3.2
Rewiring the Nervous System, Without Wires
D. A. Borton
School of Engineering and the Brown Institute for Brain Science, Brown University, Providence,, RI, 02912, USA
1 Introduction
The human nervous system is arguably the most advanced information-processing network in existence. Multiarea, recurrent networks in our brains enable flexible behavior in a dynamic and uncertain world. Amazingly, small-scale networks of the spinal cord can regulate intelligent responses on a millisecond timescale to a poorly placed foot on a stair, initiating an ever-useful correction loop and avoiding catastrophe. Large-scale networks across vast landscapes of the brain regulate everything from the decision to take a step forward to knowing when to take a step backward.
In the healthy nervous system, the development of intention and motor execution is a dynamic and highly distributed process that originates in the brain. The intended action is transmitted along the axon super highway of the spinal cord and down to smart circuits in the spinal cord that transform the descending command into coordinated patterns of muscle activation. A continuous stream of sensory and motor information is actively coalesced in the brain to ensure the accurate and sequenced execution of movement. The output of the motor cortex and brainstem motor regions is under the continuous influence of other structures of the brain, including the cerebellum and basal ganglia, which are essential for producing smooth movements. These contextual information streams form loops interacting with one another at key integration centers, such as the association cortices and the thalamus. Failures of function in one seemingly insignificant processing loop can, and often does, lead to dramatic consequences that induce transient or permanent deficits in cognitive ability or motor control.
For example, in Parkinson's disease (PD), the premature loss of dopaminergic transmission in the substantia negra disturbs the basal ganglia loops and motor output circuitry of the brain, causing tremor, gait disturbance, rigidity, and deficits in cognitive function. Demyelination – the phenotype of multiple sclerosis (MS) – reduces the conductivity of axons, which alters their ability to convey information to distant circuits. Stroke, while focal in nature, induces complex and far-reaching effects on the central nervous system (CNS). Less subtle, but particularly palpable is the dramatic consequence resulting from severe spinal cord injury (SCI), which, in extreme cases, can make a person completely unable to control or interact with the world around them. Nervous system disorders have long-term health, economic, and social consequences. Despite the best available medical treatments, millions of individuals endure a long life with sensorimotor and cognitive deficits that dramatically affect their quality of life.
The goal of this chapter is to discuss the potential impact that advances in wireless neurotechnology may provide persons with neuromotor disease and insult, but also to explore fundamental basic research avenues enabled by leveraging wireless telemetry in human and nonhuman brain science. Although work in progressing across many labs and in many corners of the world to address the devastating effects of neuromotor disease, here we will focus our discussion on the restoration of ambulatory function after SCI, as it exemplifies how state-of-the-art wireless microelectronic technology is being applied to solve biomedical therapeutic challenges for both reading from and writing into the nervous system. A conceptual schematic of our approach is presented in Fig. 1.

Figure 1 Conceptual illustration of a brain–machine interface technology. Neural data is collected from a variety of neural interfaces, then translated (decoded) into meaningful output control signals through machine learning, and finally used to control a motor prosthetic.
(Source: https://commons.wikimedia.org/wiki/File:Brain-Controlled_Prosthetic_Arm_2.jpg. Public domain; https://commons.wikimedia.org/wiki/File:Prosthetic_Limbs_at_Headley_Court_MOD_45157827.jpg. Photo: Cpl Richard Cave RLC (Phot)/MOD).
2 Why go wireless?
Interestingly, the computational power and multiscale connections between short (cortical) and long-range (spinal cord) neural circuits that produce movement remain poorly understood. Many of our best insights into neural computation derive from studying how single brain areas enable trained animals to solve simple, highly constrained tasks with fully observable sense data. Tethered and constrained experimental paradigms for nonhuman primates, for example, have enabled computational neuroscientists to develop sophisticated data-driven models of cognitive processing, yet have been limited principally to studying brain activity within an isolated context. To understand the dynamic neural processes that mediate fluid, natural human behavior, such as dexterous manipulation of objects with multiple parallel sensory cues, we must study recurrent neural computations spanning many interacting brain regions during complex and naturalistic behaviors. This cannot be accomplished with today's tethered electronic connections. Such connections not only physically limit the areas of implantation due to their size and cabling mechanics but also functionally limit the behavioral repertoire possible to explore. Decoding strategies for brain-controlled interfaces must coalesce dynamic data entering the body along multiple sensory pathways in order to synthesize a stable decision. In order to achieve this, synergistic advances must be made in the key areas of neurotechnology and computational neuroscience so that researchers can interpret neural dynamics at multiple scales in real time during unconstrained movements applicable to human brain–computer communication.
3 One wireless recording solution used to explore primary motor cortex control of locomotion
Considerable investment and decades of intensive research have brought great insight into how movement1–5 and proprioception or sensation6–9 are accomplished in upper limb processing pathways. Such insight has brought about impressive demonstrations of brain interfaces to external effectors replacing upper limb control.10–13 Conversely, lower limb systems remain the poorly understood cousins of their biologically elevated counterparts. Primate locomotion relies on dynamic interactions between cortical, brainstem, and spinal circuits. While the contribution of primary motor cortex to skilled hand movement has been studied extensively, its role during natural locomotor behaviors remains largely unknown.14 Early work performed in cat quadrupedal locomotion was focused on active forelimb control and accommodation after external perturbation.15–19 Only recently have hind limb areas of primary motor cortex recordings been the subject of electrophysiological experiments in rodents,20 with the anatomical area still under some debate.21 Perhaps this limited basic research on locomotor centers in the cortex is due to the prevailing dogma that walking is a highly automated process largely organized within the spinal cord, putting into question whether the primate motor cortex participates meaningfully in locomotor control.
While few attempts have been made to access neuronal modulation from cortical regions of nonhuman primates during locomotion,22 such recordings were performed under highly constrained conditions due to the need for cabled electronics. Recently, a limited data set of spiking activity was collected from premotor cortical areas of a rhesus monkey walking on a treadmill.23 24
In order to move beyond constrained conditions, we designed, built, and deployed a miniaturized wireless high data rate neurosensor platform, shown in Fig. 2. This device leveraged numerous earlier advances from several laboratories25–27 and integrates custom amplification microelectronic circuitry that amplifies and multiplexes 100 channels of broadband neural signals, transmits data digitally at high sampling rates (20 kSps/channel) up to a 5 m distance via a single-input multiple-output (SIMO) wireless link for extended spatial coverage; and operates on a single one-half AA Li-ion battery continuously for more than 48 hours. The neurosensor weighs only 46.1 g and incorporates three ultralow-power, custom-designed ASICs for signal amplification, packaging, and transmission. The assembled components are protected from mechanical and electrical impact by a static-dissipative, carbon-fiber-reinforced poly-ether-ether-ketone enclosure.28

Figure 2 A silicon-based microelectrode array (MEA) was implanted into primary motor cortex of the nonhuman primate brain. A transcutaneous pedestal connection exits the skin for sampling of the signal. Traditionally, a cable is connected to this pedestal. Here, we attach a wireless neurosensor capable of transmitting the high-bandwidth data meters, untethering the subject from large recording electronics.
(Source: Reproduced with permission of Blackrock Microsystems).
This wireless technology enabled acquisition of full spectrum neuronal population dynamics in untethered freely moving monkeys. In an experiment to more fully characterize hind limb area of primary motor cortex, we implanted a 96-electrode microelectrode array (MEA) in the hind limb area of the primary motor cortex along with bipolar electrodes into a pair of agonist and antagonist muscles for each joint of the contralateral leg in order to record electromyography (EMG) activity (a total of eight muscles were acquired wirelessly). The EMG activity and neural data were collected in conjunction with whole-body kinematics while the monkeys were walking quadrupedal, without behavioral constraints, on a treadmill across a range of velocities – see Fig. 3. All animals displayed robust and reproducible modulation of motor cortex spiking activity that was distributed across the entire gait cycle. The temporal structure of neuronal ensemble modulation coevolved with speed-dependent changes in joint angles and leg muscle activity patterns.28 We found that the leg area in primary motor cortex contains information that can be leveraged to build a locomotor prosthetic. Cross-task neural and kinematic data were recorded from five nonhuman primates, underscoring the robustness of such a platform prosthetic in a diverse human population. What is likely, given the results of that study, is that there is at least a shared control by the motor cortex in the production of locomotion, enough to inspire us to propose a platform to bypass a spinal lesion and enable active, dynamic modulation of gait in closed-loop, that is, gait modulation controlled by the subject's intention: a brain–spinal interface.29
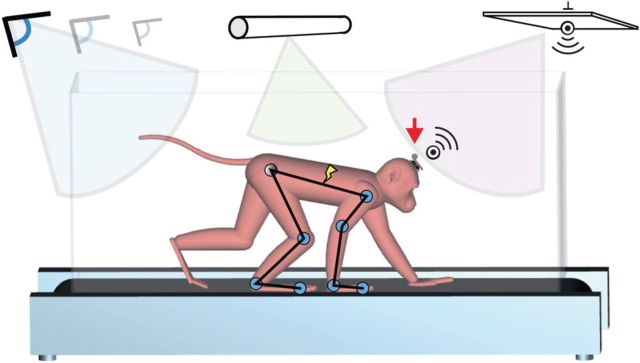
Figure 3 A wireless neuromotor recording and stimulation platform: neural data is recorded wirelessly and transmitted meters away to a control center, where decoding and interpretation drive outgoing commands for spinal stimulation. In parallel, muscle activity and kinematic movements are recorded for basic research.
But how should we modulate gait?
4 Writing into the nervous system with epidural electrical stimulation of spinal circuits effectively modulates gait
Thousands of years ago (and until the 20th century in some countries) humans used the electric eels to treat pain – one of the first documented uses of electricity to deliver therapy. Since then, advances in material science, charge storage, and control of charge delivery have enabled more precise perturbations of the nervous system. The challenge for the next generation of neuroprosthetic treatments is to capitalize on the myriad technologies and methods at our disposal to initiate a productive conversation with the nervous system wherein devices not only deliver therapy but also continuously titrate themselves based on neural states and detected motor impairments.
Let us first take a step back and explore the historical interpretations of spinal dynamics. The spinal sensorimotor infrastructure has traditionally been viewed as an assembly of reflex subsystems and central pattern generators that produce automated and stereotypical motor activity in response to sensory input or descending command.30 The high degree of automaticity embedded in spinal circuits enables the execution of complex motor behaviors with considerable precision without conscious thought. The spinal brain acts as a smart information-processing interface that integrates dynamic input from sensory ensembles and, on this basis, makes decision on how to continuously adjust motor output in order to meet environmental constraints while maintaining stability. Despite these advanced properties, the markedly depressed state of the spinal cord postinjury prevents the production of standing and walking. Consequently, much effort has been invested in developing paradigms to replace the missing sources of neuromodulation and excitation that are normally delivered to spinal sensorimotor circuits from higher cortical areas to coordinating movement. Electrical stimulation has been the primary strategy used to compensate for the interrupted source of spinal excitation after injury – see Fig. 4. Continuous electrical stimulations applied to the dorsal roots,31 over the dorsal aspect of the spinal cord,32 or directly into the ventral horn of lumbar segments, have showed the ability to elicit standing and stepping patterns in animal models and humans with SCI,33 PD,34 and MS.35 In combination with monoamine agonists, epidural electrical stimulation (EES) of lumbosacral circuits has been able to restore full weight-bearing locomotion in rats with complete SCI.36 This electrochemical neuroprosthesis replaces the missing source of neuromodulation and excitation after the interruption of descending pathways, although the exact mechanisms remain unclear.

Figure 4 Biophysical models of spinal anatomy enable accurate estimation of electrical stimulation effects on spinal roots and motor reflexes.
Neurotechnology and stimulation protocols are at the early stages of development. Empirical knowledge and visual observations have guided electrode positioning, as well as the selection of electrode configurations and stimulation parameters. Extensive mappings revealed that various locations and stimulation profiles are necessary to facilitate standing, stepping, and isolated movements. This manual tuning is inherently impractical and suboptimal. These experiments emphasize the need to establish a mechanistic framework to personalize multisite stimulation algorithms, and develop closed-loop control systems that take full advantage of this paradigm to facilitate movement in motor-impaired subjects.
Integration of lower limb sensorimotor information into generalized control after limb loss or disease stands to drastically improve postinjury success in everyday tasks, for example, walking, control of balance, removal of phantom limb sensation, and “simple” control of the sit to stand transition. Rapid reintegration of stretch, pressure, and texture information into the ongoing proprioceptive dynamics will enable limb replacements that truly integrate with the host. Recent technological advancements have made read and write access to the nervous system possible in untethered and freely moving subjects,28 37 leading us to envision a novel, chronic open communication window for sensorimotor prosthesis to restore proprioception from and control of lower limb prostheses.29
Often, in order to gain access to the spinal-specific and cortical-specific mechanisms controlling motor function, lesion studies are conducted to remove the contribution of a specific pathway to functional recovery, for example, pyramidal tract lesions, hemisectioning of the spinal cord, and induced stroke in the motor cortex. Experiments conducted in nonhuman primates have significantly contributed to identifying primate-specific mechanisms of recovery after partial SCI. For example, it was shown38 that the recovery of fine manual skills and locomotion after a cervical hemisection in rhesus monkeys is associated with the extensive sprouting of spinal cord midline crossing corticospinal fibers, which are abundant in primates39 but rare in rodents.40 Along the same lines, Isa and coworkers41 showed that recovery of finger dexterity after a partial cervical SCI in monkeys relies on extensive regions of the contralesional primary motor cortex and bilateral premotor cortex, which have no equivalent in rodents.
However, current nonhuman primate injury-based models for studying the dysfunctional locomotor system limit our ability to observe, quantify, and enhance recovery of function in a few critical ways: (i) after a surgical or impact injury, the kinematic and nervous system state is constantly in flux and competing with inflammatory and compensatory responses; (ii) intrasubject variability cannot be assessed as the lesion only occurs once; (iii) as recovery invariably occurs, an iterative development process cannot be used, hampering the development of restorative interfaces; and (iv) physical lesions have systemic effects, complicating care and experimental progress.42 These experiments also highlight the limitations of current methodologies of studying recovery in within the spinal system as well as the dynamics of reorganization in the forebrain, and fail to provide a stable platform for the development of restorative neurotechnology.
5 Genetic technology brings a better model to neuroscience
If we imagine a reversible, noninjuring lesion model of the nervous system, many of the physical injury model limitations described above disappear. This problem could be solved if it were possible to block the corticospinal or reticulospinal pathways specifically and temporarily. Such a method would abolish inflammatory complications, its reversibility will allow for multiple trials and developmental iterations within the same subject, and specific targeting will greatly reduce the systemic effects of current lesion models. The last decade has produced a toolbox of methods for genetic dissection of the nervous system.43–45 The combination of both retrograde and anterograde viruses (e.g., double-virus) enables unprecedented neural pathway selectivity and specificity. Control of gene expression in mammalian cells by tetracycline-locked promoters can enable lasting inhibition of many days.46 47 Isa and coworkers of the National Institute for Physiological Sciences in Osaka, Japan used these viral-based methods to show temporary shutdown of the propriospinal (PN) connections in the ventral horn between C6 and T1. Sensitivity of the expressed protein to the antibiotic doxycycline48 enabled the elaboration of the contribution of PN neurons toward hand dexterity. In order to specifically remove the contributions of supraspinal structures to locomotor pattern generation, we use these methods to block the linkage among cortical, midbrain, and spinal targets. We believe this will yield a significant contribution to both basic science methodologies and lay the foundation for the demonstration of a motor restorative interface to gain fundamental knowledge about the nervous system and to use that knowledge to reduce the burden of neurological disease.
6 The wireless bridge for closed-loop control and rehabilitation
Many years of prior research within the scientific community has culminated in the proof-of-concept demonstration that neural information from the motor cortex could be used to drive stimulation signals to the spinal cord. However, there currently exists no dynamically adapting electrical neuromodulation therapy to alleviate or modulate gait function. While recent studies by our group have shown that sufficient data exists to control such modulation, the integration of real-time, closed-loop technologies into a brain–spinal interface platform has not previously been accomplished and is nontrivial. Critically, in order to drive stimulation pulses in meaningful spatiotemporal patterns, we must use a wireless implantable stimulator. Fortunately, we found such a solution in industry – a modified commercial implantable pulse generator (or IPG).
These devices were wirelessly connected with a common control state-machine running with complete system latencies near 100 ms from neural and kinematic recording, to decoding of intention, and finally modification of spinal stimulation parameters sufficient for therapeutic effect. The resulting innovation, shown in Fig. 5, is a platform that will soon be evaluated for clinical translation.

Figure 5 A brain–spinal interface timing diagram illustrating key changes in stimulation profile based on neural activity anticipating gait modification. Neural recordings enabled adjustment for stimulation command transmission delay.
The brain–spinal interface platform will also be a resource for the neuroscientific community. Such a system enables untethered perturbation of the nervous system, with simultaneous high fidelity and high-resolution reporting of neural and kinematic correlates. Short-term, single trial use of the BSI technology may temporarily improve gait, but it is unclear what lasting impact closed-loop spinal neuromodulation will have on recovery and compensation of function.
Furthermore, continuous use may expose functionalities previously unexpected. Given the long battery life and untethered nature of the platform, researchers will study subjects using the brain–spinal interface while in their home environment. In addition, neuroscientists will have the ability to record neural data continuously, over extended periods of time from days to weeks. Such recordings enable the evaluation of “prosthetic integration” or how the subject uses the brain–spinal interface beyond primary objective to restore gait. “Prosthetic learning”49–52 may be revealed as long-term experiments are performed. Through advances in neurotechnology, computational neuroscience, and closed-loop interactions between electrophysiological recording and stimulation systems, we hope to advance in a nonincremental way our ability to understand continuous and dynamic changes of neuromotor circuits with unprecedented temporal and spatial breadth. Such advancements will have vast implications on biobehavioral research, neuromotor disease diagnosis, and neurorestorative therapies.
7 Conclusion
This chapter discusses a platform of technologies that push neuroscience into the wireless age with the hope of opening new experimental and therapeutic pathways. As we progress toward therapeutic approaches, we are mindful of the patient population. For example, there have been over 1573 major limb amputations to-date in military operations in Afghanistan and Iraq. In addition, in 2013 alone there were nearly 27,000 veterans with spinal injuries and related disorders cared for by Veterans Affairs. These veterans stand to benefit directly from the research discussed. In addition, millions of people worldwide live with SCI. There currently exists no clinical electrical neuromodulation therapy to alleviate or modulate gait function of an individual after amputation or SCI. Functional electrical stimulation (FES) has been the most productive tool to temporarily restore stance to paralyzed individuals,53–55 although peripheral nerve stimulation is being studied.56 57 To achieve transformational change for these patients, prosthetic technologies must advance beyond tonic stimulation of muscles and into biophysical, model-based multisegment stimulation modulated across time and space. Temporal modulation will integrate more effectively into the ongoing dynamics of remaining lumbar spinal circuits;58 spatial modulation will enable activation of multiple spinal segments, based on computational models of anatomical activation59 through EES technology. Engagement and, as needed, direct control by the brain of therapeutic stimulation will enable neural plasticity, important for rehabilitation over long periods, and more natural control limb kinematics. Further, by combining EES with cortical stimulation of the sensory areas (in conjunction to recording from the motor area), one envisions a true internally closed-loop sensorimotor prosthetic system.
Acknowledgments
This work was supported in part by the International Foundation for Research in Paraplegia (IRP Grant P152), the DARPA Young Faculty Award (YFA, Grant D15AP00112), and Brown University.
References
- 1. J. M. Carmena, M. A. Lebedev, R. E. Crist, et al., “Learning to control a brain–machine interface for reaching and grasping by primates,” PLoS Biol. 1, E42 (2003).
- 2. M. Velliste, S. Perel, M. C. Spalding, A. S. Whitford, and A. B. Schwartz, “Cortical control of a prosthetic arm for self-feeding,” Nature 453, 1098–1101 (2008).
- 3. C. T. Moritz, S. I. Perlmutter, and E. E. Fetz, “Direct control of paralysed muscles by cortical neurons,” Nature 456, 639–642 (2008).
- 4. C. Ethier, E. R. Oby, M. J. Bauman, and L. E. Miller, “Restoration of grasp following paralysis through brain-controlled stimulation of muscles,” Nature 485, 368–371 (2012).
- 5. M. D. Serruya, N. G. Hatsopoulos, L. Paninski, M. R. Fellows, and J. P. Donoghue, “Instant neural control of a movement signal,” Nature 416, 141–142 (2002).
- 6. M. Bergenheim, E. Ribot-Ciscar, and J. P. Roll, “Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements,” Exp. Brain Res. 134, 301–310 (2000).
- 7. S. N. Baker, M. Chiu, and E. E. Fetz, “Afferent encoding of central oscillations in the monkey arm,” J. Neurophysiol. 95, 3904–3910 (2006).
- 8. G. A. Tabot, J. F. Dammann, J. A. Berg, et al., “Restoring the sense of touch with a prosthetic hand through a brain interface,” Proc. Nat. Acad. Sci. 110, 18279–18284 (2013).
- 9. M. C. Dadarlat, J. E. O'Doherty, and P. N. Sabes, “A learning-based approach to artificial sensory feedback leads to optimal integration,” Nature Neurosci. 18, 138–144 (2015).
- 10. G. R. Muller-Putz, R. Scherer, G. Pfurtscheller, and R. Rupp, “EEG-based neuroprosthesis control: A step towards clinical practice,” Neurosci. Lett. 382, 169–174 (2005).
- 11. L. R. Hochberg, D. Bacher, B. Jarosiewicz, et al., “Reach and grasp by people with tetraplegia using a neurally controlled robotic arm,” Nature 485, 372–395 (2012).
- 12. J. L. Collinger, M. A. Kryger, R. Barbara, et al., “Collaborative approach in the development of high-performance brain–computer interfaces for a neuro-prosthetic arm: Translation from animal models to human control,” Clin. Transl. Sci. 7, 52–59 (2014).
- 13. V. Gilja, C. Pandarinath, C. H. Blabe, et al., “Clinical translation of a high-performance neural prosthesis,” Nature Med. 21, 1142–1145 (2015).
- 14. R. N. Lemon, “Descending pathways in motor control,” Ann. Rev. Neurosci. 31, 195–218 (2008).
- 15. T. Drew, “Motor cortical cell discharge during voluntary gait modification,” Brain Res. 457, 181–187 (1988).
- 16. T. Drew, W. Jiang, and W. Widajewicz, “Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat,” Brain Res. Rev. 40, 178–191 (2002).
- 17. D. M. Armstrong and T. Drew, “Discharges of pyramidal tract and other motor cortical neurons during locomotion in the cat,” J. Physiol. 346, 471–495 (1984).
- 18. I. N. Beloozerova and M. G. Sirota, “The role of motor cortex in the control of accuracy of locomotor movements in the cat,” J. Physiol. 461, 1–25 (1993).
- 19. I. N. Beloozerova, B. J. Farrell, M. G. Sirota, and B. I. Prilutsky, “Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and nonaccurate stepping,” J. Neurophysiol. 103, 2285–2300 (2010).
- 20. W. Song, and S. F. Giszter, “Adaptation to a cortex-controlled robot attached at the pelvis and engaged during locomotion in rats,” J. Neurosci. 31, 3110–3128 (2011).
- 21. S. B. Frost, M. Iliakova, C. Dunham, et al., “Reliability in the location of hindlimb motor representations in Fischer-344 rats: Laboratory investigation,” J. Neurosurg. 19, 248–255 (2013).
- 22. N. A. Fitzsimmons, M. A. Lebedev, I. D. Peikon, and M. A. Nicolelis, “Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity,” Front. Integr. Neurosci. 3, article no. 3 (2009).
- 23. J. D. Foster, P. Nuyujukian, O. Freifeld, et al., “A freely-moving monkey treadmill model,” J. Neural Eng. 11, 046020 (2014).
- 24. D. A. Schwarz, M. A. Lebedev, T. L. Hanson, et al., “Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys,” Nature Methods 11, 670–676 (2014).
- 25. W. R. Patterson, Y. K. Song, C. W. Bull, et al., “A microelectrode/microelectronic hybrid device for brain implantable neuroprosthesis applications,” IEEE Trans. Biomed. Eng. 51, 1845–1853 (2004).
- 26. Y.-K. Song, W. R. Patterson, C. W. Bull, et al., “A brain implantable microsystem with hybrid RF/IR telemetry for advanced neuroengineering applications,” IEEE Eng. Med. Biol. Soc. 2007, 445–448 (2007).
- 27. Y.-K. Song, D. A. Borton, S. Park, et al., “Active microelectronic neurosensor arrays for implantable brain communication interfaces,” IEEE Trans. Neural Syst. Rehabil. Eng. 17, 339–345 (2009).
- 28. M. Yin, D. A. Borton, J. Komar, et al., “Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior,” Neuron 84, 1170–1182 (2014).
- 29. D. A. Borton, M. Bonizzato, J. Beauparlant, et al., “Corticospinal neuro-prostheses to restore locomotion after spinal cord injury,” Neurosci. Res. 78, 21–29 (2014).
- 30. S. Grillner, “Biological pattern generation: the cellular and computational logic of networks in motion,” Neuron 52, 751–766 (2006).
- 31. D. Barthelemy, H. Leblond, and S. Rossignol, “Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats,” J. Neurophysiol. 97, 1986–2000 (2007).
- 32. K. Minassian, B. Jilge, F. Rattay, et al., “Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials,” Spinal Cord 42, 401–416 (2004).
- 33. V. R. Edgerton, and S. Harkema, “Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges,” Expert Rev. Neurother. 11, 1351–1353 (2011).
- 34. M. B. Santana, P. Halje, H. Simplicio, et al., “Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease,” Neuron 84, 716–722 (2014).
- 35. L. S. Illis, A. E. Oygar, E. M. Sedgwick, and M. A. Awadalla, “Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis,” Lancet 307, 1383–1386 (1976).
- 36. R. van den Brand, J. Heutschi, Q. Barraud, et al., “Restoring voluntary control of locomotion after paralyzing spinal cord injury,” Science 336, 1182–1185 (2012).
- 37. D. A. Borton, M. Yin, J. Aceros, and A. Nurmikko, “An implantable wireless neural interface for recording cortical circuit dynamics in moving primates,” J. Neural Eng. 10, 026010 (2013).
- 38. E. S. Rosenzweig, G. Courtine, D. L. Jindrich, et al., “Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury,” Nature Neurosci. 13, 1505–1512 (2010).
- 39. E. S. Rosenzweig, J. H. Brock, M. D. Culbertson, et al., “Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys,” J. Comp. Neurol. 513, 151–163 (2009).
- 40. C. Brosamle and M. E. Schwab, “Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract,” J. Comp. Neurol. 386, 293–303 (1997).
- 41. Y. Nishimura and T. Isa, “Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys,” Exp. Neurol. 235, 152–161 (2012).
- 42. L. H. Sekhon and M. G. Fehlings, “Epidemiology, demographics, and pathophysiology of acute spinal cord injury,” Spine 26, S2–S12 (2001).
- 43. G. Nagel, T. Szellas, W. Huhn, et al., “Channelrhodopsin-2, a directly light-gated cation-selective membrane channel,” Proc. Nat. Acad. Sci. 100, 13940–13945 (2003).
- 44. F. Zhang, L.-P. Wang, E. S. Boyden, and K. Deisseroth, “Channelrhodopsin-2 and optical control of excitable cells,” Nature Methods 3, 785–792 (2006).
- 45. I. Diester, M. T. Kaufman, M. Mogri, et al., “An optogenetic toolbox designed for primates,” Nature Neurosci. 14, 387–397 (2011).
- 46. M. Gossen and H. Bujard, “Tight control of gene expression in mammalian cells by tetracycline-responsive promoters,” Proc. Nat. Acad. Sci. 89, 5547–5551 (1992).
- 47. M. Yamamoto, N. Wada, Y. Kitabatake, et al., “Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain,” J. Neurosci. 23, 6759–6767 (2003).
- 48. M. Kinoshita, R. Matsui, S. Kato, et al., “Genetic dissection of the circuit for hand dexterity in primates,” Nature 487, 235–238 (2012).
- 49. J. M. Carmena, “Advances in neuroprosthetic learning and control,” PLoS Biol. 11, e1001561 (2013).
- 50. S. Dangi, A. L. Orsborn, H. G. Moorman, and J. M. Carmena, “Design and analysis of closed-loop decoder adaptation algorithms for brain–machine interfaces,” Neural Comput. 25, 1693–1731 (2013).
- 51. K. Ganguly, and J. M. Carmena, “Emergence of a stable cortical map for neuroprosthetic control,” PLoS Biol. 7, e1000153 (2009).
- 52. K. V. Shenoy and J. M. Carmena, “Combining decoder design and neural adaptation in brain–machine interfaces,” Neuron 84, 665–680 (2014).
- 53. V. K. Mushahwar, P. L. Jacobs, R. A. Normann, R. J. Triolo, and N. Kleitman, “New functional electrical stimulation approaches to standing and walking,” J. Neural Eng. 4, S181–S197 (2007).
- 54. R. J. Triolo, S. N. Bailey, M. E. Miller, L. M. Lombardo, and M. L. Audu, “Effects of stimulating hip and trunk muscles on seated stability, posture, and reach after spinal cord injury,” Arch. Phys. Med. Rehabil. 94, 1766–1775 (2013).
- 55. M. L. Audu, L. M. Lombardo, J. R. Schnellenberger, et al., “A neuroprosthesis for control of seated balance after spinal cord injury,” J. Neuroeng. Rehabil. 12, article no. 8 (2015).
- 56. L. E. Fisher, D. J. Tyler, J. S. Anderson, and R. J. Triolo, “Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve,” J. Neural Eng. 6, 046010 (2009).
- 57. K. H. Polasek, H. A. Hoyen, M. W. Keith, R. F. Kirsch, and D. J. Tyler, “Stimulation stability and selectivity of chronically implanted multicontact nerve cuff electrodes in the human upper extremity,” IEEE Trans. Neural Syst. Rehabil. Eng. 17, 428–437 (2009).
- 58. N. Wenger, E. M. Moraud, S. Raspopovic, et al., “Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury,” Sci. Transl. Med. 6, 255ra133 (2014).
- 59. M. Capogrosso, N. Wenger, S. Raspopovic, et al., “A computational model for epidural electrical stimulation of spinal sensorimotor circuits,” J. Neurosci. 33, 19326–19340 (2013).
