Chapter 12
Aqueous Two-Phase Systems for Micropatterning of Cells and Biomolecules
Stephanie L. Ham and Hossein Tavana
The University of Akron, Department of Biomedical Engineering, Akron 44325, OH USA
12.1 Introduction
Studying cells enables a better understanding of the human physiology and exploring a multitude of diseases to develop diagnostics and therapeutics methods and tools. Microengineering approaches facilitate cellular studies through the development of novel assays to mimic the cellular environments in various contexts. To address the need for microengineering in such studies, microfluidic technologies were developed to model various physiologic and pathologic cellular processes, enable drug testing, perform bioanalyses, and allow sorting and separation of cells [1–8]. The possibility of compartmentalizing different cells in microfluidic devices enabled recapitulating tissue and organ level functions not feasible before [9, 10]. Despite the power and capabilities of microfluidic systems, the need for microfabrication of microfluidic chips that often utilizes cleanroom facilities and specialized equipment is a major drawback for users and laboratories unfamiliar with these methods or without access to them. Additionally, the operation of microfluidic devices, especially for long-term experiments, is difficult even for trained users. Therefore, techniques that allow micropatterning capabilities of microfluidic systems without a need for device fabrication would greatly benefit the research community. Aqueous two-phase systems (ATPS) provide a unique tool for channel-free cell and reagent micropatterning and eradicate the complications of microfluidic systems.
An ATPS results from the separation of aqueous solutions of two structurally different polymers at specific concentrations. The ATPS forms when separation of unlike polymer molecules is more energetically favorable than interactions between them [11]. Several factors influence the phase separation of two aqueous polymer solutions including molecular weight (MW) of polymers, temperature of polymer solutions, and the composition (pH and ionic content) of the separation media [12]. Phase diagrams are frequently utilized to characterize the ATPS and determine concentrations that render immiscible polymer solutions (Figure 12.1). The curved line in the phase diagram is known as the binodal curve that divides the graph into two regions: above the binodal curve, any pair of concentrations of the two polymers results in a two-phase system, whereas compositions below the binodal curve give a one-phase system. When an ATPS develops, a top phase enriched with one polymer and a bottom phase enriched with the second polymer result [14]. Each point above the binodal curve (e.g., initial ATPS composition in Figure 12.1) is associated with compositions of top and bottom phases on the binodal curve determined by a unique tie line (dashed line in Figure 12.1) [15]. When mixed with an ATPS, biomolecules (such as cells, proteins, and nucleic acids) can preferentially partition to one of the two aqueous phases, distribute between them, or collect at their interface. Partition of biomolecules depends on different factors including their surface charges, MWs of phase-forming polymers, pH and temperature of the separation medium, and the interfacial tension between the aqueous polymer phases. The potential of ATPS for separation and fractionation of biomolecules was first discovered in 1956 by Albertsson [16] and have since been utilized in a wide variety of applications including industrial scale protein purification [17, 18].
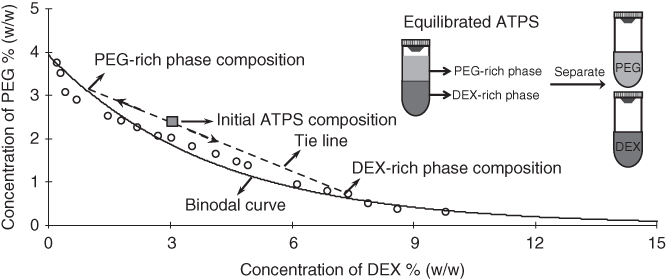
Figure 12.1 A phase diagram for ATPS made with PEG (MW: 35 kDa) and DEX (MW: 500 kDa) polymers. The solid curved line is the binodal curve. Only those combinations of PEG and DEX above the binodal curve will form a two-phase system. The dashed line represents a tie line that provides the compositions of each segregated polymer phase of the ATPS formed from concentrations of PEG and DEX displayed as the initial ATPS composition point. The schematic illustrates the separation of PEG and DEX phases from an ATPS to be used for micropatterning applications.
(Reprinted from Ref. [13].)
While a variety of polymer pairs dissolved in aqueous solutions facilitate ATPS formation, polyethylene glycol (PEG) and dextran (DEX) are the most frequently used [19]. The PEG-DEX ATPS results in a PEG-rich top phase and a denser DEX-rich bottom phase. This ATPS has many unique properties that make it beneficial for biomolecular partitioning studies. The PEG-DEX ATPS is inexpensive and allows phase separation with low polymer concentrations to maintain the separation medium highly aqueous and in a wide range of temperatures [12]. This chapter provides a summary of applications utilizing the PEG-DEX ATPS as a convenient and inexpensive molecular and cellular micropatterning tool.
12.2 Small Molecules Applications
12.2.1 Bioreagent Patterning
Conventional lithography-based biopatterning techniques require physical contact of an elastomer stamp with a dry substrate to transfer adhesive molecules and subsequently overlaying them with cells. These techniques are limited with direct patterning onto cells. Micropatterning with ATPS eradicated this limitation by providing a convenient, inexpensive, contact-free, and straight forward method to selectively deliver reagents to cells in culture [20]. This capability was the first demonstrated in 2009 by direct micropatterning of nucleic acids and viruses on live cells. Bioreagents of interest were mixed with the aqueous DEX phase solution and then patterned dropwise on a monolayer of cells immersed in the aqueous PEG phase solution using pipetting tools. The bioreagents remained partitioned to the nanoliter-volume DEX phase drops, resulting in specific and localized delivery of reagents to cells residing under patterned DEX phase drops (Figure 12.2a–c). This approach allowed patterning of different shapes as well as in standard high throughput array formats. The shape of patterns remained stable during incubation due to an ultralow interfacial tension between the two aqueous polymer phase solutions and interactions of their interface with the cell surface.
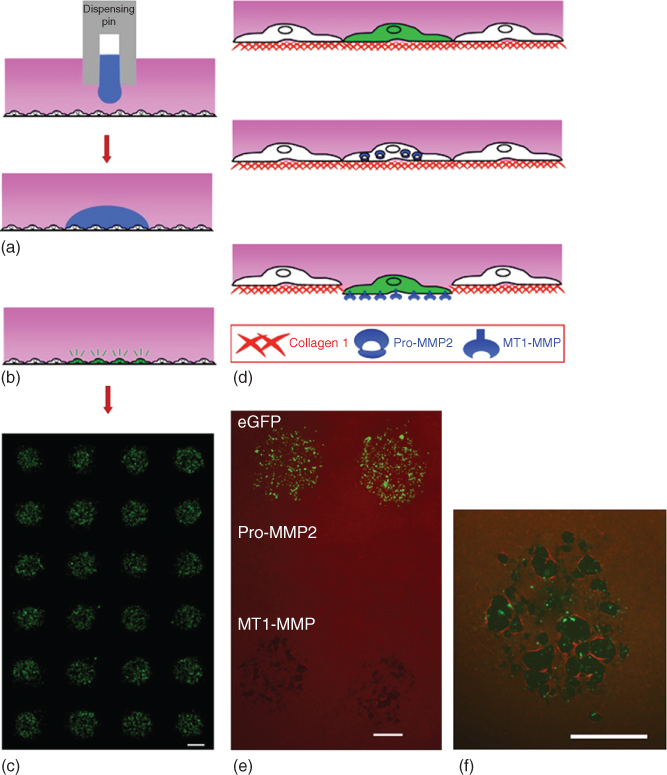
Figure 12.2 (a) Bioreagent delivery to cells in culture. Cells were immersed in the PEG phase (pink). Bioreagents in the DEX phase were delivered to the cells by slow dispensing of DEX phase drops (blue). Distinct droplets containing the bioreagent over the cells resulted. (b) Only the cells underneath the DEX phase droplet were exposed to the bioreagent. (c) ATPS mediated fluorescence microarray of eGFP-expressing cell clusters. (d,e) Location-specific collagen I degradation only by MT1-MMP expressing cells and not pro-MMP2 (matrix metalloproteinase-2, type IV collagenase) or control (eGFP) expressing cells. MT1-MMP expressing HEK 293H cells degraded type I collagen (red) whereas pro-MMP2 and eGFP expressing cells lacked this activity. (f) Degradation of collagen matrix (red) by MT1-MMP protein (green) expressing cells through antibody staining. Scale bars are 700 µm.
(Tavana et al. [20]. Copyright 2009. Reproduced with permission of Nature Publishing Group.)
This bioreagent patterning technique could conveniently be adapted to commercially available dispensing systems to perform high throughput dispensing of DEX phase droplets. This method was demonstrated useful for localized delivery of bioreagents to cells for liposomal-mediated gene delivery and expression analysis and viral infection of defined subpopulations of cells for gene expression and knockdown studies. This contact-free patterning method was uniquely suited to investigate interactions of cells and their underlying extracellular matrix (ECM) substrate. To demonstrate this capability, cells cultured on a type I collagen matrix were micropatterned with lentiviral vectors encoding for membrane type 1-matrix metalloproteinase (MT1-MMP) to enable localized activation of the MT1-MMP enzyme in cells. Immunofluorescence confirmed patterned degradation of collagen I fibrils only by the MT1-MMP expressing cells (Figure 12.2d–f). Considering that matrix degradation is a key process metastatic cancer cells use to infiltrate tissue stroma [21], this contact-free gene delivery approach provides unique opportunities to unravel the roles of various MMPs in cancer metastasis. Biopatterning with ATPS additionally allowed localized delivery of a combination of bioreagents within the patterning DEX phase solution for co-expression studies. In addition to its direct patterning capabilities, this nanoliter-scale delivery system used only small amounts of expensive biological reagents and reduced producing of toxic waste (e.g., viral vectors solutions).
12.2.2 Antibody Assays
Antibody assays detect the amount of specific antigens expressed in cells leading to informative protein expression data. Standard immunostaining methods to explore protein expression in cells require a large volume of expensive antibodies. Multiple primary antibodies are used for multiplexed immunostaining and often produce high background signal from primary antibody cross-reactions. Few methods exist to improve the efficiency of multiplex immunostaining but have their own drawbacks such as the need for specific antibody combinations (imaging and probing methods), difficulty to integrate into laboratory or clinical settings (microfluidic methods), and incompatibility with imaging of subcellular protein localization (antigen transfer methods). Multiplexed immunostaining was significantly improved through the use of an ATPS in standard immunostaining protocols [22]. Using a PEG-DEX ATPS, primary antibodies were diluted within the DEX phase solution and then micropatterned on fixed cell samples immersed in the PEG phase solution containing 0.1% BSA (blocking solution). Once the primary antibodies were patterned on samples with the ATPS approach and following an overnight incubation at 4 °C, the ATPS was replaced with a solution of secondary antibodies. This localized immunostaining significantly reduced antibody cross-reactions since primary antibody solutions were retained in the DEX phase drops, allowed multiple antigens to be analyzed on one sample with detection in a single imaging channel, and facilitated micropatterned detection of proteins on a sample cell surface. ATPS-mediated patterning of primary antibodies allowed quick determination of optimal primary antibody concentrations and resulted in cost-saving due to reduced amount of expensive immunostaining reagents. This antibody micropatterning technique allowed protein detection on different types of samples (cell cultures, explants, and tissue sections) and using standard modes of detection such as chemiluminescent and immunofluorescence.
ATPS have been utilized similarly with detection antibody patterning [23]. Cross-reactivity is a major drawback to multiplexed enzyme-linked immunosorbent assays (ELISAs) and a cause of false positives or negatives in antigen testing of samples. Micropatterning with the PEG-DEX ATPS resolved this issue by localizing detection antibodies to capture antibodies immobilized on a surface (Figure 12.3a). The ATPS eliminated diffusion and dispersion of the detection antibodies, which could cause non-specific antigen detection, due to their favorable partition to the aqueous DEX phase. The DEX phase drops created a dome over corresponding antibody spots, allowing specific detection with no cross-reactivity. This was exemplified with the lack of cross-reaction of goat detection antibodies with a neighboring spot coated with an anti-goat capture antibody (Figure 12.3c). However, using the standard ELISA technique (Figure 12.3b), cross-reaction occurred at the coated neighboring spot (Figure 12.3d). The ATPS-ELISA technique was validated through multiplexed detection of four different graft-versus-host-disease (GVHD) antigen markers used for diagnosis and prognosis purposes. In addition to eradicating cross-reactions in traditional multiplex ELISA, this method required less antibody and patient-derived samples.

Figure 12.3 Similar procedures are performed for ATPS-ELISA detection antibody patterning (a) and conventional bath applied detection antibody (b). The use of a PEG-DEX ATPS for containment of detection antibodies (dashed line antibody symbols) in specific locations of capture antibodies (solid line antibody symbols) resulted in elimination of cross reactivity of antibodies. (c) When using ATPS-ELISA methods, neighboring anti-goat coated spots were not detected by goat detection antibodies due to the containment of antibodies in the DEX phase droplet. (d) However, with conventional methods, cross reactivity occurred in which the detection antibodies detected the neighboring anti-goat coated spot.
(Reprinted from Ref. [23]. No changes made. Image included in article's Creative Commons license (http://creativecommons.org/licenses/by/3.0/).)
Comparable to the enhanced multiplex ELISA application [23], a PEG-DEX ATPS system also showed advantages in amplified luminescent proximity homogenous assays (AlphaLISAs) [24]. An ATPS allowed AlphaLISA multiplexing with increased sensitivity, elimination of washing steps, reduction of reagents and costs, and prevention of antibody non-specificity. Antibody-bead reagents were mixed with a DEX phase solution dissolved in AlphaLISA assay buffer. Samples of human plasma or cells supernatant were mixed with a PEG phase solution (dissolved in 25 mM HEPEs buffer with 0.1 wt% casein) and preferentially partitioned to DEX phase droplets that were subsequently dispensed into the PEG-antigen solution. Proteins diffused from the PEG phase solution into the DEX phase solution due to their high affinity to the DEX phase. These separation effects resulted in containment of the proteins and antibody reagents in the DEX phase drops, allowing target proteins to be matched with high affinity and specificity to corresponding antibody-bead pairs. For detection, photo-sensitizing donor beads were mixed with a new DEX phase solution that was added to wells dropwise to merge with the antigen- and antibody-containing DEX phase drops. This approach allowed simultaneous detection of four different chemokine and cytokine biomarkers associated with GVHD.
12.2.3 Collagen Microgels
Another unique application of ATPS micropatterning is the formation of collagen microgels to study collagen contraction by cells in various physiological processes [25]. A number of cell types (fibroblast, smooth muscle, epithelial, and endothelial) cause collagen matrix contraction, which is a characteristic of cell motility and affects tissue structures [26]. An ATPS was used for this application to eliminate difficulties of microgel production with conventional methods such as the need for expensive equipment and high throughput experimentation to analyze effects of a multitude of biological factors on collagen contraction [27, 28]. Collagen was mixed with a DEX phase solution prepared in phosphate buffered saline (PBS) solution and dispensed into the PEG phase solution made in a complete cell culture medium. Collagen collected at the interface of the ATPS and polymerized to develop a hemispherical collagen dome. This resulted in a distinct collagen microgel in an aqueous solution that showed similar structural and contractile properties when compared to collagen macrogels. This technique conveniently allowed the incorporation of cells into the collagen microgels by adding them to the DEX phase solution and patterning them onto existing cells in culture (Figure 12.4a). By patterning collagen microdroplets containing pre-osteoblast cells on an adherent breast epithelial cell layer (Figure 12.4b), the pre-osteoblast cells remained confined within the collagen drops and caused collagen matrix contraction (Figure 12.4c). The contraction of the cell-containing collagen microgel resulted in mechanical disruption and consequent damage to the breast epithelial cell layer (Figure 12.4d). Soluble growth factors that may affect collagen contraction could easily be added to the collagen microgels to elucidate their roles on contractile behavior of collagen. This ATPS collagen microgel approach allowed building physiologically relevant tissue-like structures to understand biological processes involving matrix interactions with cells and soluble factors.
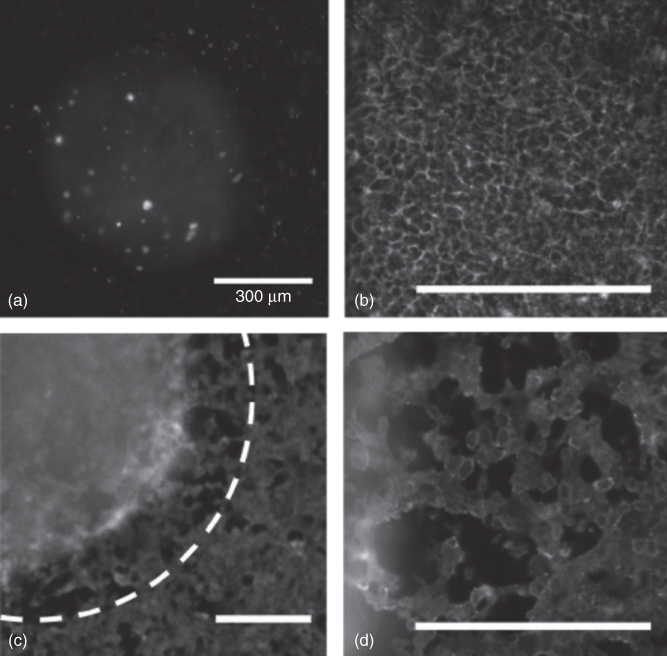
Figure 12.4 (a) A collagen DEX phase droplet containing NIH 3T3 cells dispensed on an existing cell layer of HEK 293 cells. (b) Prior to collagen patterning, MCF10A cells formed an interconnected epithelial monolayer. (c) When MC 3T3 cells were mixed in the collagen-DEX phase droplet and dispensed on the MCF10A epithelial layer, the collagen droplet remained separated and collagen matrix contraction resulted (initial boundary of the collagen droplet depicted in a white dashed line). (d) Damage to the MCF10A epithelial layer occurred within 4 h as a result of the mechanical disruption from the collagen matrix. Scale bars are 300 µm.
(Moraes et al. [25]. Copyright 2013. Reproduced with permission of Elsevier.)
12.3 Cell Patterning
12.3.1 Bacterial Cells
In addition to its numerous benefits when used for localizing biomolecules such as nucleic acids and proteins, ATPS micropatterning has significantly expanded bioengineering approaches for bacterial and mammalian cellular research. Using ATPS, bacteria populations were grown in distinct locations in suspension eliminating detachment, pattern outgrowth, and hindered growth problems with surface immobilized bacteria colony patterning [29]. Both aqueous polymer solutions of PEG and DEX were made in bacterial cell culture media. Bacteria cells were mixed in the DEX phase solution and dispensed as droplets into the PEG phase solution where the cells were restricted to and remained in the DEX phase (Figure 12.5a). This approach uniquely supported printing and adjacently orienting multiple bacterial populations in one culture dish without bacteria dispersion out of the aqueous DEX phase (Figure 12.5b). This enabled bacterial microarrays for use as biosensors, demonstrated with an array of bacteria suspensions that fluorescently or luminescently responded to different chemical stimulations. This simple ATPS-mediated bacterial micropatterning also facilitated convenient imaging of bacterial cultures and growth of bacteria in their normal media formulations.
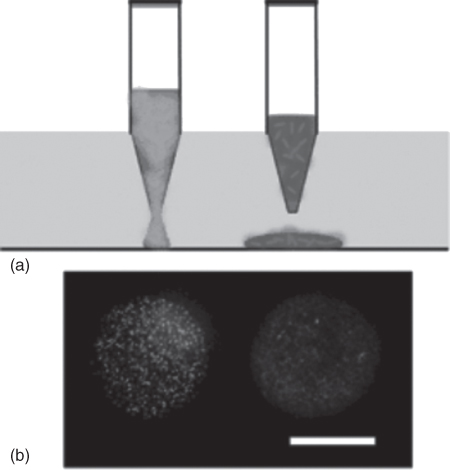
Figure 12.5 (a) Bacteria were mixed with DEX phase solution and pipetted into PEG-phase containing culture dish. This resulted in ATPS formation and different localized bacterial suspensions at specific positions. (b) A fluorescent image of patterned bacterial suspensions. Scale bar is 500 µm.
(Yaguchi et al. [29]. Copyright 2010. Reproduced with permission of Royal Society of Chemistry.)
Expansion of ATPS-mediated bacteria culturing methods has led to patterning of bacterial biofilms with high precision and specific localization [30]. The exclusion of bacteria to a DEX phase droplet immersed in a PEG phase solution allowed formation of varying and multiple biofilms next to and on top of each other. A biofilm layer was initially developed by culturing bacteria in a dish. Then, the media was replaced with a PEG phase solution in which bacteria-containing DEX phase droplets were dispensed on the existing biofilm layer to form biofilms overtime (Figure 12.6a). The diffusion of molecules took place between the PEG and DEX phase solutions – therefore, chemical interactions were maintained between bacterial biofilms. Through these experiments, it was exemplified that an antibiotic-sensitive bacteria colony (MGI655/pLacCherry) became significantly more resistant to antibiotic treatment when cultured on an antibiotic-resistant bacteria biofilm (MG1655/pAmCyan) (Figure 12.6b).
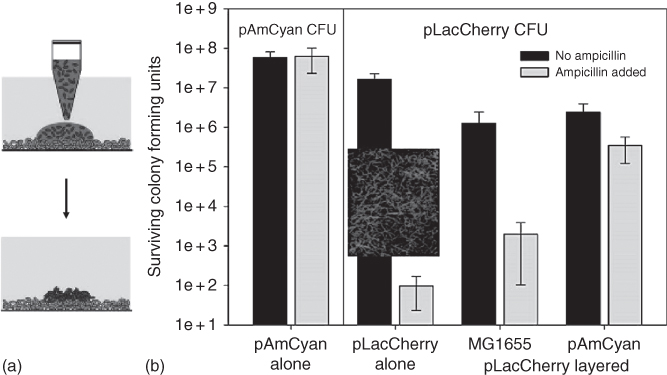
Figure 12.6 (a) Schematics of patterning bacteria on an existing biofilm. (b) Colony forming units (CFU) with pAmCyan and pLacCherry transformed E. coli (MG1655) strains treated with (gray bars) and without (black bars) ampicillin. The MG1655/pLacCherry strain was the ampicillin sensitive strain where as MG1655/pAmCyan strain was ampicillin resistant as demonstrated by their responses to ampicillin treatment. Biofilms of MG1655 (non-transformed, control) and MG1655/pAmCyan were prepared with ATPS technique and after 24 h, another DEX phase suspension of MG1655/pLacCherry was dispensed and layered on top of the original bacteria colonies. When in single and co-culture with the non-transformed control MG1655 E. coli strain, the MG1655/pLacCherry survival was very low when treated with ampicillin. However, its survival increased when cultured on top of the ampicillin resistant bacteria, MG1655/pAmCyan. The inset represents the abnormal morphology of the E. coli MG1655/pLacCherry strain after treatment with ampicillin. This data indicated the influence of the ampicillin resistant E. coli strain (MG1655/pAmCyan) on the ampicillin sensitive strain (MG1655/pLacCherry) from co-culturing the two bacterial species together.
(Reprinted with permission from [30]. Copyright 2012. American Chemical Society.)
The ability to create spatially-segregated bacterial colonies that could chemically communicate was further utilized to study the correlation between bacteria colony localization and chemical communication [31]. In this patterning application, bacteria cells were introduced into the DEX phase solution where they were retained when added to the PEG phase solution. The ATPS allowed confinement of the bacteria as microcolonies while molecules such as acyl-homoserine lactone (AHL) or oxygen could freely diffuse and interact with the bacteria. Further control over location of bacteria colony-containing DEX phase droplets was achieved by the incorporation of magnetic particles that could be utilized to magnetically move bacteria colonies. In a validation study, it was found that when two bacterial colonies (one producing AHL and another responsive to AHL, detected fluorescently) were formed in the same Petri dish, the inter-colony distance played a critical role in the interactions between colonies and AHL responsiveness. For example, decreasing the distance between the two colonies resulted in an increase in inter-colony communications.
Bacterial micropatterning is a useful approach to culture bacterial colonies on epithelial cells and evaluate the interactions between the two cell types [32]. Current culturing methods to study the interactions of these two cell types have difficulties associated with bacteria cells that are freely suspended preventing their association with epithelial cells, bacteria overgrowth that depletes the epithelial cell culture media, and limited exposure time of the two cell types. An ATPS could eradicate these difficulties by localizing bacterial cultures on epithelial cells while allowing communications between the two cell types. This was achieved by growing monolayer cultures of epithelial cells and immersing them in the PEG phase solution. Then, bacteria-containing DEX phase drops were dispensed on the monolayer to form distinct bacterial colonies over the epithelial cells (Figure 12.7). When MCF-10A human mammary epithelial cells were grown as a monolayer with invasive Escherichia coli cells grown as colonies on top, epithelial cells beneath the invasive bacteria colonies were killed. In the control experiment with noninvasive E. coli cells, there was no effect on the MCF-10A cells. This approach uniquely supported the culturing of three different bacterial species simultaneously on one epithelial cell monolayer culture. Such ATPS-enabled bacterial micropatterning on epithelial cells may lead to a better understanding of intercellular communications between bacteria and epithelial cells and new infection therapies.
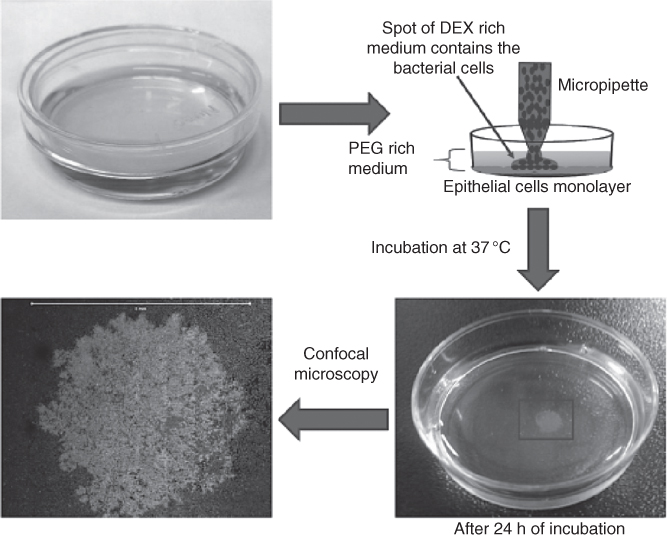
Figure 12.7 ATPS technique for formation of bacterial colonies on an epithelial monolayer. MCF-10A cells were grown in a Petri dish until monolayer formation. The culture medium was replaced with the PEG phase solution and droplets containing a mixture of DEX phase and bacteria were dispensed on the monolayer. This resulted in formation of a localized bacteria colony on the monolayer after 24 h of incubation. Scale bar is 5 mm.
(Reprinted from Ref. [32]. No changes made. Image included in article's Creative Commons license (http://creativecommons.org/licenses/by/4.0/).)
12.3.2 Mammalian Cells
12.3.2.1 Cell Exclusion and Cell Island Patterning
ATPS can be conveniently used to localize cell on surfaces as cell exclusion patterning and cell island patterning. The cell exclusion patterning was used in several studies to facilitate high throughput cell migration niches and determine anti-migratory effects of various chemical compounds [33–35]. Drops of the DEX phase solution (prepared in standard cell culture media) were dispensed into the center of wells of a cell adherent 96-well plate and allowed to dehydrate for about 24 h. Cells of interest were mixed at an optimum cell density with the PEG phase solution prepared in standard cell culture media. This mixture was then dispensed into each well containing a DEX disk resulting from drying of the DEX phase drop. The drop became rehydrated upon contact with the PEG phase solution and an ATPS resulted. Cells in the PEG phase solution attached around the rehydrated DEX phase drop. This patterning created a circular gap within a monolayer of cells in each well (Figure 12.8, left). The ATPS could then be removed and replaced with cell culture media to observe cell migration into the empty gap of each well. Addition of drug solutions facilitated studying the inhibitory effects of different drugs against cell migration. This technique was much simpler to use than existing migration assays, generated high throughput migration niches with highly reproducible migration niche size, and eliminated any external factors that could influence migratory behaviors. This cell exclusion patterning was used to study migration of breast cancer cells and anti-migratory effects of a collection of phytochemical compounds. Treating patterned triple negative breast cancer cells with phytochemicals reduced cell migration to different extents. Fisetin (Figure 12.9) and quercetin showed the largest inhibition of migration of cancer cells in a dose-dependent manner.
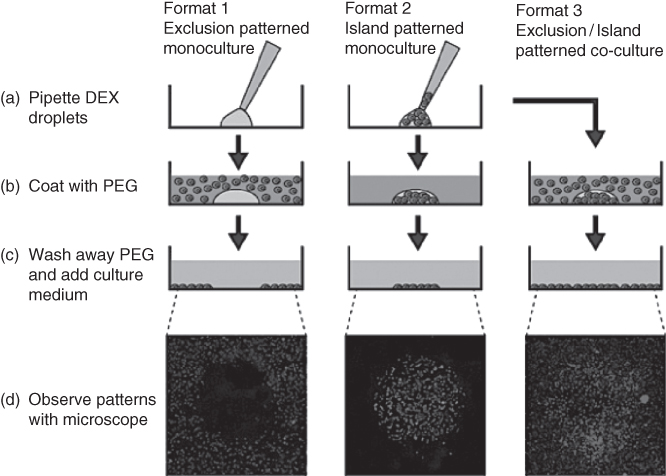
Figure 12.8 Three different cell patterning modes (exclusion (left), island (middle), and co-culture (right)) with HeLa cells were performed with the ATPS approach. All three modes began with (a) dispensed DEX phase droplets that do (island and co-culture) or do not (exclusion) contain cells. Then these DEX phase droplets were (b) coated with PEG phase solution that do (exclusion and co-culture) or do not (island) contain cells. When cells successfully attached, the ATPS solutions were (c) removed and replaced with standard cell culture media. The resulting cell patterns were observed with (d) fluorescent imaging for monocultures that were post-stained and cell specific pre-stained co-cultures.
(Frampton et al. [12]. Copyright 2013. Reproduced with permission of JoVE.)
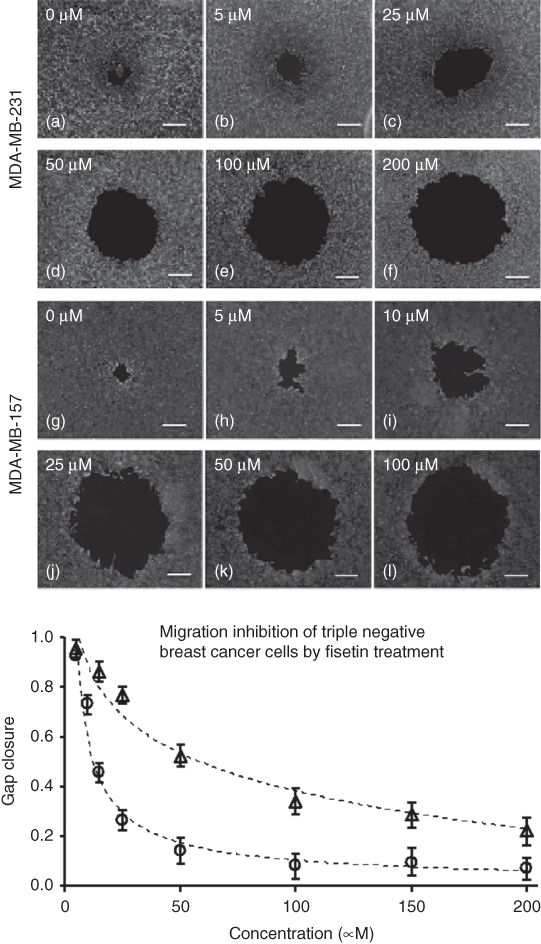
Figure 12.9 ATPS-based migration assay performed with MDA-MB-231 cells (a–f) and MDA-MB-157 cells (g–l) utilized for analysis of fisetin effects on cell migration. Displayed by the decrease in gap closure from left to right in the images, increasing fisetin concentration successfully blocked cell migration. Scale bar is 500 µm. The bottom graph is a dose-dependent anti-migratory effect of fisetin at non-toxic concentrations normalized against control (no treatment) for both cell types, that is, MDA-MB-157 (circles) and MDA-MB-231 (triangles).
(Reprinted from Ref. [34].)
Cell island patterning involved a similar concept as the cell exclusion patterning except that cells were mixed with the DEX phase solution [12]. This cell-containing DEX phase solution was then dispensed on a dry cell substrate and quickly covered with the PEG phase solution (Figure 12.8, middle). Unlike the cell exclusion patterning technique, drying of the DEX phase droplet was not favorable for patterning cells as an island. Cells in the DEX phase solution remained partitioned and attached as circular clusters in the middle of the substrate. The PEG phase solution was replaced with cell culture media to analyze cell proliferative behaviors. Cervical cancer cells exhibited outward proliferation from initial island patterns that was quantifiable with image analysis tools.
12.3.2.2 Cell Co-Culturing
The combination of cell exclusion and island patterning techniques can be utilized for co-culturing of cells to create a more physiologically relevant cell model. In this technique, different cell types were included in each polymer phase dissolved in cell culture media of the PEG-DEX ATPS (Figure 12.8, right) [12]. Submicroliter cell-containing DEX phase droplets were dispensed onto a substrate and then immediately covered with a cell-containing PEG phase solution. After 12 h of incubation, a cell pattern resulted where a monolayer of one cell type on the substrate was surrounded by the second cell type. The ATPS could be removed and replaced with cell culture media to observe the interactions of the two cell types. This method rapidly and conveniently allowed the co-culturing of two cell types to examine the influence signaling between different cells compared to single culture cell exclusion or cell island patterning results. Using an ATPS-mediated liver-fibroblast cell co-culture, it was demonstrated that the presence of fibroblasts increased levels of albumin production by the liver cells.
12.3.2.3 Heterocellular Stem Cell Niche Engineering
The ability to incorporate different cell types into two aqueous polymer phases of an ATPS has demonstrated useful in the formation of heterocellular stem cell niches [36]. An ATPS made cell patterning possible on existing monolayer cell cultures – a limitation of many other cell patterning methods. For heterocellular niche formation, cell suspension was mixed with a DEX phase solution and dispensed onto a monolayer of cells immersed in the PEG phase solution (Figure 12.10a). The suspension cells remained confined to the DEX phase solution and adhered to the underlying cells. The two-phase system was removed and replaced with cell culture media for long-term studies. This non-contact development of a heterocellular niche supported full cell viability and functionality. This approach was used to create heterocellular niches comprising of mouse embryonic stem cells (mESCs) and stromal PA6 cells and study neural differentiation of mESCs due to stromal cells signaling. mESCs of defined densities were microprinted in DEX phase drops onto a monolayer of stromal PA6 cells and allowed to proliferate to form individual colonies on PA6 cells. The size of colonies was determined by the initial number of mESCs within each DEX phase drop. It was found that colony size played a critical role in guiding neural differentiation of mESCs; increase in the colony size disproportionately enhanced extension and density of neurite processes quantified by the expression of neural cell maker beta-III tubulin, TuJ1 (Figure 12.10b–d).

Figure 12.10 (a) ATPS-mediated localized micropatterning of different densities of mESCs on existing stromal cell layer to form heterocellular niches. Scale bar is 250 µm. (b) TuJ1 staining of differentiated mESCs on day 8 of culture. Scale bar is 500 µm. (c) The fluorescent intensity of TuJ1 increased with larger mESC colony area. (d) When TuJ1 expression was normalized by the colony area, it was found that neural differentiation was colony size-dependent and increased with larger colony size.
(Reprinted from Ref. [36].)
To demonstrate the role of feeder cells signaling on the fate of mESCs, two different types of feeder cells and mESCs were used to create heterocellular niches [37]. PA6 cells and mouse embryonic fibroblast (MEF) cells were suspended in two separate DEX phase solutions and microprinted in an alternating array format with pre-defined center-to-center distance on a culture dish containing the aqueous PEG phase. After formation of islands of PA6 and MEF cells, the ATPS medium was removed and mESCs were added in standard media. mESCs randomly landed on islands of feeder cells as well as on the bare culture surface. It was shown that mESCs on PA6 islands displayed neurogenesis (shown by positive TuJ1 expression), whereas mESCs on MEF islands remained pluripotent (shown by positive Oct4 expression). Therefore, cell microprinting with ATPS enabled assessing the role of different support cells on stem cell phenotypes.
12.3.2.4 Skin Tissue Engineering
Patterning with ATPS provided a method to produce non-circular cell constructs in a simple and controlled manner that is advantageous for numerous applications such as tissue engineering for wound healing [38]. Desired cell patterning shapes were first developed by dehydration of DEX dissolved in a buffer solution on a polydimethyl siloxane (PDMS) substrate. The dehydrated DEX phase patch was then introduced to a cell monolayer culture containing the PEG phase solution (prepared in a buffer solution). The patterned DEX phase patch became rehydrated from the bulk PEG phase solution while remaining immiscible due to polymer phase separation and created a micro-domain. It was demonstrated that fluorescent probes or enzymes could be introduced to the DEX phase patch for multi-layer reagent fabrication. Additionally, the incorporation of trypsin in DEX phase patches resulted in selective cell detachment based on the DEX phase patch shape for use as a wound healing assay. This method allowed reproducible wound healing patterns to be formed across different monolayers of cells. Importantly, this approach provided an opportunity to correctly model the geometrical characteristics such as area and perimeter of wounds that have been suggested to influence the healing process [39]. This wound healing assay was exemplified with breast cancer cells (MCF-7) and JNK and P38 inhibitors where the inhibitors significantly decreased wound closure – similar to other findings with cancer cell migration inhibition studies [40, 41].
Cell partition in ATPS is dependent on the physiochemical properties of the separation medium and cells. While ATPS-engineered formulations have been adjusted to preferentially partition cells to one aqueous polymer phase, different formulations enable collecting cells at the interface of the two phases. One application uses a PEG-DEX ATPS prepared in cell culture media to preferentially partition cells at the interface between the DEX and PEG phases for formation of macroscale tissue-like constructs (Figure 12.11a) [42]. A suspension of cells in the PEG phase solution was dispensed on top of a DEX phase solution in microcentrifuge tubes. The ATPS formed in which cells collected at the flat ATPS interface where they were confined and encouraged to form cell–cell contacts and assemble into stable planar constructs. This technique was simple, inexpensive, and adaptable with many different cell types. The application of these tissue constructs was demonstrated with the production of monolayer and bilayer cells and keratinocyte constructs to successfully integrate with a dermal matrix to develop into skin equivalents (Figure 12.11b).

Figure 12.11 (a) ATPS-mediated formation of skin tissue-like constructs by preferential partition of cells to the interface of ATPS. DEX phase solution was added to microcentrifuge tube and cells mixed with PEG phase solution was layered on top. Bilayer constructs resulted from the sequential cell seeding in the PEG phase solution to the DEX phase-containing tube. Confocal imaging revealed the two separately labeled cells in the bilayer construct. Scale bar is 100 µm. (b) The cellular constructs formed with primary human keratinocytes resemble skin when on decellularized dermal matrices as demonstrated by the hematoxylin & eosin (H&E) staining of an attached keratinocyte construct to a dermal matrix on day 3. Scale bars are 20 µm.
(Frampton et al. [42]. Copyright 2015. Reproduced with permission of John Wiley & Sons.)
12.3.2.5 Three-Dimensional Cellular Models
Two-dimensional cell culture methods remain popular for cellular studies due to their simplicity of production and maintenance. However, the need for three-dimensional (3D) cellular systems has increasingly become apparent due to their more physiologically relevant structure and biological behavior. To eliminate the difficulty of conventional 3D cultures, a PEG-DEX ATPS was formulated to allow culturing 3D constructs using a simple method. Cells were suspended in the aqueous DEX phase and dispensed onto varying biological surfaces (cell monolayer or decellularized matrices), immersed in the PEG phase solution, as controlled, pre-defined patterns. Cells remained within printed patterns and adhered to their underlying substrate (Figure 12.12a). Temporal and spatial control over printing allowed patterning of cells on top of each other to generate multi-layer constructs [43]. This method was adapted to an in-house robotic printing device for consistent cell patterning with controllable and precise interspacing (Figure 12.12b,c). The possibility of mimicking heterotypic cellular interactions was explored with mouse myoblast cells printed as cell layers in varying geometries on a cell monolayer or decellularized matrix (Figure 12.12d–f).
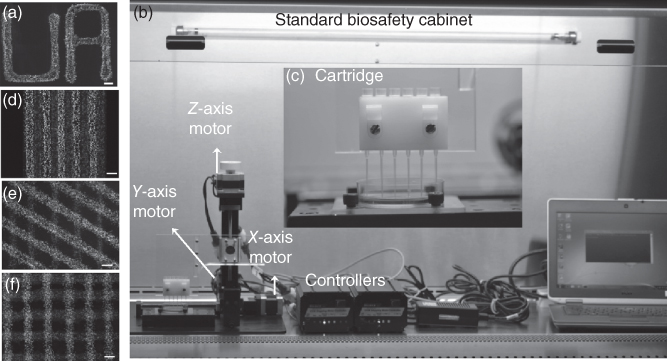
Figure 12.12 (a) The ATPS microprinting technique allowed generating cellular patterns of user-defined shapes. Scale bar is 500 µm. (b) The automated cell printer for ATPS printing of cells. The printer includes 3-axis motors, controller units, and computer. (c) A cartridge system is used to include cells as “bio-ink” suspended in DEX phase solution within narrow pipette tips. (d–f) The ATPS printing of cells allowed co-culturing on a cell monolayer or decellularized matrix in various directions and patterns. Scale bar is 500 µm.
(Reprinted from Ref. [43].)
Availability of 3D culture is of great importance in cancer research to model tumors and study their growth behaviors and drug responses. Recently, the ATPS technique was used to conveniently generate 3D clusters of cancer cells known as spheroids. The denser DEX phase solution (prepared in cell culture media) containing cancer cells was dispensed as a submicroliter droplet into a non-adherent microwell containing the immersion PEG phase solution (Figure 12.13a, left) [44, 46, 47]. The drop sank to the bottom of the microwell. The cancer cells preferentially remained partitioned to the DEX phase droplet where cell–cell interactions promoted aggregation of cancer cells into a single spheroid (Figure 12.13a, middle and right). Free diffusion of nutrients from the immersion phase supported growth of the cellular spheroid. This approach eliminated many difficulties with other spheroid formation techniques such as use of physical forces to promote cell aggregation, formation of multiple spheroids in wells, and incompatibility of the techniques with standard biochemical analysis tools [45]. The use of an ATPS caused spontaneous spheroid formation within 24 h of incubation. Successful spheroid formation with this method was demonstrated with various breast, skin, brain, and colon cancer cells. Additionally, drug response analyses illustrated the importance of incorporating 3D cancer cell spheroid cultures in anti-cancer drug screening to elicit more physiologically-relevant responses. For example, using a standard chemotherapeutic drug (paclitaxel), triple negative breast cancer spheroids displayed complete drug resistance compared to their monolayer counterparts (Figure 12.13b).

Figure 12.13 (a) The ATPS approach used for three-dimensional spheroid formation. Cancer cells were mixed with the DEX phase and a drop of the mixture was dispensed into wells containing the PEG phase solution. Scale bar is 250 µm.
(Reprinted from Ref. [44].)
(b) Drug testing of paclitaxel with MDA-MB-157 breast cancer cell spheroids (circles) resulted in significantly different percent viabilities (normalized against control, non-treated conditions) compared to monolayer (triangles) culture of cells. Spheroids showed complete drug resistance whereas monolayer cultures displayed drug effectiveness, emphasizing the influence of three-dimensional morphology on drug responses of cancer cells.(Reprinted from Ref. [45].)
12.4 Conclusions
PEG-DEX ATPS enabled the development of various molecular and cellular assays through convenient micropatterning of biomolecules. Standard antibody assays (immunostaining, ELISA, and AlphaLISA) were improved, simplified, and advanced by adapting them to a PEG-DEX ATPS to provide more efficient and specific antibody delivery assays. Microgel formation difficulties were eradicated by utilizing the phase separation properties of PEG and DEX polymer solutions to conveniently fabricate distinct collagen microgels for contractile studies. Additionally, bioreagents such as liposomal constructs and lentiviral vectors were successfully delivered to cells in a controlled and localized manner while preserving cellular viability in highly aqueous two-phase solutions. The biocompatibility of the PEG-DEX ATPS also made it ideal for a multitude of applications involving bacterial and mammalian cells. Bacterial cell colonies were formed due to their preferential partitioning in the DEX phase where biochemical interactions between distinct bacteria colonies or biofilms were maintained. Different mammalian cellular patterning techniques (cell exclusion and cell island) for migration and proliferation studies were adjusted for co-culturing and heterocellular niche modeling. Non-spherical cell patterns, advantageous for wound healing applications, were created with DEX phase patches dehydrated on PDMS molds. By adjusting for cellular partitioning to the interface of the PEG-DEX ATPS, macroscale tissue-like constructs in aqueous solutions were formed. ATPS with PEG and DEX phase solutions also facilitated modeling of 3D heterocellular layers and cancer cell spheroids. The main advantages of PEG-DEX ATPS for molecular and cellular applications exemplified throughout these various applications included ease and simplicity, cost-effectiveness, biocompatibility, and adjustable partitioning properties to tailor for specific applications. Micropatterning with ATPS in molecular and cellular applications has been well-adopted by the scientific community and continue to be explored for further applications.
Acknowledgments
Financial support was provided by the National Institutes of Health under grant R21CA182333.
References
- 1 Whitesides, G.M. (2006) The origins and the future of microfluidics. Nature, 442, 368–373.
- 2 Wheeler, A.R. et al. (2003) Microfluidic device for single-cell analysis. Anal. Chem., 75, 3581–3586.
- 3 Wlodkowic, D., Faley, S., Zagnoni, M., Wikswo, J.P., and Cooper, J.M. (2009) Microfluidic single cell array cytometry for the analysis of tumour apoptosis. Anal. Chem., 81, 5517–5523.
- 4 Yi, C., Li, C.W., Ji, S., and Yang, M. (2006) Microfluidics technology for manipulation and analysis of biological cells. Anal. Chim. Acta, 560, 1–23.
- 5 Brouzes, E. et al. (2009) Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. U.S.A., 106, 14195–14200.
- 6 Khandurina, J. and Guttman, A. (2002) Bioanalysis in microfluidic devices. J. Chromatogr. A, 943, 159–183.
- 7 Gossett, D.R. et al. (2010) Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem., 397, 3249–3267.
- 8 Bhagat, A.A.S. et al. (2010) Microfluidics for cell separation. Med. Biol. Eng. Comput., 48, 999–1014.
- 9 Clausell-Tormos, J. et al. (2008) Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol., 15, 427–437.
- 10 Park, J., Koito, H., Li, J., and Han, A. (2009) Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomed. Microdevices, 11, 1145–1153.
- 11 Atefi, E., Joshi, R., Mann, J.A., and Tavana, H. (2015) Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Appl. Mater. Interfaces, 7, 21305–21314.
- 12 Frampton, J.P., White, J.B., Abraham, A.T., and Takayama, S. (2013) Cell co-culture patterning using aqueous two-phase systems. J. Vis. Exp., 16–20. doi: 10.3791/50304
- 13 Atefi, E. et al. (2014) Ultralow interfacial tensions of aqueous two-phase systems measured using drop shape. Langmuir, 30, 9691–9699.
- 14 Albertsson, P.-Å. and Tjerneld, F. (1994) Phase diagrams. Methods Enzymol., 228, 3–13.
- 15 King, R.S., Blanch, H.W., and Prausnitz, J.M. (1988) Molecular thermodynamics of aqueous two-phase systems for bioseparations. AIChE J., 34, 1585–1594.
- 16 Albertsson, P.-A. (1956) Chromatography and partition of cells and cell fragments. Nature, 177, 771–774.
- 17 Hatti-kaul, R. (2001) Aqueous two-phase systems. Mol. Biotechnol., 19, 697–713.
- 18 Asenjo, J.A. and Andrews, B.A. (2012) Aqueous two-phase systems for protein separation: phase separation and applications. J. Chromatogr. A, 1238, 1–10.
- 19 Schindler, J. and Nothwang, H.G. (2006) Aqueous polymer two-phase systems: effective tools for plasma membrane proteomics. Proteomics, 6, 5409–5417.
- 20 Tavana, H. et al. (2009) Nanoliter liquid patterning in aqueous environments for spatially-defined reagent delivery to mammalian cells. Nat. Mater., 8, 736–741.
- 21 Chambers, A.F. and Matrisian, L.M. (1997) Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst., 89, 1260–1270.
- 22 Frampton, J.P., Tsuei, M., White, J.B., Abraham, A.T., and Takayama, S. (2015) Aqueous two-phase system-mediated antibody micropatterning enables multiplexed immunostaining of cell monolayers and tissues. Biotechnol. J., 10, 121–125.
- 23 Frampton, J.P. et al. (2014) Aqueous two-phase system patterning of detection antibody solutions for cross-reaction-free multiplex ELISA. Sci. Rep., 4, 4878.
- 24 Simon, A.B. et al. (2014) Aqueous two-phase systems enable multiplexing of homogeneous immunoassays. Technology, 2, 176.
- 25 Moraes, C., Simon, A.B., Putnam, A.J., and Takayama, S. (2013) Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials, 34, 9623–9631.
- 26 Grinnell, F. and Petroll, W.M. (2010) Cell motility and mechanics in three-dimensional collagen matrices. Annu. Rev. Cell Dev. Biol., 26, 335–361.
- 27 Hong, S., Hsu, H.-J., Kaunas, R., and Kameoka, J. (2012) Collagen microsphere production on a chip. Lab Chip, 12, 3277.
- 28 Gullberg, D. et al. (1990) Beta1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp. Cell. Res., 186, 264–272.
- 29 Yaguchi, T. et al. (2010) Micropatterning bacterial suspensions using aqueous two phase systems. Analyst, 135, 2848–2852.
- 30 Yaguchi, T. et al. (2012) Aqueous two-phase system: derived biofilms for bacterial interaction studies. Biomacromolecules, 13, 2655–2661.
- 31 Byun, C.K. et al. (2013) Productive chemical interaction between a bacterial microcolony couple is enhanced by periodic relocation. J. Am. Chem. Soc., 135, 2242–2247.
- 32 Dwidar, M., Leung, B.M., Yaguchi, T., Takayama, S., and Mitchell, R.J. (2013) Patterning bacterial communities on epithelial cells. PLoS One, 8, 1–14.
- 33 Lemmo, S., Nasrollahi, S., and Tavana, H. (2014) Aqueous biphasic cancer cell migration assay enables robust, high-throughput screening of anti-cancer compounds. Biotechnol. J., 9, 426–434.
- 34 Ham, S.L. et al. (2015) Phytochemicals potently inhibit migration of metastatic breast cancer cells. Integr. Biol., 7, 792–800.
- 35 Tavana, H. et al. (2011) Polymeric aqueous biphasic system rehydration facilitates high throughput cell exclusion patterning for cell migration studies. Adv. Funct. Mater., 21, 2920–2926.
- 36 Tavana, H., Mosadegh, B., and Takayama, S. (2010) Polymeric aqueous biphasic systems for non-contact cell printing on cells: engineering heterocellular embryonic stem cell niches. Adv. Mater., 22, 2628–2631.
- 37 Tavana, H., Mosadegh, B., Zamankhan, P., Grotberg, J.B., and Takayama, S. (2011) Microprinted feeder cells guide embryonic stem cell fate. Biotechnol. Bioeng., 108, 2509–2516.
- 38 Bathany, C., Park, J., Cho, Y.-K., and Takayama, S. (2013) Dehydrated aqueous two-phase system micro-domains retain their shape upon rehydration to allow patterned reagent delivery to cells. J. Mater. Chem. B, 1, 6020.
- 39 Gorin, D.R., Cordts, P.R., LaMorte, W.W., and Menzoian, J.O. (1996) The influence of wound geometry on the measurement of wound healing rates in clinical trials. J. Vasc. Surg., 23, 524–528.
- 40 Mao, L. et al. (2010) Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res., 12, R107.
- 41 Huang, C., Rajfur, Z., Borchers, C., Schaller, M.D., and Jacobson, K. (2003) JNK phosphorylates paxillin and regulates cell migration. Nature, 424, 219–223.
- 42 Frampton, J.P. et al. (2015) Rapid self-assembly of macroscale tissue constructs at biphasic aqueous interfaces. Adv. Funct. Mater., 25, 1694–1699.
- 43 Petrak, D., Atefi, E., Yin, L., Chilian, W., and Tavana, H. (2014) Automated, spatio-temporally controlled cell microprinting with polymeric aqueous biphasic system. Biotechnol. Bioeng., 111, 404–412.
- 44 Lemmo, S., Atefi, E., Luker, G.D., and Tavana, H. (2014) Optimization of aqueous biphasic tumor spheroid microtechnology for anti-cancer drug testing in 3D culture. Cell. Mol. Bioeng., 7, 344–354.
- 45 Ham, S.L., Joshi, R., Thakuri, P.S., and Tavana, H. (2016) Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp. Biol. Med. (Maywood), 241, 939–954.
- 46 Ham, S.L., Atefi, E., Fyffe, D., and Tavana, H. (2015) Robotic production of cancer cell spheroids with an aqueous two-phase system for drug testing. J. Vis. Exp., 1–9. doi: 10.3791/52754
- 47 Atefi, E., Lemmo, S., Fyffe, D., Luker, G.D., and Tavana, H. (2014) High throughput, polymeric aqueous two-phase printing of tumor spheroids. Adv. Funct. Mater., 24, 6509–6515.
