10.4.1 Deducing a Rate Law from the Experimental Data
Assuming that the reaction is essentially irreversible (which is reasonable after comparing runs 3 and 5), we ask what qualitative conclusions can be drawn from the data about the dependence of the rate of disappearance of toluene, ![]() , on the partial pressures of toluene, hydrogen, methane, and benzene.
, on the partial pressures of toluene, hydrogen, methane, and benzene.
![]()
- Dependence on the product methane. If methane were adsorbed on the surface, the partial pressure of methane would appear in the denominator of the rate expression and the rate would vary inversely with methane concentration:
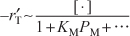
However, from runs 1 and 2 we observe that a fourfold increase in the pressure of methane has little effect on
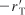 . Consequently, we assume that methane is either very weakly adsorbed (i.e.,
. Consequently, we assume that methane is either very weakly adsorbed (i.e., 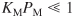 ) or goes directly into the gas phase in a manner similar to propylene in the cumene decomposition previously discussed.
) or goes directly into the gas phase in a manner similar to propylene in the cumene decomposition previously discussed.
- Dependence on the product benzene. In runs 3 and 4, we observe that, for fixed concentrations (partial pressures) of hydrogen and toluene, the rate decreases with increasing concentration of benzene. A rate expression in which the benzene partial pressure appears in the denominator could explain this dependency:

The type of dependence of
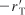 on PB given by Equation (10-68) suggests that benzene is adsorbed on the clinoptilolite surface.
on PB given by Equation (10-68) suggests that benzene is adsorbed on the clinoptilolite surface. - Dependence on toluene. At low concentrations of toluene (runs 10 and 11), the rate increases with increasing partial pressure of toluene, while at high toluene concentrations (runs 14 and 15), the rate is essentially independent of the toluene partial pressure. A form of the rate expression that would describe this behavior is
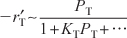
A combination of Equations (10-68) and (10-69) suggests that the rate law may be of the form

- Dependence on hydrogen. When we examine runs 7, 8, and 9 in Table 10-6, we see that the rate increases linearly with increasing hydrogen concentration, and we conclude that the reaction is first order in H2. In light of this fact, hydrogen is either not adsorbed on the surface or its coverage of the surface is extremely low (1 » KH2PH2) for the pressures used. If H2 were adsorbed,
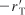 would have a dependence on PH2 analogous to the dependence of
would have a dependence on PH2 analogous to the dependence of 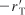 on the partial pressure of toluene, PT [see Equation (10-69)]. For first-order dependence on H2,
on the partial pressure of toluene, PT [see Equation (10-69)]. For first-order dependence on H2,

Combining Equations (10-67) through (10-71), we find that the rate law
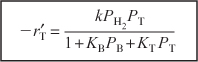
is in qualitative agreement with the data shown in Table 10-6.
