sello, 1983):
1. Type of cross-linking agent
2. Amount of cross-linking agent
The various ways of reducing liberation of formaldehyde are:
1. Use of selected cross-linking agent
2. After-wash of the finished fabric
3. Addition of formaldehyde acceptors such as cyclic urea, cyclic carboxylic acid amides and cyclic carbamates in the finishing bath
4. Treatment of finished fabric with formaldehyde acceptors. The BASF fog chamber technique involves a spray-mist (fog. application to the finished textile fabric with a solution of formaldehyde binding substances as mentioned above.
The important effects of resin finishing on fabric properties are:
1. Crease recovery – a linear relationship between crease recovery and the logarithm of resin content has been demonstrated for cyclic ethylene urea and for a modified melamine at solid contents 2–10% on cotton (Foster, 1957). A similar relationship probably exists for dimethylolurea. For a certain amount of resin the improvement in crease recovery is higher on viscose than on cotton.
2. Strength – on application of resin, cotton is invariably weakened, while viscose always gains somewhat in dry strength and substantially in wet strength. It is probably due to differences in the fine structures of the two fibres. Cotton has an intrinsically strong, highly ordered structure in which less ordered regions, the fibrillar boundaries, act as areas in which slippage can occur, allowing transfer and release of strains. Cross-linking in these regions prevents such slippage and results in a less perfect distribution of the applied stress, leading to localised stresses and consequently early failure. Rayon, on the other hand, has an intrinsically weak, less ordered fine structure; the molecular chain is much shorter and the system is easily torn apart. Insertion of cross-links has the effect of increasing the molecular weight and hence the cohesiveness and strength increase as a whole. With the introduction of a very large number of cross-links (which may not arise during normal resin treatment), the structure becomes hard and brittle.
3. Elongation – due to resin treatment a large reduction in elongation (50% or more) occurs for both cotton and rayon with consequent loss in tearing strength. Simultaneous application of softeners and lubricants restores tearing strength completely for viscose, but restoration on cotton largely depends on fabric construction and resin content.
The three methods of application of resin on textile materials are as follows:
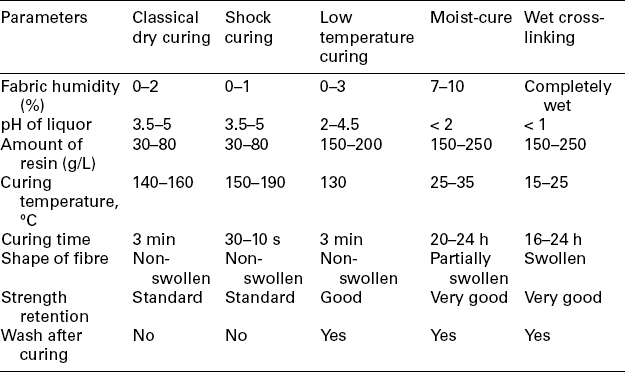
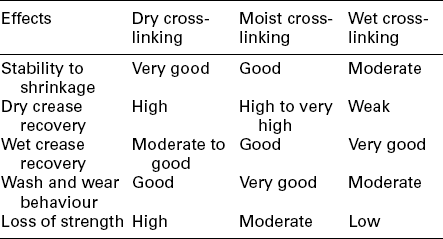
Comparison of the processing parameters and the effects obtained by the above three resin finishing methods is shown in Tables 15.2 and 15.3, respectively.
The controlling factors in resin finishing are:
• Concentration of resin in pad bath (depends on type of fibre, fabric structure and application). More the resin better is the performance. But the restricting factor is the loss in strength. 30 to 45% loss of tensile strength has to be taken in account.
• pH of the pad bath (should be acidic).
• Wet pick-up (should be as low as possible).
• Moisture content of dried fabric (should be 5–7%).
• Time and temperature of curing (depend on resin and type of catalyst).
• With strongly acidic catalyst – curing may be carried at lower temperature; with metal salts 5–3 min at 140–160 °C respectively is required.
• For better performance the fabric should be made of good quality long staple cotton fibres.
• Performance will also depend on yarn construction and fabric construction (weaving and design).
• The residual alkalinity on the fabric before treatment should be less than 0.04 g caustic soda/100 g of fabric.
The efficiency of curing may be assessed by measuring the nitrogen content of a sample before and after washing (i.e. after removing unreacted resin). The efficiency should be at least 80%.
Quality control tests: crease recovery angle, tensile strength, tear strength, free-formaldehyde content.
15.11 Protection from fire damage and water penetration
Repellency to fire and water are two most important finishes for textile materials. Fire retardancy is an important characteristic of textile materials in order to protect consumers from unsafe apparel. Fire fighters and emergency personnel require protection from flames. Floor coverings, upholstery and drapery also need protection, especially when used in public buildings. The military and airlines industries have multiple needs for fire-retardant textiles. The term flame retardant is used to describe fabrics which will not support combustion and are self-extinguishing. In case of accidental fire, this type of fabric will not contribute to the spreading of flame. The resistance of fabric to water may be of two types – water-proof and water-repellent. In the former type both water and air cannot penetrate into the fabric, while in case of water-repellency, only air can penetrate into the fabric.
15.11.1 Fire retardant finishes
Properties that characterise burning behaviour of the products can be listed as follows:
The physical and chemical properties of the fabric that influence burning are weight, weave, density, yarn size, twist, and ply, degree of purification of the cotton, presence of certain dyes, moisture content, characteristics of the fibres in a blend, and type of finishing agent.
The factors which promote the rate of propagation of combustion of textiles (Rouette, 2000) are:
• Rate of the pyrolysis reaction
• Melting behaviour of the fibre material
• Oxygen requirement of the decomposition products during burning
• Weight per unit area of the material
• Fit of clothing (tight or loose)
• Number and nature of the layers of under and outer clothing
The effect of dyes is of minor importance in this connection. It is possible that they (e.g. metal complex dyes) may occasionally have a significant wicking effect, which does not significantly increase the rate of propagation.
In testing and evaluating the burning behaviour of textiles, the following variables are important:
1) Minimum ignition time – the time that is required to ignite a test sample of the textile material under defined conditions, that is, to produce persistent self-supporting combustion.
2) Burning time – also known as the after-burn time or the after-burn period, that is, the time over which the test sample continues to burn independently after ignition and removal of the source of ignition.
3) Rate of flame spread – the distance that the flame travels on the burning test sample in unit time.
4) Glow time – also known as afterglow time or afterglow period, that is, the time over which the material continues to glow after the source of ignition is removed.
Quality control tests: inclined and vertical flammability testers, cigarette burning test.
15.11.2 Water repellent finishes
Many terms are used to describe the water-repellency of textile materials, particularly fabrics. However, they are mostly classified into two unambiguous groups – water-proof and water-repellent.
Treated or untreated textile materials that prevent the absorption and penetration into their structure are called water-proof. In practice, a waterproof fabric shows no penetration by water below hydrostatic pressure of 100 cm (10 kPa).
The main differences between water-repellent and water-proof fabrics are:
1) The pores in water-proof fabrics are filled, while they are open in water-repellent fabrics.
2) Air and water vapour permeability are zero or negligible in the case of water-proof fabrics, while they are usually high in the case of water-repellent fabrics.
3) Water-proof fabrics are resistant to penetration by water, while water can penetrate water-repellent fabrics under external hydrostatic pressure.
Water-repellent finishes resist wetting. If the fabric becomes very wet, water will eventually pass through.
When a drop of oil in contact with a textile surface forms a contact angle with it, the following three cases are possible:
| Contact angle | Property on surface |
| > 90° | no wetting (formation of droplets) |
| < 90° | surface wets |
| 0° | instant wetting of surface |
The contact angle depends on the surface energy of substrate (SE) and the surface tension (ST) of the liquid. Wetting occurs only when ST is less than SE.
ST of some liquids and SE value of some fabrics are as given in Table 15.4.
Table 15.4
Surface tension and surface energy of a few liquids and a few fibres respectively
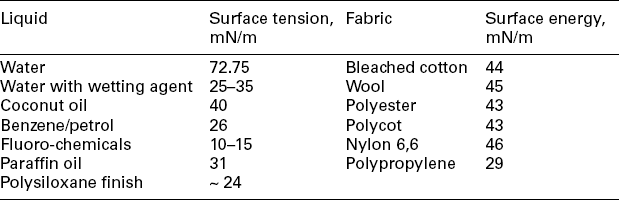
Source: Goyal and Prabhu, 2009.
Hence, it is necessary to design proper structure of water repelling agent keeping in mind the surface characteristics of the material. The structure of the fabric is also equally important from the point of view of the ability of fabric to shed water resting on its surface and its ability to resist penetration of water.
Water repellents commonly available in the market fall under different categories as follows:
• Wax based repellents (20–25% paraffin and 5–10% zirconium salt, aluminium based salts) are cheap and can provide good water-repellency and resistant to water-pressure but they are not wash-fast and cannot impart oil repellency. The breathability of the finished fabric is low.
• Resin based repellents – products of condensation of fatty compound (acids, amides or amines) with methylated melamines.
• Silicon repellents – aqueous emulsions of polydimethylpolysiloxane – the finished fabrics have good water-repellency, water vapour permeability, soft handle, but cannot provide soil- or oil-repellency.
• Fluorochemicals – mostly copolymers of fluoroalkyl acrylates and meth-acrylates – they provide good water and oil repellency, resistance to washing and dry cleaning, and good soil-repellency but the price level is high.
For water-repellency, the fabric needs to have a rough surface and a loose structure while ensuring best water shedding properties and the right construction for offering resistance to penetration. Fabric for water repellent finish must be free from sizes, detergents, alkali and acids.
There is considerable growth in the use of more expensive fluorochemical finishes which can be applied at low add-on, for example 0.15–0.3% (on the weight of fabric). Fluorochemical-based water repellents give durable finish. The carbon–fluorine bond is extremely strong and stable. Chemical substances containing fluorinated carbons, ‘fluorochemicals’, have special physical and chemical properties such as chemical stability, thermal stability, and low surface energy. Fluorochemical products provide performance benefits not achievable with other substances. For example, textile fluorochemicals provide oil and water-repellency and stain and soil resistance without affecting the hand, appearance, or breathability of a fabric.
High fabric repellency depends on the following factors:
1) Fine yarns and compact textile structure
2) A thoroughly prepared fabric free from impurities such sizes, lubricants, surfactants with rewetting action
3) Uniform application of a chemical finish to provide a low-energy surface having lower critical surface energy than the surface tension of liquids likely to be encountered.
Three main test methods are used to evaluate the water-repellency of fabrics, namely:
1) Spray tests, to simulate exposure to rain
2) Hydrostatic pressure tests, measuring water penetration as a function of pressure exerted by water standing on the fabric
3) Wettability tests – sorption water by the fabric when immersed in water
Oil repellency is assessed by hydrocarbon resistance test noting contact angle or collapse time of drops of solvents falling on the fabrics. Various solvents namely n-hexadecane, n-tetradecane, n-decane, n-octane, n-heptane etc. may be used for the testing purpose.
15.12 Anti-pilling finish
Experience has shown that generally pure cotton fabrics are not prone to develop objectionable pilling. Some cotton fabrics may become hairy and some pills may form, but the rate of pill break-off is greater than the rate of formation leaving the surface void of pills. The reason for this is because cotton anchor fibres are relatively weak. Experience has also shown that some fabric constructions made with polyester/cotton yarns will exhibit severe pilling. When these pills are magnified, the anchor fibres are seen to be polyester filaments. The strong polyester anchor fibres do not break off easily so pills continue to build-up and not wear away.
The following factors affect the formation of pills on the textile materials (Tomasino, 1992).
15.12.1 Fibre variables
1) Fibre fineness – yarns made from fine denier synthetic filaments (≤ 1.5 dpf) pill more than yarns made from coarse filaments (≥ 2.5 dpf). This is because yarn twist imparts greater cohesive forces onto larger diameter fibres than finer fibres. As the filament denier decreases, the total number of synthetic filaments in any given weight percentage of a blend increases. This creates many more fibre ends that serve as anchor fibres. Finer fibres, because they are more in number, will move more easily through a yarn assembly than stiff thicker fibres which are in fewer number.
2) Fibre strength – fibre tenacity is a major contributor to fabric pilling because fabrics made from weaker synthetic fibres pill less than fabrics made from their stronger counterparts. Synthetic fibre producers offer pill resistant varieties that are based on lower molecular weight polymers, resulting in lower tenacity and flex life. Low flex life alone is not enough to produce pill-free fabrics; fibre migration must also be controlled.
15.12.2 Yarn variables
1) Yarn twist – yarns with low twist will pill more than yarns with high twist. The degree of twist will influence the ability of fibres to migrate to the surface. The lower the twist, the easier it is for fibres to migrate.
2) Hairy yarns – hairy yarns pill more than smooth yarns. Low twist contributes to yarn hairiness.
3) Yarn spinning methods – open end spun yarns pill more than ring-spun yarns because the yarn structure is more uneven, allowing for greater fibre mobility. Air-jet spun yarns with low flex life fibres result in relatively pill-free fabrics. Air-jet yarns have wrapper fibres holding the yarn assembly together. These act to keep the body fibres from migrating to the surface.
15.12.3 Fabric construction
Tightly constructed knits and woven fabrics pill less than loosely constructed knits and wovens. Tighter constructions reduce the migration tendencies of the fibres within the yarns.
15.12.4 Preparation and dyeing
Some wet processes make pilling worse while others provide substantial improvement. Preparation and dyeing processes that overwork the fabric will cause excessive hairiness and lead to poor pill performance. Long preparation and dyeing cycles are especially bad. In some instances pills may arise from just these processes alone. High-temperature dyeing of fabrics containing low flex life, high shrinkage fibres improves pilling performance. Auxiliaries such as carriers also have a positive effect on these fibres.
1) Singeing and shearing: singeing and shearing are methods of reducing fabric hairiness. In many cases, pill ratings are improved because of the reduced hairiness. This improvement may last for some fabrics for many wash-dry cycles; however, for others the onset of pilling is only temporarily postponed. Pills will start to form after several wear-wash cycles.
2) Heat setting: heat setting of fabrics containing thermoplastic fibres is often beneficial in improving pilling performance. For some fabrics, the improvement may be temporary and deteriorate after multiple wash cycles. On the other hand, heat setting is definitely helpful with those fabrics made with synthetic fibres having higher heat shrinkage. When high heat shrinkage is combined with low flex life, heat setting can enhance pilling performance to the point where the fabric appears to be pill-free.
3) Certain softeners, which decrease fibre-to-fibre friction by internal lubrication, such as non-ionic organo-modified silicone microemulsions and amino functional polysiloxanes, result in a decrease in fabric pilling performance.
4) For polyester/viscose blended fabrics, Sanforizing treatment also results in an increase in pilling propensity (Hussain et al., 2008).
15.12.5 Fabric finishing types
1) Finishing procedures have a pronounced positive effect on fabric pilling. Many fabrics can be improved by selection of proper finishing conditions.
2) Film-forming binders – finishing with film-forming latexes will improve the pilling performance of nearly all fabrics. If the final fabric hand is acceptable, these film-forming finishes may be used for a universal solution of pilling tendency. These finishes reduce fibre migration by bridging across filaments binding them together. About 1.5% solids deposited on the fabric is needed to do much good.
3) Latex binders applied to fabrics utilising low flex life (pill resistant) fibres produce a dramatic improvement in pill ratings. Whereas the base fabric alone is only marginally better than one made from conventional polyester fibres (still rated objectionable), the latex finished fabric is virtually pill-free. The performance of latex finished conventional fibre fabric is improved but not to the same degree as the low flex-life-fibre fabric.
4) Durable press reactants – cellulose cross-linking resins applied to low flex-life fibres also produce dramatic improvement in pill rating. However, they have very little effect on conventional polyester fibres.
5) Fabric softeners – materials that reduce the coefficient of friction between fibres will make pilling worse. Fabric softeners increase the pilling propensity of a given fabric. Those applied after dyeing and/or in the finish bath make matters worse. Laundry added softeners may also interfere with pill resistance. These materials operate on the fibre migration portion of the pilling mechanism making it easier for the filaments to move. Softeners co-applied with latexes tend to overcome the improvements noted with film former. Those softeners that provide a soft hand (silicones, ditallow quats) are the worst offenders.
15.13 Other types of finishing: antistatic, soil release, antimicrobial and UV protection
In this section remaining four finishes namely anti-static, soil release, antimicrobial and UV protection are discussed. While the first two finishes are inter-related and mostly applicable for synthetic textile materials only, the other two have increasingly gained momentum in recent years. Static charges are produced during friction in synthetic materials due to their hydrophobic nature which create discomfort to the user as well as helping soils to be retained by the substrate. Antimicrobial finishes are applied to protect against accumulation of microorganisms on textile material which can cause rot and odour formation on textile materials and may also spread bacterial infection. Sun-induced skin damage may be caused by increased exposure to UV light. UV Protection fabrics may save us from such damage.
15.13.1 Antistatic finish
Static electricity is generated in various ways; however, most static generation originates from triboelectric charge generation. When two materials come in contact, for thermodynamic reasons there is a redistribution of electric charges at each surface. Rubbing is not necessary, but it will generally increase charge transfer.
Triboelectric series is the ranking of materials in order of their ability to produce static charge. Air, asbestos, glass, nylon, wool, cotton, wood can produce positive charge while rubber, acetate, polyester, acrylic, polyurethane, Teflon can produce negative charge. When a material from the first group is brought into contact with a material from the latter group both acquire respective charges. The amount of static charge build-up depends on the electrical resistances of the contacting surfaces. Static charge generation is high if the resistances are high. If conductivity is increased the charges will run back along the separating surfaces to the point of contact. In the textile industry, especially in dry or low-humidity weather, electrostatic charges may generate causing repulsion between fibres, roller lapping, ballooning of yarn while running in the machine, discomfort while wearing charged garment or walking over a carpet.
Virtually all textile fibres, except metal and carbon fibres, have high electrical resistivity when completely dry. On increase in relative humidity, the resistivity as well as static charge generation decreases. Due to very low moisture, synthetic fibres are prone to static charge generation – the problem can be minimised by blending with conductive metal or carbon fibres or by finishing with suitable antistatic agents. The antistatic agent should have the following properties (Heywood, 2003):
1) Easy to apply, readily dilutable and applicable by conventional methods such as exhaust, spraying, kiss-roll method and padding
2) No effect on dyeing, colour and fastness of dyed materials
3) Stable to decomposition and vaporisation and should not diffuse inside the material
The chemical classes suitable for use as antistatic agents include polyglycols, fatty glycol esters, fatty amide glycol ethers, ethoxylated amines, amine oxide, quaternary polymers and ammonium salts, phosphoric ethers and esters, etc.
Antistatic effective chemicals are largely chemically inert and require thermosol or heat treatment for fixing on polyester goods. Numerous types of chemicals are used as antistatic finishes, including polyglycols, fatty polyglycol ethers, ethoxylated amines, amine oxides, quaternary compound and polymers and phosphoric esters. Thermostable antistatic agents also have a good soil-release action.
Fabrics are generally treated with antistatic agents by padding followed by drying in a stenter or a suitable oven.
Quality control test: conductivity meter (Honestometer). The surface resistivity is easy and relatively reproducible to determine. But it is a static test with no information about the important charging and discharging behaviour of textiles. Therefore, combination with a charge dissipation test is favoured.
A charge dissipation test was performed with static voltmeter. The fabric was fixed vertically and charged with direct voltage or by rubbing with a glass rod. Maximum charge generated, as well as decayed, was measured. The time necessary for charge to fall to one half of its maximum value, called field intensity half-life time, was recorded. The relation between half-life time and the antistatic property of the textile materials is shown in Table 15.5.
Table 15.5
Relation between half-life time for decay of charge intensity and antistatic property of textiles
| Half-life time (seconds) | Antistatic property |
| 0–0.3 | Very good |
| 0.3–1.0 | Good |
| 1.0–2.0 | Satisfactory |
| 2.0–3.0 | Sufficient |
| > 3.0 | Insufficient |
Source: Schindler and Hauser, 2004.
15.13.2 Soil-release finish
The purpose of soil-release finishing for textile is to facilitate the removal of soiling matter during laundering. Soils deposited on textile materials have complex composition containing both oily and particulate matters, namely clay, soot, street dirt, etc. Electrostatic attractive forces are primarily responsible for the deposition of air-borne particulate soils.
The degree of retention after laundering of a mixture of clay and six fats representative of human sebum on bleached cotton sheeting has been studied, with the conclusion that the presence of fats leads to a build-up of soiling, possibly as a result of the fats acting as adhesives for the clay (Sontag et al., 1970).
Work on the deposition of fatty soil on polyester-fibre fabric during washing resulted in the following recommendations (Grindstaff et al., 1970):
1) Non-ionic surfactants should be used rather than anionic.
2) The surfactant concentration should be kept above the critical micelle concentration when appreciable quantities of fatty soil are present.
3) The temperature of the first rinsing water should be kept well below 60 °C.
Factors affecting soiling and soil-release (Heywood, 2003) are as follows:
Nature of soil
One of the most frequently occurring difficult-to-remove soils is sebum (shirt collar soil), which contains mostly fatty acids, triglycerides and fatty alcohols. Normally it can be removed by washing below 50 °C. But if allowed to remain on the fabric for long time, fatty acids tend to oxidise to produce viscous material that requires higher temperature for its removal.
Nature of substrate
Soil removal from hydrophobic synthetic fibres is far more difficult than from hydrophilic fibres. The extent of soiling largely depends on the area of contact between soil and substrate. Hence, fibre geometry, yarn and fabric parameters play important roles in soil-release.
Effects of finishing
A linear correlation was observed between the extent of soiling by oily soil and the hydrophobicity of the fabrics. Hydrophobic finishes such as durable press finish, silicone and fluorocarbon finishes increase soil-retention. Soiling increases with decreasing moisture regain of cotton, particularly in the region from 5% to 4.3%, by cross-linking with DMDHEU. This corresponds to increase in nitrogen content of 1.6% to 3.4% respectively (Fiebig and Rezk, 1973). Durable press finish decreases the ability of cotton to resist both aqueous and oily soiling.
Soil-release finishes are mostly amphiphilic polymers, containing both hydrophobic and hydrophilic groups. The appropriate hydrophilic-hydrophillic balance is necessary for soil-release activity.
15.13.3 Antimicrobial finishing
Microbes, namely bacteria, virus, fungi and yeast, are present almost everywhere. Whereas human beings have an immune system to protect against accumulation of microorganisms, material such as textiles can easily be colonised by high numbers of microbes or even decomposed by them. Textiles are carriers of microorganisms, such as pathogenic bacteria, odour generating bacteria, mould and fungi. If the environment is favourable, certain bacteria can grow in a short time from a single germ to millions.
Microorganisms are small living forms of life that we cannot see with the naked eye, such as:
1) Tiny bacteria having rod, spiral or ball shape
2) Primitive plants such as fungi
An ideal antimicrobial treatment of textiles should satisfy the following requirements (Vishnu and Bhagyashri, 2010):
1) Effective against a broad spectrum of bacterial and fungal species
2) Non-toxic to consumers, no irritation or allergy to the user
3) Durable to dry cleaning, laundering and hot pressing processes
4) No negative effect to the fabric quality (e.g. physical strength and handle) or appearance
5) Compatible with other finishing agents
6) Not destructive of the resident flora of non-pathogenic bacteria on skin of the wearer.
Antimicrobial finishes may be of three types:
1) Rot proofing finishes provide long term or short term material protection against physical deterioration.
2) Hygiene finishes are concerned with the control of infection and unwanted bacteria; a specialised development is the prevention of dust mites.
3) Aesthetic finishes are used to control odour development and staining.
Some examples of antimicrobial agents are cross-linked polyethylene glycol, quaternary ammonium compounds (chains of 12–18 carbon atoms), triclosan, silver based antimicrobials, etc. Chitosan, neem, alovera and clove oil are some examples of natural antimicrobial agents.
Antibacterial efficiency may be evaluated by two types of tests:
15.13.4 UV protection finish
In recent years, consumers have become increasingly aware of the need for sun protection, which is related to the incidence of sun-induced skin damage and its relationship with increased exposure to UV light. UV radiation can lead to acute and chronic reactions and damage, such as acceleration of skin ageing and sunburn. Billions of people live on the earth, and each has his or her own colour of the skin. In the human body the skin colour depends on the quantities of melanin, carotene and oxygenated or reduced haemoglobin combined in the skin, as well as thickness, water content, etc. Among other factors, the quantity of melanin distributed in the skin determines its fairness or darkness and greatly influences the human complexion; at the same time melanin plays an important role in minimising the damage that UV rays cause in the skin.
Protection of the skin against the action of solar radiation is a relatively new objective of textile finishing, since the textile does not always guarantee adequate protection. The unfinished fabric is limited as a guarantee of adequate protection. Thus, a special additional sun protection finish is applied in the form of UV stabilisers. Electromagnetic radiation of wavelength between 150 and 400 nm are classed as ultraviolet rays. Approximately 10% of sun’s energy is in the form of ultraviolet radiation. Atmosphere absorbs most of the noxious radiations emitted by the sun, only 5% of the harmful radiations reach to the surface of the earth.
UV absorbers, such as benzotriazole and phenyl benzotriazole, molecules are able to absorb the damaging UV rays of sunlight. UV absorbers convert UV energy into harmless heat energy. This transformation is regenerative and can be repeated indefinitely.
Solar protection factor (SPF), can be defined as the ratio of the time taken for a patch of skin to develop erythema, or reddening, with and without protection. The larger the SPF, the more protective is the fabric to UV radiation. Typically, a fabric with an SPF of > 40 is considered to provide excellent protection against UV radiation. In Europe and Australia, SPF is referred to as the ultraviolet protection factor (UPF). The SPF of textile fabrics can be assessed by measuring transmission through a screen (a fabric or chemical) with the aid of a spectrophotometer, after necessary correction for the nature of the solar spectrum in comparison with instrumental characteristics and to compensate for the fact that not all portions of the spectrum are actually harmful.
Cotton and silk fibres offer little protection to UV radiation since the radiation can pass through without being markedly absorbed. Wool and polyester, on the other hand, have significantly higher SPF since these fibres will absorb UV radiation. Nylon falls in between these extremes. One factor influencing nylon and polyester absorbance is the presence of the delustrant TiO2, a material that strongly absorbs UV radiation.
Tight microfibre fabrics provide better UV protection than fabrics made from normal sized fibres with the same specific weight and type of construction. Many dyes absorb UV radiation as well as visible light. An SPF of 50 or higher can be achieved by dyeing cotton fabric to a deep shade. Since fashion and comfort often dictate the use of lightly coloured fabrics for summer apparel, the need arose for UV absorbing materials that could be applied to fibres to provide the desired SPF in light shades. Dyestuff and auxiliary manufacturers have responded by developing a variety of materials suitable for use as UV protection finishes. Benzotriazole derivatives and oxalic acid dianilide derivatives provide UV absorbers for natural fibres, while phenyl salicylates, benzophenones, benzotriazoles, phenyltriazines and cyanoacrylates provide UV absorbers for synthetic fibres.
UV absorbers should have sufficient wash fastness and light fastness as dyestuffs. Laundering trials should be carried out with all new formulations to confirm that the claimed UV protection is actually active during the life of the garment. If a UV absorber is also present in the fibre, the brightening effect from the OBA can be greatly diminished or even absent. Proper choice of an appropriate OBA can minimise this problem.
15.14 Wool treatment and enzyme finishes
This section deals with decatising and crabbing of wool fabric and enzymatic finish called bio-polishing. Decatising, also known as crabbing, blowing, and decating, is the process of making permanent a textile finish on a cloth, so that it does not shrink during garment making. The word comes from the French word ‘décatir’, which means to remove the ‘cati’ or finish of the wool. Though used mainly for wool, the term is also applied to processes performed on fabrics of other fibers, such as cotton, linen or polyester. Crabbing and blowing are minor variations on the general process for wool, which is to roll the cloth onto a roller and blow steam through it.
Crabbing is a treatment used to set the cloth and yarn twists permanently in woolens and worsted goods. In the crabbing process the fabric goes through hot and then cold water in order to set the cloth.
Bio-polishing removes the protruding fibers of a fabric through the action of an enzyme. Enzymes, such as cellulase for cotton, selectively remove protruding fibers. These enzymes may be deactivated by an increase in temperature.
15.14.1 Decatising of wool
Pressure decatising is a setting process for wool and wool-mix fabrics that provides conditions for achieving high levels of set. The principal objectives are:
The process involves winding the fabric under controlled tension onto a perforated beam previously covered with a wrapper) interleaved with a decatising wrapper. When the batch is complete, it is loaded into a steaming chamber, sealed and steamed under pressure. Typical conditions for wool fabrics are 0.8–1.0 bar pressure at a temperature of 120–125 °C for two minutes, followed by cooling to arrest the setting process. High levels of permanent set can be imparted, producing a ‘permanent’ finish. This is important for stability of finish (to steaming during making up), for control of fabric dimensional properties, and for handle and lustre. It is important that wool fabrics are at the correct pH and moisture content prior to all pressing and decatising procedures, particularly pressure decatising. Fabric pH is ideally around pH 6 – stronger acidity reduces set, and stronger alkalinity may cause yellowing. Moisture content for all-wool fabrics is ideally 12–15%, since low moisture levels can result in low levels of set.
Decatising effects can be influenced by the following factors (Rouette, 2000):
• Pressure, generated by backcloth tension and pressure from the pressure roller during the winding of the decatising cylinder
If these parameters act for an appropriate period they influence the surface pattern and the handle of the product according to the following rules:
1) Brilliant sheen (permanent) and solid handle: high steam pressure, medium steaming time, long cooling in tightly wound state, satin backcloth
2) Brilliant sheen (permanent) and soft handle: high steam pressure, long steaming time, short cooling in less tightly wound state, satin backcloth
3) Lower sheen (permanent) and solid handle: medium steam pressure, medium steaming time, long cooling in less tightly wound state, satin or molleton backcloth
4) Low sheen (permanent) and soft handle: medium steam pressure, long steaming time, no cooling, winding with low tension, satin or molleton backcloth
Modern pressure decatising machines offer a variety of steam cycles and choice of steam directions (in to out, out to in) to produce variations in handle, lustre and finish. Computers are used to store and retrieve data, including fault monitoring. Most modern decatising machines offer continuous processing. They generally provide lower levels of set than batchwise pressure decatising (Myers, 2002).
15.14.2 Crabbing of wool
Setting or crabbing is mainly applicable to worsted fabrics and is required to relax and set the strains introduced into the yarns and fabric during spinning and weaving. If some weave structures are not set in this way, they may be susceptible to the formation of distortions (e.g. ‘crows-footing’) during subsequent wet finishing. The crabbing operation is carried out in the presence of heat and moisture, during which the intermolecular bonds in the wool are broken and then reformed in a more relaxed configuration. Setting is arrested by shock cooling.
In a traditional batch crabbing machine, the fabric is wound onto a cylinder (covered with a cotton wrapper) which is rotated, whilst half immersed in hot or boiling water, for a predetermined time. To ensure even treatment, the fabric is reversed and the treatment repeated. The fabric may then be steamed and finally passed through a tank of cold water to arrest the setting process.
Two basic types of continuous crabbing machine are available. In cylinder types, the fabric is initially wetted through a trough of hot water and then passed around a large, rotating, heated cylinder. The fabric is pressed at high pressure against the heated cylinder by a specially engineered impermeable belt. Special seals resist escape of steam and entry of air at the edges of the belt. Fabric operating temperatures as high as 135–140 °C are claimed and superheated steam is created in situ, setting the fabric. Setting is arrested by shock cooling.
Superheated water machines differ in design from the cylinder types in having no pressure belt to maintain fabric/cylinder contact and use superheated water to facilitate setting. A possible advantage for fabric quality is that yarns are claimed to fully swell with minimal fabric compacting.
The fabric enters and exits through barometric columns. The temperature of water is around 110 °C, although a series of steam battery heaters situated around the main cylinder are claimed to elevate the fabric temperature during its contact time with the cylinder, promoting fabric set.
15.14.3 Enzyme finishing
Bio-polishing is a process to remove the protruding fibres of a fabric through the action of an enzyme. This cellulase enzyme selectively acts on the protruding cellulose fibres and ceases to work after finishing the job by a simple raise in temperature of the treatment bath.
Cellulases are multi-component enzyme systems that are commonly produced by soil-dwelling fungi and bacteria. The effects achieved by treating with these enzymes are pilling reduction, increase in softness and amelioration of handle, surface structure, fabric appearance and dyeing yield. The most important cellulase-producing organisms are fungi of the genera Trichoderma, Penicillium and Fusarium. The cellulases used are chemically complex and consist of at least three enzyme systems working synergistically (Holme, 1998).
Acid cellulases exhibit the greatest activity generally in the pH range 4.5–5.5 at 45–55 °C, whereas neutral cellulases require a pH of 5.5–8.0 at 50–60 °C. Generally a treatment of 45–120 min is appropriate, as prolonged treatment time may significantly increase the fibre loss. Where acid cellulase predominates, required washing time is short (20–45 min), whereas for neutral cellulase a longer wash time (45–120 min) is required. Excessive cellulose dosage and vigorous agitation may also increase weight loss.
For cotton, restriction of the enzyme to the fibre surface is easily achieved, because cellulose is a highly crystalline material with only a small amorphous region, making the diffusion of enzymes into the interior of the fibre nearly impossible. Thus, by regulating enzyme dosage and choosing the right type of enzyme, the catalytic action of the enzyme can be confined to the surface of cotton and to the amorphous regions, leaving the fibres, as a whole, intact. Surface modification of cellulosic fabrics conferring cooler and softer feel, brighter luminous colour and more resistance to pilling using cellulases, is often known as bio-polishing – a term created by Novo Nordisk.
Bio-polishing is affected by many factors. the major ones are enzyme type, type of fabric and process variables. The predominant process variables that control bio-polishing are temperature, pH, duration of treatment, material to liquor ratio, enzyme concentration and mechanical agitation.
The bio-polishing process requires:
Enzyme dosage: 1–5% on fabric weight (depending on the activity of the enzyme)
Liquor ratio: 5–15 L/kg of fabric.
Time: 60–120 min (depending upon the amount of hydrolysis required).
After treatment, the enzyme must be deactivated either by alkali treatment at pH 9–10 or by increasing the temperature to 70–80 °C and giving a treatment for ten minutes.
15.15 Low-liquor finishing
Foam finishing, a low liquor finishing technique, depends on the following factors.
15.15.1 Foam properties
Foam properties are governed to a large extent by the method of generation and by the treatment liquor composition, plus other factors, such as the purpose for which the foam is generated and the method of foam application. The principal properties of foam for the textile finisher are the foaming degree, foam stability, viscosity, wetting power, and the bubble size and size distribution (Elbadawi and Pearson, 2003).
15.15.2 Foaming degree
This is the measure of the extent of foaming. It is an indication of the volume of foam in litres that has been produced from one litre of liquor. The foaming degree is normally expressed in terms of the foam density (g/mL), which is calculated from weighing a known volume of foam. Care must be exercised during sample collection by inserting the foam-discharge hose inside the container to prevent any air pockets from being trapped within the sample (Equation [15.8]).
Foam densities of between 0.07 and 0.14 g/mL and between 0.20 and 0.33 g/mL have been used for foam finishing and foam printing, respectively (Turner, 1981). The selection of the foam density to be used depends on the fabric mass per unit area and is directly proportional to it. It is reported that low foam densities yield low wet-pick-up values (Weber and Tuschen, 1980).
Another expression of the foaming degree is the blow ratio (Equation [15.9]), which is defined as the ratio of the mass of a given volume of liquid before foaming to the mass of the same volume of foam.
The blow ratio varies with the density of the liquor, whereas the foam density is independent of the liquor density. The blow ratio is altered through control of the amount and pressure of injected air or alternatively through the liquor-pump speed. Although blow ratio is widely used as a measure of the foaming degree, it is more usefully considered as a foam generation parameter than a foam property. Moreover, it assumes 100% dispersion of the injected air in the liquor, which cannot be true, because air pockets are often seen at the foam exit.
15.15.3 Foam stability
Foam stability is defined as the time that foam will maintain its initial properties as generated. Foam stability is required during generation, transportation, and application to the fabric and has to be lost thereafter. Foams that are too stable are difficult to collapse; hence penetration into the fabric is poor. On the other hand, relatively unstable foams collapse before the point of application, resulting in an uneven distribution of chemicals on the fabric.
Foam stability can be expressed in terms of foam half-life, which is the time required for half of the volume of liquid contained in the foam to revert to the bulk-liquid phase. The shorter the time, the lower is the stability of the foam. It is reported that foam half-lives vary according to the type of process for which they are used: for instance, 30–45 min for finishing, 30–180 min for dyeing, and 8–10 h for printing (Jerg, 1979).
Another method is the drop-emergence test, in which the time taken for one drop of liquid to emerge from foam is measured. For the determination of the drop-emergence time, a given volume of foam is placed in a measuring cylinder. The measuring cylinder is then inverted and the time noted until the first drop emerges.
Other less-used methods of foam stability are determination of the change in foam viscosity and measurement of the electrical conductance of the foam between two fixed electrodes (Cooke, 1983). As the foam drains, the bubble lamellae become thinner and the electrical conductance decreases, giving a measure of foam stability.
Foam stability can be attributed to several factors, which include the following.
1) Surface viscosity – high viscosity of the liquid film that forms the bubble walls contributes to film strength and durability, owing to the increased resistance to deformation; that is, there is a reduced thinning and drainage rate. However, the higher the surface viscosity, the harder it is to remove the entrapped air and to break the foam.
2) Film elasticity is defined as the ability of the liquid film to resist mechanical disturbance. Film elasticity is influenced by change in the surface tension, which is affected by the type and the concentration of the surfactant. An explanation of this phenomenon is that the surfactant flows from regions of higher concentration to those of lower concentration when the bubble is deformed. This is a self-healing mechanism that helps to maintain more uniform film elasticity and leads to better foam stability.
3) Zeta potential of the film – this is an electric or entropic effect due to repulsion of charged ionic surfactants in the film surfaces. Ionic surfactants are adsorbed at the bubble wall, with charged groups oriented towards the liquid and the nonpolar sections protruding into the air. As the inner and outer bubble walls (which have similar charges) approach each other, because of the drainage of liquid between them, the electrical double layers can overlap, and the resultant repulsive forces prevent collapse of the bubble walls, retarding destruction of the foam.
The other factors that contribute to foam instability are:
1) Buoyancy of the bubbles, which causes them to rise at a rate proportional to the bubble radius and density of the surrounding liquid medium, and inversely proportional to the liquid viscosity;
2) Thinning of the bubble walls as the liquid drains down the inside the bubble walls through gravity and collects at the intersections of the bubble faces, known as Plateau's borders; the rate of drainage, and hence foam stability, is dependent upon the liquid viscosity, and variation in bubble size, which results in pressure differences between bubbles.
15.15.4 Foam viscosity
This is a measure of the foam stiffness or resistance to flow when the bubble walls are still in the liquid state. The viscosity increases as the number of air bubbles increases. It is also affected by the addition of stabilisers and other foam ingredients. Higher foam viscosities result in greater stability through reduced foam-drainage rates. In practice, viscosity modifiers are added to bring about foam viscosities in the range of 2000–3000 centipoise (cP) for textile utilisation. Measurement of foam viscosity is a delicate process, since the foams may be either thixotropic (i.e. they possess the property of showing a temporary reduction in viscosity when shaken or stirred) or pseudoplastic (i.e. they show an instantaneous decrease in the apparent viscosity with an increase in the shear rate) in nature (Shaw, 1980).
There are various methods available for the measurement of foam viscosity, the selection of which depends on the type of foam produced.
Foam viscosity may be measured using a rotary Brookfield viscometer operating at a low shear rate, or by using a coaxial Brabender viscometer or a U-type Ostwald viscometer in a temperature-controlled water bath.
15.15.5 Bubble size and size distribution
Bubble size and bubble-size distribution are important criteria of foam behaviour. Foam of finer bubble size is more stable than foam of the same density with coarser bubbles. As foam ages, the bubble size increases and the distribution of sizes broadens owing to diffusion of air from the smaller (high pressure) to the larger bubbles (low pressure) through the thin lamellae. Uniform bubble size in the range of 50–100 μm in diameter is a necessity for textile applications (Ghosh, 1988).
Observing and measuring the complex structure of foam is extremely difficult, as the nascent bubbles jostle one another to seek a stable arrangement before they burst and collapse. Techniques such as photomicrographs of a foam sample in a recessed microscope slide and freezing the foam have been used to determine the bubble size and distribution.
The size of the photographically recorded bubbles is measured and classified in different categories, and the distribution is characterised by the mean and standard deviation of the bubble size (Kroezen and Wassink, 1987).
A visual technique has also been reported in which foam with a narrow distribution of bubble sizes is selected by visual inspection. This is then laid on a board, frozen, and used as a standard for comparison. This technique is quite subjective.
15.15.6 Foam wetting power
Textile substrates are not readily wettable by foam. The foam has to spread out on the substrate first and then break, releasing the liquid to penetrate the fibres. The wetting power can be assessed by the spreading or penetration rate, which is governed by the foam-drainage rate.
Liquid can drain out of the lamellae films between foam bubbles owing to gravity and surface-tension differences between the least and the most sharply curved parts of the bubble. The drainage rate is affected by the foam density, viscosity, and temperature and by the film thickness.
Rapid wetting by foam, or rather by the liquid released on collapse of the foam upon contact with the textile material, is a very important property where application as textile finishing is concerned. The wetting power of the foam can be assessed by the measurement of either the spreading or the penetration rate.
Foam spreading time is evaluated by measuring the growth in the wetted area. In practice, a measuring cup is completely filled with foam and inversely placed on a standard fabric material. After a specified time, the area of the wetted-out fabric outside the circumference of the original measuring cup is recorded.
15.16 Plasma treatments
Plasma is defined as a state in which a significant number of atoms and/or molecules are electrically, thermally or magnetically charged or ionised. Plasma, in general, refers to the excited gaseous state consisting of atoms, molecules, ions, metastables, and the excited state of these, and electrons such that the concentration of positively and negatively charged species is roughly the same. Theoretically, plasma is referred to as a ‘fourth state of matter’.
Plasmas create a high density of free radicals by disassociating molecules through electron collisions and photochemical processes. This causes disruption of the chemical bonds in the fibre polymer surface, which results in the formation of new chemical species. Plasma treatment on fibre and polymer surfaces results in the formation of new functional groups such as — OH, — C=O, — COOH, which affect fabric wettability as well as facilitate graft polymerisation that, in turn, affects liquid repellence of treated textiles and nonwovens.
Gases commonly used for plasma treatments are:
• Chemically inert (e.g. helium and argon)
• Reactive and non-polymerisable (e.g. ammonia, air and nitrogen)
• Reactive and polymerisable (e.g. tetrafluoroethylene, hexamethyldisiloxane).
The potential use of plasma treatments for finishing of fibres, yarns and fabrics is as follows:
• Removing the surface hairiness in yarn
• Hydrophilic enhancement for improving adhesive bonding, wetting and dyeing
• Hydrophobic enhancement of water and oil repellent textiles
• Antibacterial fabrics by deposition of silver particles in the presence of plasma
• Graft plasma polymerisation for producing fabrics with laundry-durable oleophobic, hydrophobic and stain-resistant finishes
• Atmospheric plasma-based graft polymerisation of textiles and nonwovens having different surface functional properties on the face and back side of the fabric
• Flame-retardant coating using monomer vapours (halogen and/or phosphorus) in combination with nitrogen and/or silicone
• Durable antistatic properties using PU-resin and plasma processing
• Shrink resistance of woollen fabrics and preparation of machine-washable wool by plasma processing without resin
• Electro-conductivity of textile yarns by surface plasma deposition.
The advantages of plasma treatment include the following (Chan et al., 1996):
• Modification can be confined to the surface layer (a few hundred angstrom units) without modifying the bulk properties of the polymer.
• Excited species in plasma can modify the surfaces of all polymers, irrespective of their structures and chemical reactivity.
• By the selection of the feed gas to plasma reactor, it is possible to achieve the desired type of chemical modification for the polymer surface.
• The use of plasma can avoid the problems encountered in wet chemical treatments, such as residual chemical in the effluent and swelling of the substrate.
However, the processing parameters are highly system dependent, that is the optimal parameters developed and optimised for one system usually need to be modified for application to another system. The process and its set-up are complicated. It is very difficult to control precisely the amount of a specific functional group formed on the sample surface (Shishoo, 2007).
The effectiveness of plasma treatment depends on different factors namely (Kan and Yuen, 2007):
15.16.1 Nature of gas used
The result of plasma treatment depends strongly on the nature of the gas or the vapour used in glow discharge. Most organic, organosilicone or organometallic vapours tend to form a thin film on the surfaces, which are subjected to glow discharge, and the deposition of these films is the main factor modifying the polymer surface. On the other hand, glow discharges of non-polymerising gases, for example noble gas, nitrogen, oxygen, hydrogen, ammonia or water vapour, modify polymer surfaces through processes, such as oxidation, chemical etching, cross-linking and perhaps grafting.
Helium, neon and argon are the three inert gases commonly used in plasma technology. Because of its relatively lower cost, argon is by far the most common inert gas being used. Inert gas plasma has been used for the pre-treatment of substrates for cleaning purposes before reactive gases are applied.
If a plasma reaction is to be carried out with a high-system pressure but at a low reactive gas flow rate, an inert gas can serve as a diluent.
Oxygen and oxygen-containing plasma are most commonly employed to modify polymer surfaces, (it is well known that the oxygen plasma can react with a wide range of polymers to produce a variety of oxygen functional group, including C–O, C=O, O–C–O and C–O–O at the surface).
Nitrogen-containing plasma is widely used to improve wettability, print-ability, bondability and biocompatibility of polymer surfaces.
15.16.2 System pressure
The system pressure used may affect the energy of the plasma species. If the pressure is high, the probability of collision between plasma species will be increased leading to the loss of energy of the species before interacting with the material.
15.16.3 Discharge power
The intensity of plasma is a combined factor of pressure and discharge power. The breakdown energy necessary to produce plasma varies from one gas to another. Normally, the higher the discharge power applied, the more kinetic energy the plasma species will carry, resulting in strong intensity of plasma action.
15.16.4 Duration of treatment
The duration of treatment plays an important role in the plasma treatment. Generally speaking, the longer the duration of treatment, the more severe the modification of the material surface (e.g. sputtering or etching) will be.
A longer duration will not only affect the material surface but also provide an opportunity for the plasma species to penetrate the interior region of the material. This may alter the morphology of the polymeric material. However, when the treatment duration is too long, this will adversely affect the material and therefore careful control of treatment duration is required.
15.16.5 Ageing of the plasma-treated surface
In general, the concentration of functional groups introduced to a polymer surface by the plasma treatment may change as a function of time depending on the environment and temperature. This is because polymer chains have much greater mobility at the surface than in the bulk, allowing the surface to reorient in response to different environments.
Surface orientation can be accomplished by the diffusion of low-molecular weight oxidised materials into the bulk and the migration of polar function groups away from the surface. Ageing of plasma-treated polymer surfaces can be minimised in a number of ways.
An increase in the crystallinity and orientation of a polymer surface enhances the degree of order and thus reduces mobility of polymer chains, resulting in slower ageing. A highly cross-linked surface also restricts the mobility of polymer chains and helps to reduce the rate of ageing.
15.16.6 Effects of environment
When a polymer is exposed to the oxygen-containing plasma, the surface will change to a high-energy state with increased surface tension, as a result of the formation of polar groups. Various surface studies indicate that when the treated surface is placed in a low-energy medium, such as air or vacuum, the decrease in surface energy is caused by the rotation of the polar groups in the bulk or the migration of low-molecular weight fragments to the surface to reduce the interfacial energy. When a low-energy surface formed by treating a polymer in fluorine-containing plasma is placed in a high-energy medium, such as water, the apolar groups will tend to minimise the interfacial energy by moving away from the surface into the bulk. This phenomenon is usually described as ageing of a treated surface.
15.17 Future trends
The general trend in chemical finishing is towards the use of more sophisticated chemical finishes that are more environmentally friendly and are specifically formulated for ease of application on automated machinery and equipment. Higher quality chemical finishes for the more discerning and demanding requirements of the market will in turn create pressures on manufacturers of chemical finishes to engineer their products to provide right-first-time processing.
Major driving forces will be (Holme, 1993):
• The need for higher quality, higher added value products
• Right-first-time, right-on-time, right-every-time processing
• Increased levels of automation and process control in machinery and equipment
• The increased use of process integration
• Greater emphasis on cost reduction by minimising the use of water, energy and all utilities
• The challenges posed by environmental issues, e.g. minimising discharges of chemicals onto land, into air and particularly into water
Quality reveals itself in daily use and is defined by the user as the situation when the expected and the experienced quality are as close as possible. The important aspects of quality policy in textile finishing plan design are:
• Avoid potential sources of risk
• Built-in freedom from maintenance
All these measures are intended to increase process reliability and reproducibility. Higher quality results in gaining customer trust in the supplier and develops loyalty towards him, mutually benefiting each other (Strohle, 2009).
15.18 References
spa, Biancalani. The ‘Natural Touch’ Quality and sustainability in fabric finishing. Colourage Supplement. 2009; 56(1):33–34.
Chan, C.M., Ko, T.M., Hiraoka, H. Polymer surface modification by plasmas and photons. Surf Sci Rep. 1996; 24(1):1–54.
Clapp, T.G., Godfrey, A.B., Greeson, D., Johnson, R.H., Rich, C., Seastrunk, C., Weaving a quality textile industry. Quality Digest. 2001 October. http://www.quality-digest.com/oct01/html/textile.html
Cooke, T.F. Foam Wet Processing in the Textile Industry. Text. Chem. Col.. 1983; 15(5):74–85.
Duckworth, C. Engineering in textile coloration, Dyers Company pub. Trust, SDC. UK: Bradford; 1983.
Elbadawi, A.M., Pearson, J.S. Foam technology in textile finishing. Textile Progress. 2003; 33(4):1–31.
Fiebig, D., Rezk, A.A. The Effect of Crosslinking Conditions on the Wet-Soiling Behavior of Cotton. Textile Research J. 1973; 43:438.
Foster, S.H., A Simplified Relationship between the Properties of Resin-Finished Cotton Fabric and the Solids Applied. Textile Research J. 1957; 27:129–135, doi: 10.1177/004051755702700206.
Fulmer, T.D. Computer supported controls for finishing ranges, America’s Textiles, August. Vol.. 1983; 12:15.
Ghosh, D.K. Wet processing by foam technology, Man-made Text. India. 1988; 31(11):483.
Goyal, R., Prabhu, C.N. Water repellent finishes. Colourage, February, issue. 2009; 2:88–90.
Grindstaff, T.H., Patterson, H.T., Billica, H.R., Studies of Soiling and Detergency: Part IV: Deposition and Transfer Experiments With Fatty Soils. Textile Research J. 1970; 40:35–42, doi: 10.1177/004051757004000106.
Hayakashi, M., Dyeing Technology on Polyester Micro-Fibre. presentation at Aleppo University on 26th April. 2008. www.arabytex.com/forum/
Heywood, D. Textile finishing, Woodhead. England: Cambridge; 2003.
Holme, I. New Developments in the Chemical Finishing of Textiles. Journal of the Textile Institute. 1993; 84(94):520–533.
Holme, I. Recent Advances in Chemical Processing. Colourage Annual. 41–56, 1998.
Hussain, T., Ahmed, S., Qayum, A. Effect of different softeners and sanforising treatment on pilling performance of polyester/viscose blended fabrics. Coloration Technology. 375–8, 2008.
International Textile Reports. Process control system for textile finishing. Melliand Textilberichte. 1991; 72:144–145.
Ishikawa, K. What is Total Quality Control?. Englewood Cliffs, New Jersey, USA: Prentice Hall; 1985.
Jerg, G., Foam Printing, Ciba Geigy Publication F-C.5. 1979:24.
Kan, C., Yuen, C.M. Plasma technology in wool. Textile Progress. 2007; 39(3):121–187.
Kehry, S., Uhl, H., Improving the efficiency of textile machinery with intelligent data management, 2010.. www.benningergroup.com
Kroezen, A.B.J., Wassink, J.G. Bubble size distribution and energy dissipation in foam mixers. J. Soc. Dyers Col.. 1987; 103(11):386.
Lewin, M., Sello, S.B. Functional finishes, part A, Handbook of fibre science and Technology: vol. II. New York and Basel: Marcel Dekkar; 1983.
Marsh, J.T. An introduction to textile finishing. India: B.I. Publications; 1979.
Merritt, D.S. Energy savings by dryer exhaust control, Am. Dyestuff Rep.. 1982; 71(9):26.
Myers, S.A., Finishing in Wool: Science and Technology. W.S. Simpsion. Woodhead Cambridge, England, 2002:314–332.
Pehl, R. Surface finish of knitted fabrics – faults and options in the event of damage during the raising process. International Textile Bulletin (Dyeing/ Printing/Finishing). 1991; 37(1):27.
Hans-Karl, Rouette. Encyclopedia of Textile Finishing, Springer. Germany: Aachen; 2000.
Roy Choudhury, A.K., Chatterjee, B., Saha, S., Shaw, K. Comparison of Performances of Macro. Micro and Nano Silicone Softeners, DOI: 10.1080/00405000.2012.654666, Journal of the textile institute, Available online: 03. (Feb 2012):2012.
Satees G (2012) www.scribd.com/doc/29869975/Alkaline-Weight-Reduction, accessed on 27 August 2012.
Schindler, W.D., Hauser, P.J. Chemical Finishing of Textiles, Woodhead. England: Cambridge; 2004.
Schreiber, W., Maschen, S. Process automation for continuous wet finishing ranges. Melliand Textilberichte. 1992; 73(10):835–836.
Shah D R, ‘Process control and safety in chemical processing of textiles’, www.sul-phurdyes.com/knowledgewall.html
Shishoo, R. Plasma Technologies for Textiles, Woodhead. England: Cambridge; 2007.
Shaw, D.J., I ntroduction to Colloid and Surface Chemistry. 3rd Edition. Butterworth, London. England, 1980.
Sontag, M.S., Purchase, M.E., Smith, B.F., A double-label radioactive tracer analysis of the retention of selected fats on cotton and its relation to yellowing. Textile Research J.. 1970;40(6):529–536, doi: 10.1177/004051757004000607.
Ströhle, J. Increasing the Process Reliability of Textile Finishing Processes. Supplement to Colourage. 2009; 56(4):28–29.
Tomasino, C. Chemistry & Technology of Fabric Preparation & Finishing. North Carolina, USA: College of Textiles, North Carolina State University; 1992.
Turner, G.R. Foam Technology: What’s It All About? Textile Chemist & Colorist. 1981; 13(2):13–18.
Vigneswaran, C., Anbumani, N. Effects of Weight Reduction Treatment by Partial Alkaline Hydrolysis of Physical Properties of Polyester Rotor Spun Yarns, Institute of Engineers (India) Journal, TX. August issue. 2007; 88:14–18.
Vishnu, A.D., Bhagyashri, K. Antimicrobial finishing of textiles, Man-made Textiles in India. March. 2010; 53(3):89–95.
Weber, R., Tuschen, J. U.K. develops new dyeing for the continuous dyeing of carpets. ChemiefasernTextilindustrie (Eng. Edn). 30, 1980. [82,745.29.].