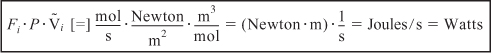11.2.2 Evaluating the Work Term
It is customary to separate the work term, ![]() , into flow work and other work,
, into flow work and other work, ![]() . The term
. The term ![]() , often referred to as the shaft work, could be produced from such things as a stirrer in a CSTR or a turbine in a PFR. Flow work is work that is necessary to get the mass into and out of the system. For example, when shear stresses are absent, we write
, often referred to as the shaft work, could be produced from such things as a stirrer in a CSTR or a turbine in a PFR. Flow work is work that is necessary to get the mass into and out of the system. For example, when shear stresses are absent, we write
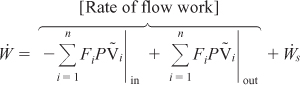
where P is the pressure (Pa) [1 Pa = 1 Newton/m2 = 1 kg·m/s2/m2] and ![]() is the specific molar volume of species i (m3/mol of i).
is the specific molar volume of species i (m3/mol of i).
Let’s look at the units of the flow work term, which is
![]()
where Fi is in mol/s, P is Pa (1 Pa = 1 Newton/m2), and ![]() is m3/mol.
is m3/mol.
We see that the units for flow work are consistent with the other terms in Equation (11-3), i.e., J/s.
In most instances, the flow work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries. Substituting Equation (11-4) into (11-3) and grouping terms, we have
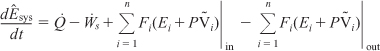
The energy Ei is the sum of the internal energy (Ui), the kinetic energy ![]() , the potential energy (gzi), and any other energies, such as electric or magnetic energy or light:
, the potential energy (gzi), and any other energies, such as electric or magnetic energy or light:
![]()
In almost all chemical reactor situations, the kinetic, potential, and “other” energy terms are negligible in comparison with the enthalpy, heat transfer, and work terms, and hence will be omitted; that is,
![]()
We recall that the enthalpy, Hi (J/mol), is defined in terms of the internal energy Ui (J/mol), and the product ![]() (1 Pa·m3/mol = 1 J/mol):
(1 Pa·m3/mol = 1 J/mol):
![]()
Typical units of Hi are
![]()
Enthalpy carried into (or out of) the system can be expressed as the sum of the internal energy carried into (or out of) the system by mass flow plus the flow work:
![]()
Combining Equations (11-5), (11-7), and (11-8), we can now write the energy balance in the form

The energy of the system at any instant in time, Êsys, is the sum of the products of the number of moles of each species in the system multiplied by their respective energies. This term will be discussed in more detail when unsteady-state reactor operation is considered in Chapter 13.
We shall let the subscript “0” represent the inlet conditions. Unsubscripted variables represent the conditions at the outlet of the chosen system volume.
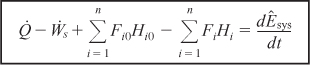
In Section 11.1, we discussed that in order to solve reaction engineering problems with heat effects, we needed to relate temperature, conversion, and rate of reaction. The energy balance as given in Equation (11-9) is the most convenient starting point as we proceed to develop this relationship.