Chapter 18
Chemistrode for High Temporal- and Spatial-Resolution Chemical Analysis
Alexander J. Donovan1 and Ying Liu1,2
1University of Illinois at Chicago, Department of Chemical Engineering, Chicago, IL, 60607, USA
2University of Illinois at Chicago, Department of Biopharmaceutical Sciences, Chicago, IL, 60607, USA
18.1 Introduction
The chemistrode is a droplet-based microfluidic device for stimulating, recording, and analyzing molecular signals with high spatiotemporal and chemical resolution. The invention of its electrochemical precursor, the microelectrode, several decades ago allowed for a transformation in the investigation of biophysical phenomena at exquisite fidelity. The pioneers in this new field of electrophysiology, Hodgkin and Huxley, utilized the enormous squid giant axon as their tool to resolve the electrochemical dynamics across an axonal membrane. This allowed for the measurement of individual ionic currents across membrane-spanning ion channels for the first time in 1952 [1, 2]. Together with John Eccles, they received the Nobel Prize in Physiology or Medicine in 1963 for their contributions in initiating this new era in neuroscience [3]. Nevertheless, voltage clamping with the squid giant axon gives inadequate signal sensitivity and stability, limiting spatial resolution above the micron length scale and effectively prohibiting the investigation of complex vertebrate cell types involved in many kinds of biophysical and electrochemical processes [4]. Two decades later Neher and Sakmann introduced a new electrophysiological tool to interrogate ion conductance with micrometer resolution and frequencies greater than 104 Hz [5]. Employing a glass micropipette filled with saline as a microelectrode, the electrophysiologists placed the pipette on a small “patch” of cell membrane and measured the current conducted through the pipette at a constant voltage potential [6]. Therefore, the patch clamp allowed for high fidelity recording of cellular electrochemical dynamics, including the action potential in the hippocampus of mammalian neurons [7] and related phenomena in other electrically excitable histologies such as cardiac pacemaker cells [8].
Despite the facility and power of the patch-clamp technique and other electrophysiological tools in the experimental models described earlier, its general adoption as an analytical tool for use across a wider range of physiologic scenarios to stimulate and record cellular signals is significantly limited, because only a minute fraction of the stimuli involved in life's processes are electrochemically active. An overwhelming majority of intracellular processes in which investigators are currently concerned are dominated by molecularly based actors, manifesting in the form of small molecule organic compounds, peptides, and proteins without any intrinsic electric charge. As such, there is a dearth of experimental tools with the requisite spatiotemporal resolution to observe the cacophony of molecular dialogue in these model chemical and/or biological systems. Certainly, as the domain of electrophysiology does not extend beyond this narrow locus of electrochemical phenomena, techniques relying on a fundamentally discrete set of detection criteria must therefore be developed to measure the molecular signals in those systems that contain biochemical actors.
Dramatic breakthroughs in micromachining and soft lithographic fabrication by Whitesides [9–13] and later others [14, 15] in the last few decades have created the rapidly burgeoning field of microfluidics. Harnessing the new microscale physics paradigm generated by these devices, scientists and engineers now have the capability to utilize microfluidics in a number of previously unfathomable applications in chemistry and biology, including in the development of analytical tools with the capacity to characterize, record, and possibly differentiate discrete molecular signals in complex chemical or biochemical systems [16–23].
Despite the great potential of microfluidics to transcend the inherent limitations of the microelectrode in its scope of application, devices relying on single-phase laminar-flow conditions fail to meet the strict spatial- and temporal-resolution standards for chemical analysis of highly complex systems [24]. It is even more challenging if the analysis has to be on a single live cell due to the high requirement of chemical resolution as well. The major problems associated with laminar-flow devices are from (i) inefficient mixing due to low Reynolds number (Re) [25] and (ii) Taylor dispersion as a consequence of diffusive transport and the parabolic velocity profile [26]. Thus, signal broadening and attenuation in the device, as it is directly coupled to the downstream location and time after initial analyte measurement, complicates the analysis of chemical actors with rapid signal frequency and the identification of relevant analytes present in a complex mixture with numerous other chemical moieties simultaneously. Furthermore, laminar-flow devices also severely limit the analytical methods that can be employed, with techniques requiring additional expertise and substantial time investment being largely excluded from practical consideration. If microfluidics is to be a feasible route forward in the development of a tool capable of such chemical analysis and recording at a resolution suitable for complex biochemical systems, novel geometries and phase regimes must be considered. The new device has to overcome the mixing and signal attenuation problems of single-phase laminar-flow devices (such as direct sampling [27], push–pull perfusion [28], microdialysis [29], and direct microinjection [30]), as well as transform the measurement of the molecular stimuli into stable chemical recordings where the fidelity is uncoupled from the location and time of its measurement. Techniques relying on microfabrication have been proposed in the literature in addition to single-phase laminar-flow devices for high-resolution chemical analysis. Labs-on-a-chip, in which the analyte measurements and chemical analyses are performed in close proximity on the same microscale device, significantly reduce signal attenuation problems as the transport distances are diminished by orders of magnitude [31, 32]. However, the interfacing of the measurement and analysis together on a single device creates additional complexities in manufacture and operation that are not present when each is done in isolation [33]. Therefore, the advantages of using multiphase flow to maintain chemical signals in discrete droplets or plugs without dilution and cross-contamination provide a possible solution for chemical transport with high temporal resolution [34–36].
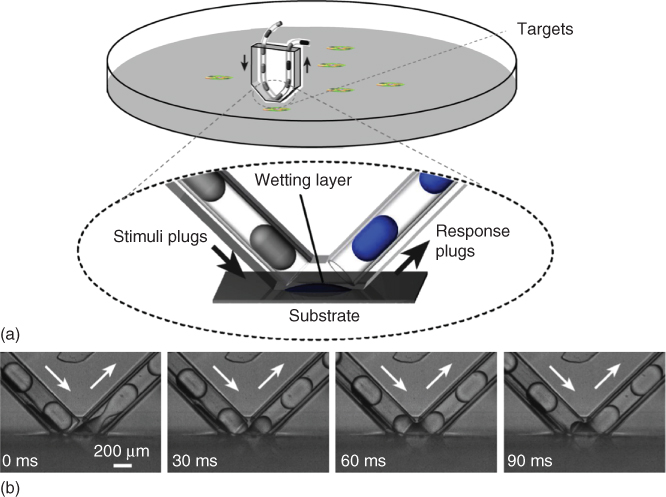
Figure 18.1 Chemistrode geometry and operation. (a) Chemistrode contacting a chemically interactive surface. Illustration of the chemistrode, a microfluidic device consisting of V-shaped channels in contact with a hydrophilic substrate. Plugs, consisting of aqueous nanoliter droplets surrounded by a fluorous carrier fluid, contain stimuli molecules that exchange chemical information in the wetting layer. This molecular information is collected in response plugs in the exiting microchannel, enabling multivariate online and offline chemical analysis at superior temporal, spatial, and chemical resolution. (b) Optical imaging exhibiting the delivery, coalescence, and reassembly of plugs at the substrate on millisecond timescales.
The Ismagilov research group at the University of Chicago (his laboratory and research group relocated to the California Institute of Technology, Pasadena, CA, USA, in 2010) recently developed the chemistrode, a veritable chemical electrode. This multiphase plug-flow device with channels shaped like the letter “V” contacts a hydrophilic substrate, where a wetting layer forms in the “open space” [37–41]. The inlet channel contains aqueous plugs with chemical stimuli separated by a fluorinated carrier fluid (Figure 18.1). Chemical information is efficiently exchanged on the wetting layer of the substrate, creating response plugs in the outlet channel that can be split up for parallel analytical studies by different techniques without loss of signal fidelity or temporal resolution. The chemistrode makes it possible to study molecular signals of many surfaces that respond to chemical stimulation at high temporal, spatial, and chemical resolution. In the following sections, we will review the fabrication and operation of the device, physical principles governing transport processes, online and offline chemical analysis techniques, and in vitro applications followed by the discussions on the challenges and possible future directions.
18.2 Chemistrode Design and Operation
18.2.1 Chemistrode Design and Fabrication
A schematic of the chemistrode interfacing with a surface is shown in Figure 18.1a. The chemistrode's major component consists of a microfluidic device with V-shaped channels measuring 300 × 300 µm, typically assembled on a poly(dimethyl)siloxane (PDMS) template by soft lithography. A silanous vapor (tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane) is applied to the microchannel walls to confer them with hydrophobic and fluorophilic surface properties. Teflon microcapillaries were also inserted into the orifices of the V-shaped microchannels. The typical angle between the inlet and outlet tubing is 90°. Infusion pumps deliver aqueous plugs containing stimuli molecules to the hydrophilic substrate via the Teflon microcapillaries. The aqueous plugs dispersed in a fluorous carrier fluid are generated by using an upstream T-junction microfluidic device or designed by using robotic automatic infusion. Similar to the electrode, the chemistrode is simply brought into contact with the hydrophilic surface of interest. Typically, fluorophilic surfactants such as triethylene glycol mono[1H,1H-perfluorooctyl]ether (RfOEG) are incorporated into the fluorous phase to prevent nonspecific protein adsorption at the interface to ensure biocompatibility and chemical sensitivity [42].
18.2.2 Chemistrode Operation
The objective of the chemistrode as a chemical analytical tool is to assemble an array of compartments containing a discrete set of stimuli molecules, deliver them to a chemically or biologically interactive surface where it captures the ensemble of chemical responses, and provide these response molecules for in situ and offline analysis in a manner properly delineated in time and space. Precisely, this sequence of events is realized with the chemistrode device in the following manner: First, the stimuli molecules are loaded into an array of discrete aqueous plugs. These plugs are aqueous droplets with volumes of several nanoliters. A fluorinated hydrocarbon fluid segregates the disparate chemical species from each other. FC3283 and FC70 are commonly chosen because of their surface tension and viscosities, in addition to their chemically inert and biocompatible properties [42, 43]. The carrier fluid prevents diffusion of chemical species across aqueous plugs. The array of stimuli molecules must then be transported to the chemically active surface – the plugs are therefore infused toward the hydrophilic substrate. Once the stimuli plugs reach the bottom of the V-shaped channel, exchange of chemical information with the substrate at the wetting layer is controlled by circulation-induced mixing, which is much more efficient than diffusion. As the substrate is hydrophilic, only aqueous species readily transfer between the aqueous droplet and the surface wetting layer, while the fluorinated carrier fluid remains in the V-shaped microchannel. Plug transport and coalescence and chemical mass transfer in this two-phase system will be discussed in the next section in greater depth. Once molecular sampling has concluded, the aqueous droplets reform in the exiting portion of the V-shaped channels. The response plugs can then be divided into parallel arrays, which will contain the same ancestral chemical species, prepared for offline analysis. The information is then aggregated together into a “chemical tableau,” yielding a complete illustration of the chemical/biological sample at the molecular scale.
18.3 Physical Principles Governing the Transport Processes
The fixed geometry of the chemistrode yields a consistent trajectory of stimuli plugs through the V-shaped microchannel and, in conjunction with the repeatable loading and formation of plug arrays, allows one to systemically investigate plug transport, coalescence, and reformation. Robust reproduction of experimental outcomes is of immense importance for the chemistrode, and indeed for any analytical tool, that is tasked with reliable delivery of chemicals to the surfaces of interest and the reassembly of plugs containing molecules generated by these surfaces. However, this physical reliability is counterbalanced by the necessity for these devices to stimulate and record at high chemical, spatial, and temporal resolutions, which is dictated by high sampling frequency. Parameters that govern the physical principles and dictate the sampling frequency limit therefore need to be identified. Elucidation of these physical determinants will not only assist operators of the chemistrode in achieving reliable and verifiable results at the desired spatial, temporal, and chemical fidelities but also aid the wider microfluidics and interfacial phenomena community, who investigate coalescence and dynamics of multiphase or plug-flow-based systems at the length scales and timescales discussed here.
18.3.1 Non-dimensional Groups
Generally, in multiphase microfluidics, three dimensionless numbers are important to control the physical transport processes: (i) the capillary number (Ca), which is the ratio of viscous and interfacial forces; (ii) the viscosity ratio (λ) between the aqueous plugs and the fluorinated carrier fluid; and (iii) the Weber number (We), which gives the relative importance of inertial to interfacial forces. Beyond these three dimensionless quantities, Re, relating inertial to viscous forces, which is usually very small for microfluidics given the length scales involved, needs to be considered for understanding of plug coalescence. The Péclet number (Pe), which gives the relationship between the rate of advection of a physical quantity by the flow and the rate of diffusion of the same quantity, needs also to be considered, since sufficient mass transfer between the plugs and the hydrophilic surface is essential and limits the sampling frequency:
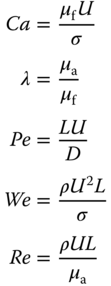
Here, U is the mean velocity of the plugs, μf is the viscosity of the fluorinated carrier fluid, σ is the interfacial tension at the aqueous/fluorous interface, μa is the viscosity of the aqueous fluid, L is the characteristic length (such as the diameter of the microcapillary), and D is the effective diffusivity of molecular actors that exchange between the plug and the hydrophilic surface.
For reliable transport of plugs of well-controlled shape, Ca is usually smaller than 0.1 [44], which establishes a limit on the maximum plug frequency allowed for exchange of signals at the substrate (fCa). Further, the stimuli plugs cannot be placed too closely together in order to prevent undesirable plug coalescence and cross-contamination of stimuli molecules. In practice, the Ismagilov research group limited the distance between adjacent plugs measured center to center to be 6 times that of the plug diameter. As an example, a typical value for fCa, equal to Ca * γ/(μf * 6d), is approximately 800 s−1 for a common geometry of the chemistrode (d = 200 µm, μf = 0.0014 Pa s, and γ = 10 mN/m for an aqueous FC3283 interface with 0.5 mg/mL RfOEG surfactant concentration) [37]. Furthermore, the pressure drop also constrains the plug frequency. Using the Hagen–Poiseuille equation to calculate pressure drop [45], which assumes that the fluid flows in an infinitely long pipe with cylindrical geometry at low Re, one can estimate the pressure drop given the channel diameter, volumetric flow rate, and dynamic viscosity of the aqueous phase. For the chemistrode of the same geometry, the maximum allowable plug frequency constrained by the pressure drop (fΔP) is therefore approximately 900 s−1. However, the chemistrode is typically operated under multiphase flow conditions, which significantly alters the velocity profile, leading to an even larger pressure drop as compared with single-phase flow devices operated at identical Re. Therefore, the single-phase convenient estimation of the fΔP gives a slightly higher value compared with the real value in multiphase flow.
18.3.2 Coalescence Dynamics of Incoming Plugs with the Hydrophilic Substrate
Coalescence of the plugs with the wetting surface is necessary to ensure mass transfer and therefore stimulation and chemical collection, which limits the upper bound of the sampling frequency (fcoal about 50 s−1) to be much lower than fΔP and fCa. Therefore, the stimuli-responsive measurements of the chemistrode are a coalescence-limited process.
The interaction of stimuli plugs with the chemically active surface and the reassembly of exiting response plugs are divided into four steps (Figure 18.2). In the first step, the stimuli plugs approach the hydrophilic substrate from above at a constant velocity, the sole major contributor to plug hydrodynamics at this early juncture. Next, the fluorous carrier fluid surrounding the aqueous droplet begins to drain, with a diminution of the plug velocity as the kinetic energy is transformed into a distortion of the plug shape. As the plug advances ever closer to contacting the hydrophilic substrate, van der Waals and other forces like hydrogen bonding at the interface between the wetting layer and the plug begin to destabilize it, leading to film breakdown. Film rupture is a very rapid event without surfactants. The presence of surfactants such as RfOEG, however, will tend to stabilize the interface. Film rupture also slows down due to surfactant molecule reorientation. Film rupture, which is essential for mass transfer, is also counterbalanced by surfactant concentration. Once chemical exchange of stimuli and response molecules occurs, plugs begin to reassemble as interfacial forces dominate once again. The response plugs can now be analyzed by offline analytical tools.
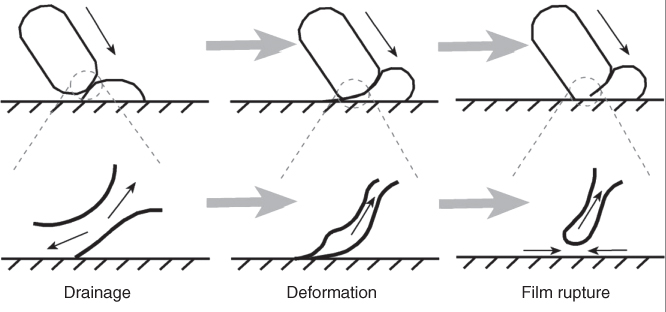
Figure 18.2 The stages of plug coalescence with the hydrophilic substrate. After traversing the first half of the V-shaped channel (first step, not shown), the stimuli plugs come in close proximity with the substrate, so that the fluorinated carrier fluid begins to drain between the plug and the wetting layer (second step, left). Next, as the distance diminishes, the plug deforms (third step, middle) until surface tension forces cause the film to finally rupture (fourth step, right) and the aqueous phase to reassemble into exiting response plugs.
Two distinct regimes have been observed with plug deformation and fluid drainage based on the value of Ca: (i) At low Ca the plugs begin to rupture at their noses. The film breakdown occurs rapidly compared with the change in the droplet shape, which leads to very little plug deformation. (ii) At moderate Ca the plug rupture is induced by the wetting surface and occurs on the substrate. The plug shape can be reproducibly generated in this regime by maintaining the same Ca. In each regime Ca correlates linearly with the coalescence time (Figure 18.3).

Figure 18.3 Plug coalescence dynamics. (a) Plug coalescence time as a function of Ca. Two regimes have been identified. In each regime, plug coalescence time correlates linearly with Ca. (b) Confocal microscopy image of plug shape immediately prior to wetting layer-induced rupture. Contrast differences between the wetting layer and the incoming stimuli plug reveal the interface (represented as a dotted line). (c) Traces of several experiments at equivalent Ca demonstrate reproducible dynamics. Changing single physical parameters like the interfacial tension but maintaining the same Ca leads to repeatability in the contour of the interface.
Above a critical Ca (Cac), the plugs no longer coalesce at the wetting layer, and molecular information is not acquired from the chemical surface. The viscosity ratio λ was not consequential in determining the failure for the plugs to coalesce at the substrate [40].
18.3.3 Mass Transfer at the Hydrophilic Substrate
The design of the two-phase chemistrode includes circulation during plug coalescence with the substrate, which overcomes the limiting behavior of molecular diffusion during mass transfer. If the sampling frequency is 50 s−1, the estimated diffusion length of glucose in aqueous solution is only 3–4 µm. Single-phase laminar-flow devices are largely precluded from stimulating and recording molecular signals as a consequence of this diffusion limitation. Moreover, molecular transport adjacent to the solid boundaries is not completely efficient. Plug coalescence with the substrate causes circulation and convection, which can sweep the chemical signals generated by/from the active surface into the plugs. The mass transfer was visualized using fluorescent markers and microscopy [40]. Despite the facility of microscopy, fluorescence detection is often preferable, which is able to more robustly visualize the transport phenomena of small molecules. Mass transfer of macromolecules and proteins is an even greater challenge, not only because of their significantly reduced diffusivity but also because polypeptides participate in numerous noncovalent associations or interactions with each other and the substrate.
To specifically investigate the mass transfer at the hydrophilic substrate total internal reflection fluorescence microscopy (TIRFM) has been employed [38]. An inherent advantage of TIRFM over other imaging techniques including confocal microscopy is that features O (100 nm) such as recirculating flows at the thin layer on the experimental surface can be readily resolved with appropriate markers. The sets of observations and measurements under the TIRFM have verified that the surface was reliably and reproducibly saturated by the plugs across a range of possible operation conditions [38]. The experimental protocol is outlined as follows: a glass Petri dish was initially rendered hydrophilic by plasma exposure and mounted on the microscope's sample stage. A chemistrode was then contacted with the hydrophilic glass surface. To ensure that efficient mass transfer was even permissible, as a control, a single-phase aqueous solution of Alexa Fluor 488 in PBS was first infused into the chemistrode with no fluorinated hydrocarbon present past the glass surface for several minutes. The fluorescence intensity was subsequently measured to determine the maximum fluorescence coverage of the glass surface. After infusion of the fluorescent dye for several minutes, the hydrophilic substrate was saturated completely with the fluorescent signal.
Mass transfer at the thin liquid layer near the solid substrate was systematically explored across a variety of operating conditions by varying Ca and Pe through changes in the flow velocity, dynamic viscosity, and interfacial tension. Five consecutive stimuli plugs with the dye (Alexa Fluor 488 in PBS buffer) were infused past the hydrophilic substrate, followed by 10 consecutive buffer plugs with no fluorescent signals. The fluorescence intensity was then measured to determine the saturation of the substrate surface. The dynamic viscosity of the aqueous plugs was increased by increasing the weight fraction of glycerol, and the interfacial tension was altered by varying the RfOEG concentration (Figure 18.4). The number of plugs to reach complete (or nearly complete, ≥90%) saturation was always less than three regardless of Ca or Pe. Capillary numbers extending across many orders of magnitude were tested, and although the hydrophilic substrate could always be reproducibly saturated with Alexa Fluor 488, a weakly log-linear correlation was observable between Ca (and Pe) and the number of plugs that were required to saturate the glass surface.
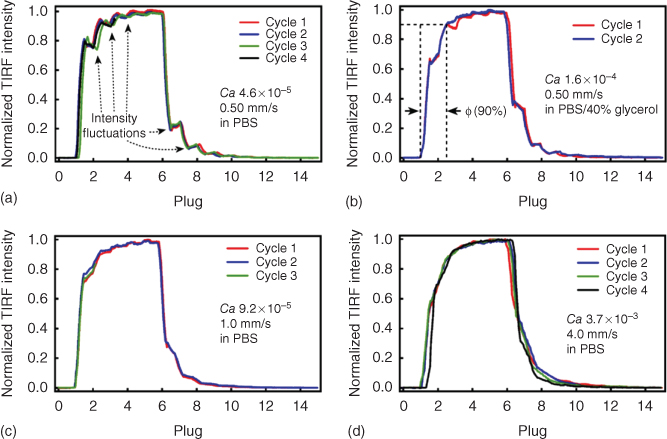
Figure 18.4 Saturation of the glass surface as a function of Ca. Fluorescence intensity fluctuations at low values of Ca, as in (a–c), indicate that eddies of fluorescent molecules are recirculating from the boundary of the wetting layer to the center, facilitating chemical exchange. Traces of various colors represent repeated experiments and demonstrate that saturation of the surface is highly reproducible. At higher values of Ca O (>10−5) as in (d), the fluorescent intensity oscillations are no longer present as mixing became slightly more efficient at the wetting layer.
At low values of Ca O (10−5), the fluorescence signal detected by TIRFM oscillated as the stimuli plugs filled with Alexa Fluor 488 coalesced with the glass surface and exchanged fluorescent signal at the wetting layer. Video recordings corroborate that this phenomenon is due to recirculation of the fluorescent signals from the wetting layer boundary to its center. When the viscous forces were increased relative to the interfacial tension, the recirculating flows began to diminish and then completely disappeared at higher values of Ca as mass transfer became complete and more efficient. Therefore, the chemistrode is capable of stimulating and recording chemical signals over a range of possible operating conditions such as flow velocity, fluid dynamic viscosity, and interfacial tension, among other physical parameters, without regard to variable mass transfer occurring at the hydrophilic substrate.
18.4 Multiform Chemical Analysis Independent in Space and Time from Data Acquisition
18.4.1 Online Analysis
The chemistrode is capable of stimulating chemically interactive substrates and recording molecular signals in real time using techniques that can be integrated with microfluidic devices. Optical imaging methods such as confocal microscopy and TIRFM can be employed to visualize the chemical and biological response of the surface (such as Ca2+ response of secreting live cells), as well as mass transfer processes upon stimuli plug coalescence as detailed in the previous section [37, 40].
Photon scattering techniques and surface plasmon resonance (SPR) can also be used to detect the surface response upon stimulation [46]. It is envisioned that the chemistrode could be integrated with real-time SPR in the following manner: the V-shaped channel of the chemistrode would be contacted with a hydrophilic, radiative substrate functionalized with an antibody. An array of aqueous stimuli plugs would be loaded with the diffusible antigens of interest. Then, a laser placed under the hydrophilic substrate would produce a surface wave when antigen–antibody binding occurred, which would subsequently be measured and characterized [47]. This potential concept manifests promise as a future tool in drug discovery and lead optimization, immunoassays, and disease diagnosis.
18.4.2 Parallel Offline Analysis
One of the biggest advantages of the chemistrode comes from its reliable, high chemical resolution and parallel offline analysis capability. As the chemistrode and other open space microfluidic devices are further developed from their initial prototypes, these tools may have potentially sweeping ramifications in the manner in which analytical chemistry and biology is conducted, totally transforming the manner in which molecular data is recorded, interpreted, shared between collaborators, and promulgated to the scientific community and the public at large.
It frequently occurs that time-resolved data emanating from chemically communicating substrates cannot be sufficiently assimilated or comprehended by the device operator. To gain a complete portrait of the molecular information exchange requires the application of downstream analytical tools in divergent scientific and engineering fields with experts trained in these specializations. As such, it is near impossible to extract much meaningful information on the chemical crosstalk if the time-resolved data must be analyzed and interpreted immediately by the device operator and cannot be shared with specialists with differing expertise in other fields. The chemistrode is additionally advantageous in that it separates the necessity for the data to be analyzed in that very moment at the same location at which it was collected. This is especially significant, as highly trained data interpreters are often dispersed across a country or even globally. The response plugs can be physically transported safely and routinely potentially internationally, meaning that the molecular data can be rendered available to scientific collaborators. These previously non-extracted data can be subsequently promulgated to the scientific community. The chemistrode, therefore, offers new ways to disseminate this molecular information to the world, which is unprecedented in its spatial and temporal resolution.
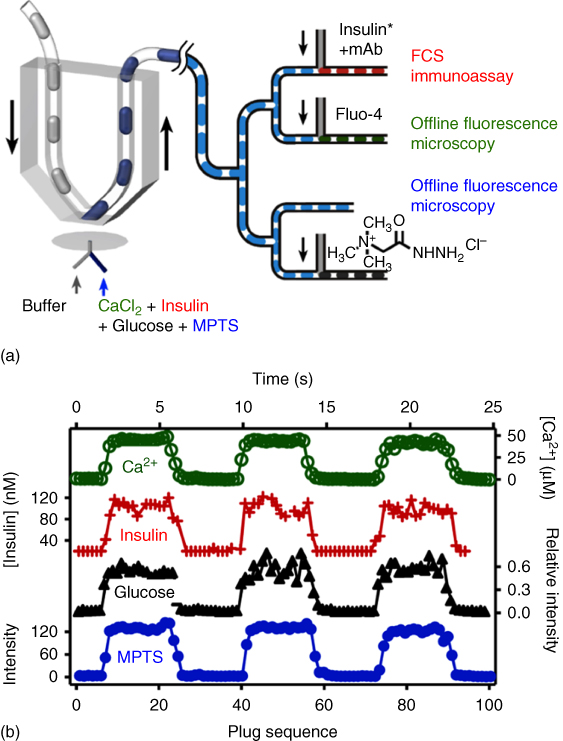
Figure 18.5 Decoding chemical signals with high temporal and chemical resolution with offline analysis. A diverse array of chemical signals can be analyzed in parallel at times and locations independent from their initial data acquisition. In the instance shown above, CaCl2, insulin, glucose, and MPTS analytes are flowed to a hydrophilic PDMS substrate in contact with a chemistrode. Response plugs containing the molecular species are generated and prepared for offline analysis. The exiting channel of the chemistrode branches twice in succession to generate four equivalent arrays of cloned daughter plugs, which are subjected to FCS coupled with an insulin immunoassay, MALDI-TOF-MS, and fluorescence microscopy.
The stimuli and response plugs can be stored with the signal preserved for days at the very least without significant attenuation (and even much longer if evaporation could be completely prevented), allowing for a myriad of disparate analytical platforms to be performed. More importantly, the plugs can be divided into ensembles of identical daughter arrays to be utilized for various analytical tools for detection of the same or different molecules (Figure 18.5). Therefore, a parallel comparison can be possible to characterize the full spectrum of molecular events. The response plugs are directed to a T-shaped junction, where the droplets and their chemical signals are bifurcated into two newly created daughter plugs, which can be subject to distinct offline analysis techniques or further bifurcated at later T-junctions downstream. In this fashion, the sum of molecular signals in individual response plugs is tremendously amplified for a diverse spectrum of analyses simultaneously while not interfering with other discrete sets of data from the substrate or diminishing resolution. Conventional or more advanced analytical tools can be applied conveniently to the plugs, such as fluorescence correlation spectroscopy (FCS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), and immunological and biomedical assays detecting a host of antigens including proteins and viruses. The reagents can be reliably injected into each of the plugs when the plugs are passed by an injection section of the device (Figure 18.5).
Equally important is the development of an analytical chemical tool with the ability to characterize a diverse spectrum of chemical and biochemical species including ions and other low molecular weight aqueous moieties, small molecule organics such as pharmaceutical drugs, peptides and proteins, and nucleic acids. Biochemical networks function on multiple parallel chemical strata to precisely control and monitor molecular dynamics, responding to stimuli (both internal and external). For example, the coagulation cascade is an exquisitely regulated nonlinear network of serine proteases regulating hemostasis. However, the presence of calcium ions and numerous other cofactors (e.g., peptides) contributes to initiation and suppression of coagulation. Hemostasis therefore encompasses multivariate interdependent communication between a diverse class of chemical moieties, ensuring that the body responds swiftly and appropriately to potential trauma. Blood sugar regulation by pancreatic beta cells is another multilevel, orchestrated molecular communication event, whose molecular actors include glucose, insulin, Ca2+, and Zn2+. The Ismagilov research group has established that the microfluidic chemistrode is capable of such independent manifold analysis of these chemical and biological signals. The parallel analysis allows for the characterization of a myriad of molecular actors decoupled from their signal acquisition in time and space. In the demonstration given by Chen et al., one injection channel was used to mimic islet response upon glucose stimulation, containing an aqueous buffer with four chemically disparate analytes, namely, CaCl2, insulin, glucose, and the fluorescent dye 8-methoxypyrene-1,3,6-trisulfonic acid trisodium salt (MPTS) as a control. Response plugs were collected and were transported to two succeeding branch points and split into four identical arrays of daughter plugs (Figure 18.5). In one channel, the concentration of ionic calcium was characterized by interaction with the fluorogenic substrate fluo-4, followed by measurement of fluorescence intensity by confocal microscopy. In another channel, a monoclonal anti-insulin antibody was added, allowing for immunological testing to be conducted. In this instance, FCS was employed to quantitate antibody–antigen binding. Glucose concentration was quantitated in the third channel: glucose was allowed to react with Girard's reagent T [(carboxymethyl)trimethylammonium chloride hydrazide] for approximately 16 h. The reaction product, a hydrazone, was then subject to MALDI-TOF-MS to identify the presence of glucose the following day. The last channel served to validate the robustness of the experiment – the fluorescence of MPTS was quantitated as a control.

Figure 18.6 Chemistrode compatibility with live cell imaging and biological substrates. A single murine pancreatic islet of Langerhans was used as an experimental model to validate the compatibility of the chemistrode with biological substrates and its ability to stimulate and record chemical signals with high spatial and temporal fidelity. The islet of Langerhans was variably exposed to a KCl buffer containing either 2 or 14 mM glucose, and the intracellular calcium concentration [Ca]i was measured indirectly after binding to a fluorogenic substrate, fluo-4.
18.5 Applicability for Stimuli–Response Surfaces
18.5.1 Single Islet Cell Stimulation and Response Analysis
Considerable scientific endeavor in recent decades has been centered around developing technologies utilizing a small collection of cells or even only a single cell as a platform for broadening our knowledge of fundamental biological processes [48]. Significant consensus has emerged that traditional macroscale analytical techniques that observe millions of cells simultaneously diminish the ability for researchers to investigate the biochemical heterogeneity of the cell population at a reasonable level of granularity. In other words, individual cells of the same species, even of the same cellular population responding to equivalent environmental stressors, were once thought to be largely identical and finely tuned bioreactors responding to stimuli in exactly the same fashion. However, new evidence suggests that individual cells may exhibit divergent, if even minute but still reasonably distinct, genotypic and phenotypic expression profiles from each other [49]. Conventional techniques thus only present a statistical average of the cellular response despite the presence of this heterogeneity, and much significant information could potentially be gained by interrogating networks of individual cellular responses to discern previously unrecognizable molecular signals that were obscured by this statistical averaging [50]. Ultimately, real-time analysis of single cells will lead to revolutionary insights into the understanding, design, and function of complex integrated biochemical systems and lead to a new generation of biomolecular diagnostic devices (Figure 18.6).
The microfluidic chemistrode is an instrument ideally suited toward such live data imaging and analysis of individual cells and provides meaningful progress toward the goal of comprehending the manner in which complex biochemical networks operate at the most fundamental level. Diabetes mellitus, a manifestation of pathologically attenuated insulin secretion and blood sugar dysregulation, is rapidly becoming a significant burden to the global healthcare system with aging populations and changes in lifestyle and diet choices strongly contributing to increased risk for disease development [51]. Insulin secretion by pancreatic beta cells into the circulation and the chemical signal's subsequent propagation is of much interest for understanding cell function. The Ismagilov research group applied the chemistrode to an experimental model consisting of a single pancreatic islet of Langerhans to correlate the complex molecular dynamics during islet response upon glucose stimulation. The islet of Langerhans was alternately exposed to a KCl buffer containing 2 and 14 mM glucose, and the intracellular calcium concentration, [Ca]i, was measured via binding to a fluorogenic substrate, fluo-4. Glucose and insulin were quantified by highly sensitive and selective methods (such as FCS and MALDI-TOF-MS as described in the previous section). This measurement on the single islet of Langerhans reveals that insulin secretion is more likely responding to the glucose concentration gradient instead of the sustained high glucose level. More importantly, the high sub-second temporal and molecular-level chemical resolution allowed the chemistrode to distinguish the characteristic oscillation events, which were hypothesized but not experimentally proved previously.
18.5.2 Isolation and Incubation of Individual Cells from Multispecies Mixtures
A dramatic surge recently in molecular biological innovations enabling for the isolation, sequencing, and manipulation of organisms' genomes with novel techniques (such as polymerase chain reaction [52], fluorescence in situ hybridization (FISH) [53], and high-throughput sequencing methods [54]) has rekindled substantial curiosity in cataloging metagenomic data on bacterial species populating a variety of ecological habitats. Isolation of genetic material from certain organisms in these challenging or unique environments may be fruitful in the creation of recombinant microbes useful for waste remediation [55], renewable energy [56], and biopharmaceutical production [57], among a myriad of other possible applications.
Despite these advancements in genetic manipulation and analysis, the protocol for selecting the desired genetic material in these heterogeneous microbial populations from the environment is complicated. The more robust species (i.e., those that possess a greater degree of fitness in terms of natural selection) typically will have its genetic, metabolomic, and proteomic products overwhelmingly skew the metadata, preventing rare species and its chemical actors from being detected. Moreover, techniques like FISH do not preserve live organisms for further downstream manipulation. Operation of the chemistrode overcomes these limitations when it is combined with stochastic confinement, a process defined here to be the individual isolation of live cells in the chemistrode's aqueous plugs. This therefore allows for the extraction of metagenomic data of interest and isolation of live specimens from microbial populations from a variety of niche habitats. A heterogeneous microbial extract cultured on a dish to form a confluent monolayer or microbial slimes like muddy or sandy slurries taken from the forest floor or the ocean bottom can be contacted with the chemistrode.
Stochastic confinement and the chemistrode were previously utilized together on a mixture of Escherichia coli and Paenibacillus curdlanolyticus [39]. The microbial suspension was introduced into the aqueous plugs and flowed through the chemistrode microchannel to the substrate. The segregated small colonies often contained only a few cells. The individual plugs were then incubated, allowing for the progenitor cells to multiply. This allowed for amplification of P. curdlanolyticus, the species of interest in this study, and its gene products, to be facilely detected since E. coli multiplies much more robustly and would outcompete P. curdlanolyticus in a traditional two-species cultivation protocol. Branching of the microchannel then allowed for the aqueous plugs to partition into daughter arrays, so that multiple platforms of chemical analysis could be performed, including cellulase assays, Gram staining, and tests that sacrifice cells like single-cell FISH, while simultaneously preserving live specimens. Future applications of the chemistrode in this arena could extend to generating metagenomes of diverse environmental habitats to screen for early-stage neoplasms and to evaluate rare bacterial samples.
18.6 Challenges and Future Directions
The power to sample chemical and biological data rapidly on timescales approximating cellular responses and further the capacity to store that information without any significant losses represent meaningful progress toward engineering a device capable of deconvoluting the molecular actors responsible for chemical communication in complex biological networks, such as the immune system and the coagulation cascade. Such an analytical tool would enable researchers to gain snapshots of these chemical and biological networks in time after a stimulus has been applied. Moreover, it would completely decouple these time points with data interpretation due to the lack of any signal decay, providing greater access to disparate analytical techniques that may not be immediately available at the time of recording.
The chemistrode, with its potential to stimulate, sample, and record chemically interactive surfaces at unprecedented chemical, spatial, and temporal resolution, fundamentally transforms the manner in which a diverse set of experimental systems can be investigated with chemical tools. For example, differentiated ex vivo tissue sections could be investigated in real time with the simultaneous deployment of numerous chemistrodes operating in synchrony, each device stimulating or recording from different cell types, resolving the chemical communication between the cells. A chemistrode integrated with SPR could take a botanical specimen and conduct a high-throughput screening for natural products with pharmacologic effects, greatly accelerating drug discovery efforts. Or the device could even be coupled with offline immunoassays to screen for the initial signs of cancer. However, all the demonstrations are currently in vitro. In order to use the chemistrode for in vivo measurements, several problems need to be solved: a membrane may need to be attached to the tip of the device to avoid losing cells into the plugs. More essential, operation conditions for reliable delivery and reassembly of plugs need to be addressed for individual systems, and fine adjustments to the chemistrode architecture are also necessary for minimally invasive processes. Moreover, the chemistrode can only stimulate and record diffusible aqueous signals. A sizeable fraction of molecular actors in complex biological systems are lipophilic or amphipathic and would be undeliverable in the chemistrode's aqueous plugs. Despite these formidable design challenges, the chemistrode's recent successful experimental applications demonstrate its role in comprehending complex chemically interactive systems at the molecular scale with unparalleled chemical, spatial, and temporal resolution.
Acknowledgments
The authors are greatly indebted to Rustem Ismagilov for his invaluable mentorship. We would also like to thank the former members of the Ismagilov research group, especially Wenbin Du, Delai Chen, and Weishan Liu, for assistance in creating figures.
References
- 1 Hodgkin, A.L., Huxley, A.F., and Katz, B. (1952) Measurement of current–voltage relations in the membrane of the giant axon of Loligo. J. Physiol., 116 (4), 424–448.
- 2 Hodgkin, A.L. and Huxley, A.F. (1952) Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol., 116 (4), 449.
- 3 Schwiening, C.J. (2012) A brief historical perspective: Hodgkin and Huxley. J. Physiol., 590 (11), 2571–2575.
- 4 Sigworth, F.J. (1980) Single Na+ channel currents. Nature, 287, 447.
- 5 Neher, E., Sakmann, B., and Steinbach, J.H. (1978) The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflügers Arch., 375 (2), 219–228.
- 6 Sakmann, B. and Neher, E. (1984) Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol., 46 (1), 455–472.
- 7 Spruston, N., Schiller, Y., Stuart, G., and Sakmann, B. (1995) Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science, 268 (5208), 297.
- 8 DiFrancesco, D. (1986) Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature, 324, 470–473.
- 9 Xia, Y. and Whitesides, G.M. (1998) Soft lithography. Annu. Rev. Mater. Sci., 28 (1), 153–184.
- 10 Kane, R.S., Takayama, S., Ostuni, E., Ingber, D.E., and Whitesides, G.M. (1999) Patterning proteins and cells using soft lithography. Biomaterials, 20 (23), 2363–2376.
- 11 Whitesides, G.M., Ostuni, E., Takayama, S., Jiang, X., and Ingber, D.E. (2001) Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng., 3 (1), 335–373.
- 12 Qin, D., Xia, Y., and Whitesides, G.M. (2010) Soft lithography for micro-and nanoscale patterning. Nat. Protoc., 5 (3), 491–502.
- 13 Anderson, J.R., Chiu, D.T., Wu, H., Schueller, O., and Whitesides, G.M. (2000) Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis, 21 (1), 27–40.
- 14 Unger, M.A., Chou, H.-P., Thorsen, T., Scherer, A., and Quake, S.R. (2000) Monolithic microfabricated valves and pumps by multilayer soft lithography. Science, 288 (5463), 113–116.
- 15 Quake, S.R. and Scherer, A. (2000) From micro-to nanofabrication with soft materials. Science, 290 (5496), 1536–1540.
- 16 Ohno, K.i., Tachikawa, K., and Manz, A. (2008) Microfluidics: applications for analytical purposes in chemistry and biochemistry. Electrophoresis, 29 (22), 4443–4453.
- 17 Brouzes, E., Medkova, M., Savenelli, N., Marran, D., Twardowski, M., Hutchison, J.B., Rothberg, J.M., Link, D.R., Perrimon, N., and Samuels, M.L. (2009) Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. U.S.A., 106 (34), 14195–14200.
- 18 Shi, W., Qin, J., Ye, N., and Lin, B. (2008) Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip, 8 (9), 1432–1435.
- 19 Joensson, H.N. and Andersson Svahn, H. (2012) Droplet microfluidics – a tool for single-cell analysis. Angew. Chem. Int. Ed., 51 (49), 12176–12192.
- 20 Köster, S., Angile, F.E., Duan, H., Agresti, J.J., Wintner, A., Schmitz, C., Rowat, A.C., Merten, C.A., Pisignano, D., and Griffiths, A.D. (2008) Drop-based microfluidic devices for encapsulation of single cells. Lab Chip, 8 (7), 1110–1115.
- 21 Srisa-Art, M., Dyson, E.C., de Mello, A.J., and Edel, J.B. (2008) Monitoring of real-time streptavidin−biotin binding kinetics using droplet microfluidics. Anal. Chem., 80 (18), 7063–7067.
- 22 Köster, S., Evilevitch, A., Jeembaeva, M., and Weitz, D.A. (2009) Influence of internal capsid pressure on viral infection by phage λ. Biophys. J., 97 (6), 1525–1529.
- 23 Schmitz, C.H., Rowat, A.C., Köster, S., and Weitz, D.A. (2009) Dropspots: a picoliter array in a microfluidic device. Lab Chip, 9 (1), 44–49.
- 24 Günther, A. and Jensen, K.F. (2006) Multiphase microfluidics: from flow characteristics to chemical and materials synthesis. Lab Chip, 6 (12), 1487–1503.
- 25 Ottino, J.M. and Wiggins, S. (2004) Introduction: mixing in microfluidics. Philos. Trans. R. Soc. Lond. Ser. A, 362, 923–935.
- 26 Beard, D. and Taylor, A. (2001) Dispersion of a solute in a microfluidic channel. J. Appl. Phys., 89 (8), 4667–4669.
- 27 Kennedy, R.T., Thompson, J.E., and Vickroy, T.W. (2002) In vivo monitoring of amino acids by direct sampling of brain extracellular fluid at ultralow flow rates and capillary electrophoresis. J. Neurosci. Methods, 114 (1), 39–49.
- 28 Cellar, N.A., Burns, S.T., Meiners, J.-C., Chen, H., and Kennedy, R.T. (2005) Microfluidic chip for low-flow push–pull perfusion sampling in vivo with on-line analysis of amino acids. Anal. Chem., 77 (21), 7067–7073.
- 29 Stuart, J.N., Hummon, A.B., and Sweedler, J.V. (2004) Peer reviewed: the chemistry of thought: neurotransmitters in the brain. Anal. Chem., 76 (7), 120 A–128 A.
- 30 Adamo, A. and Jensen, K.F. (2008) Microfluidic based single cell microinjection. Lab Chip, 8 (8), 1258–1261.
- 31 Dittrich, P.S. and Manz, A. (2006) Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug Discovery, 5 (3), 210–218.
- 32 Janasek, D., Franzke, J., and Manz, A. (2006) Scaling and the design of miniaturized chemical-analysis systems. Nature, 442 (7101), 374–380.
- 33 Chow, A.W. (2002) Lab-on-a-chip: opportunities for chemical engineering. AlChE J., 48 (8), 1590–1595.
- 34 Miller, O.J., El Harrak, A., Mangeat, T., Baret, J.-C., Frenz, L., El Debs, B., Mayot, E., Samuels, M.L., Rooney, E.K., and Dieu, P. (2012) High-resolution dose–response screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. U.S.A., 109 (2), 378–383.
- 35 Theberge, A.B., Courtois, F., Schaerli, Y., Fischlechner, M., Abell, C., Hollfelder, F., and Huck, W.T. (2010) Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed., 49 (34), 5846–5868.
- 36 Fidalgo, L.M., Whyte, G., Bratton, D., Kaminski, C.F., Abell, C., and Huck, W.T. (2008) From microdroplets to microfluidics: selective emulsion separation in microfluidic devices. Angew. Chem. Int. Ed., 47 (11), 2042–2045.
- 37 Chen, D., Du, W., Liu, Y., Liu, W., Kuznetsov, A., Mendez, F.E., Philipson, L.H., and Ismagilov, R.F. (2008) The chemistrode: a droplet-based microfluidic device for stimulation and recording with high temporal, spatial, and chemical resolution. Proc. Natl. Acad. Sci. U.S.A., 105 (44), 16843.
- 38 Chen, D., Du, W., and Ismagilov, R.F. (2009) Using TIRF microscopy to quantify and confirm efficient mass transfer at the substrate surface of the chemistrode. New J. Phys., 11 (7), 075017.
- 39 Liu, W., Kim, H.J., Lucchetta, E.M., Du, W., and Ismagilov, R.F. (2009) Isolation, incubation, and parallel functional testing and identification by FISH of rare microbial single-copy cells from multi-species mixtures using the combination of chemistrode and stochastic confinement. Lab Chip, 9 (15), 2153–2162.
- 40 Liu, Y. and Ismagilov, R.F. (2009) Dynamics of coalescence of plugs with a hydrophilic wetting layer induced by flow in a microfluidic chemistrode. Langmuir, 25 (5), 2854–2859.
- 41 Kaigala, G.V., Lovchik, R.D., and Delamarche, E. (2012) Microfluidics in the “open space” for performing localized chemistry on biological interfaces. Angew. Chem. Int. Ed., 51 (45), 11224–11240.
- 42 Roach, L.S., Song, H., and Ismagilov, R.F. (2005) Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal. Chem., 77 (3), 785–796.
- 43 Baret, J.-C. (2012) Surfactants in droplet-based microfluidics. Lab Chip, 12 (3), 422–433.
- 44 Tice, J.D., Song, H., Lyon, A.D., and Ismagilov, R.F. (2003) Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir, 19 (22), 9127–9133.
- 45 Bird, R.B., Stewart, W.E., and Lightfoot, E.N. (1960) Transport Phenomena, John Wiley & Sons, Inc., New York, 413.
- 46 Ghosh, T., Xie, Y., and Mastrangelo, C. (2013) A droplet-based novel approach for viable and low volume consumption surface plasmon resonance bio-sensing inside a polydimethylsiloxane microchip. Biomicrofluidics, 7 (4), 044122.
- 47 Pattnaik, P. (2005) Surface plasmon resonance. Appl. Biochem. Biotechnol., 126 (2), 79–92.
- 48 Andersson, H. and van den Berg, A. (2004) Microtechnologies and nanotechnologies for single-cell analysis. Curr. Opin. Biotechnol., 15 (1), 44–49.
- 49 Lecault, V., White, A.K., Singhal, A., and Hansen, C.L. (2012) Microfluidic single cell analysis: from promise to practice. Curr. Opin. Chem. Biol., 16 (3), 381–390.
- 50 Altschuler, S.J. and Wu, L.F. (2010) Cellular heterogeneity: do differences make a difference? Cell, 141 (4), 559–563.
- 51 Tuomilehto, J., Lindström, J., Eriksson, J.G., Valle, T.T., Hämäläinen, H., Ilanne-Parikka, P., Keinänen-Kiukaanniemi, S., Laakso, M., Louheranta, A., and Rastas, M. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med., 344 (18), 1343–1350.
- 52 Ottesen, E.A., Hong, J.W., Quake, S.R., and Leadbetter, J.R. (2006) Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science, 314 (5804), 1464–1467.
- 53 Liu, P., Meagher, R.J., Light, Y.K., Yilmaz, S., Chakraborty, R., Arkin, A.P., Hazen, T.C., and Singh, A.K. (2011) Microfluidic fluorescence in situ hybridization and flow cytometry (μFlowFISH). Lab Chip, 11 (16), 2673–2679.
- 54 Shembekar, N., Chaipan, C., Utharala, R., and Merten, C.A. (2016) Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip, 16 (8), 1314–1331.
- 55 Sayler, G.S. and Ripp, S. (2000) Field applications of genetically engineered microorganisms for bioremediation processes. Curr. Opin. Biotechnol., 11 (3), 286–289.
- 56 Stephanopoulos, G. (2007) Challenges in engineering microbes for biofuels production. Science, 315 (5813), 801–804.
- 57 Chang, M.C. and Keasling, J.D. (2006) Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol., 2 (12), 674–681.
