B.4 To Enhance Creative Thinking Skills
The fourth goal of this book is to help enhance creative thinking skills. This goal will be achieved by using a number of problems that are open-ended to various degrees. Here the students can practice their creative skills by exploring the example problems, as outlined at the beginning of the home problems of each chapter, and by making up and solving an original problem. Problem P5-1 gives some guidelines for developing original problems. A number of techniques that can aid the students in practicing and enhancing their creativity can be found in Fogler and LeBlanc6 and its companion Web site, www.engin.umich.edu/scps, and in the Thoughts on Problem Solving section on the DVD-ROM and on the Web sites www.umich.edu/~essen and www.essentialsofCRE.com. We will use these techniques, such as Osborn’s checklist and de Bono’s lateral thinking (which involves considering other people’s views and responding to random stimulation) to answer add-on questions such as those in Table P-3.
Table P-3. Practicing Creative Thinking
One of the major goals at the undergraduate level is to bring students to the point where they can solve complex reaction problems, such as multiple reactions with heat effects, and then ask “What if . . .” questions and look for optimum operating conditions and unsafe operating conditions. One problem whose solution exemplifies this goal is the Manufacture of Styrene, Problem P12-24C. This problem is particularly interesting because two reactions are endothermic and one is exothermic.
(1) Ethylbenzene → Styrene + Hydrogen:
Endothermic
(2) Ethylbenzene → Benzene + Ethylene:
Endothermic
(3) Ethylbenzene + Hydrogen → Toluene + Methane:
Exothermic
To summarize Section B, it is the author’s experience that both critical and creative thinking skills can be enhanced by using Tables P-1, P-2, and P-3 to extend any of the homework problems at the end of every chapter.
C. The Structure
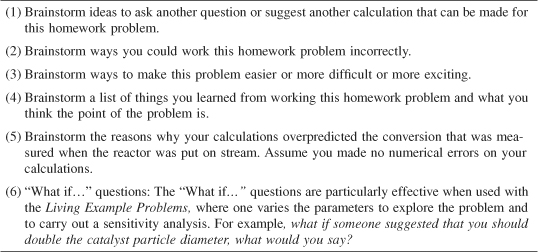
The strategy behind the presentation of material is to build continually on a few basic ideas in chemical reaction engineering to solve a wide variety of problems. These ideas, referred to as the Pillars of Chemical Reaction Engineering, are the foundation on which different applications rest. The pillars holding up the application of chemical reaction engineering are shown in Figure P-1.
Figure P-1. Pillars of Chemical Reaction Engineering.
From these pillars we construct our CRE algorithm:
Mole Balance + Rate Laws + Stoichiometry + Energy Balance + Combine
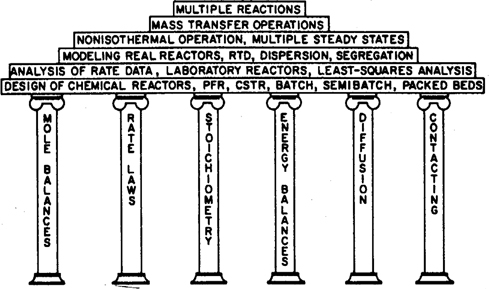
With a few restrictions, the contents of this book can be studied in virtually any order after students have mastered the first six chapters. A flow diagram showing the possible paths can be seen in Figure P-2.
Figure P-2. Sequences for studying the text.
The reader will observe that although metric units are used primarily in this text (e.g., kmol/m3, J/mol), a variety of other units are also employed (e.g., lbm/ft3, Btu). This choice is intentional! We believe that whereas most papers published today use the metric system, a significant amount of reaction engineering data exists in the older literature in English units. Because engineers will be faced with extracting information and reaction rate data from older literature as well as from the current literature, they should be equally at ease with both English and metric units.
The notes in the margins are meant to serve two purposes. First, they act as guides or commentary as one reads through the material. Second, they identify key equations and relationships that are used to solve chemical reaction engineering problems.
D. The Components of the DVD-ROM
The interactive DVD-ROM is a novel and unique part of this book. The main purposes of the DVD-ROM are to serve as an enrichment resource and as a professional reference shelf. The home page for the DVD-ROM and the CRE Web site (www.umich.edu/~essen) is shown in Figure P-3; also see the Web site www.essentialsofCRE.com.
Figure P-3. Screen shot of Web site (www.umich.edu/~essen) and DVD-ROM home page.

The objectives of the DVD-ROM are fourfold: (1) to facilitate the learning of CRE by using the DVD-ROM to actively address the Felder/Solomon Inventory of Learning Styles7 discussed in Appendix H; (2) to provide additional technical material; (3) to provide tutorial information and self-assessment exercises; and (4) to make the learning of CRE fun by using interactive games. The following components are listed at the end of most chapters and can be accessed from each chapter in the DVD-ROM.
• Learning Resources
The Learning Resources give an overview of the material in each chapter and provide extra explanations, examples, and applications to reinforce the basic concepts of chemical reaction engineering and are discussed further in Appendix E. The learning resources on the DVD-ROM include the following:
- Summary Notes
The Summary Notes give an overview of each chapter and provide on-demand additional examples, derivations, and audio comments, as well as self tests to assess each reader’s understanding of the material. We have included links to comical YouTube Videos made by students in Professor Alan Lane’s 2008 chemical reaction engineering class at the University of Alabama. Specifically, check out Fogler Zone (you’ve got a friend in Fogler) (Chapter 1), The Black Widow murder mystery and Baking a Potato by Bob the Builder and Friends (Chapter 3), CRF Reactor Video, Crimson Reactor Firm’s video of a “semi batch” reactor with Diet Coke and Mentos (Chapter 4), learn a new dance and song, CSTR to the tune of YMCA, and a rap song and Find Your Rhythm, an Ice Ice Baby remix (Chapter 5).
- Web Modules
The Web Modules, which apply key concepts to both standard and nonstandard reaction engineering problems (e.g., the use of wetlands to degrade toxic chemicals, and death from a cobra bite), can be loaded directly from the DVD-ROM. Additional Web Modules are expected to be added to the Web site (www.umich.edu/~essen) over the next several years.
- Interactive Computer Games (ICGs)
Students have found the Interactive Computer Games to be both fun and extremely useful to review the important chapter concepts and then apply them to real problems in a unique and entertaining fashion.
• Reactor Staging (Ch. 2)
• Quiz Show II (Ch. 4)
• Murder Mystery (Ch. 5)
• Tic Tac (Ch. 5)
• Ecology (Ch. 7)
• The Great Race (Ch. 8)
• Enzyme Man (Ch. 9)
• Catalysis (Ch. 10)
• Heat Effects I (Ch. 12)
• Heat Effects II (Ch. 12)
As the reader plays these interactive games, they will be asked a number of questions related to the corresponding material in the textbook. The computer will keep track of all the correct answers and at the end of the game will display a coded performance number that reflects how well the reader mastered the material in the text. Instructors will have a manual to decode the performance number.
- Solved Problems
A number of solved problems are presented along with problem-solving heuristics. Problem-solving strategies and additional worked example problems are available in the Problem Solving section of the DVD-ROM.
• Example Problems and Living Example Problems
The end of chapter problems numbered “2” (e.g., P3-2A, P11-2B) ask questions about the example problems in that chapter. These problems are a key resource. These number 2 problems should be worked before tackling the more challenging Home Problems in the chapter.

The example problems that use an ODE solver (e.g., Polymath) are referred to as “Living Example Problems” because students can load the Polymath program directly onto their own computers in order to study the problem. Students are encouraged to change parameter values and to “play with” the key variables and assumptions. Using the Living Example Problems to explore the problem and asking “What if...” questions provide students with the opportunity to practice critical and creative thinking skills.
• DVD Chapter Material
The DVD-ROM contains PDF files of the last five chapters from the fourth edition of the Elements of Chemical Reaction Engineering, which is mostly graduate material. These chapters, which were omitted from this book but are included on the DVD-ROM are: DVD Chapter 10, Catalyst Decay; DVD Chapter 11, External Diffusion Effects on Heterogeneous Reactions; DVD Chapter 12, Diffusion and Reaction; DVD Chapter 13, Distribution of Residence Times for Reactors; DVD Chapter 14, Models for Non Ideal Reactors; and a new chapter, DVD Chapter 15, Radial and Axial Temperature Variations in a Tubular Reactor.
• Professional Reference Shelf

This section of the DVD-ROM contains
- Material from the fourth edition of Elements of Chemical Reaction Engineering that is not included in the printed text of this book is included on the DVD-ROM.
- Material that is important to the practicing engineer, such as details of the industrial reactor design for the oxidation of SO2 and design of spherical reactors and other material that is typically not included in the majority of chemical reaction engineering courses.
• Software Toolbox on the DVD-ROM
Polymath. The Polymath software includes an ordinary differential equation (ODE) solver, a nonlinear equation solver, and nonlinear regression. As with previous editions, Polymath is used to explore the example problems and to solve the home problems. Polymath tutorials with screen shots are given on the DVD-ROM Summary Notes in Chapter 1 and can also be accessed from the Home Page by going to Living Example Problems and then clicking on Polymath. Most chemical engineering departments in the United States have site licenses for Polymath. If your department does not have a site license and would like one, have your instructor e-mail the CACHE Corporation at [email protected] to learn how to obtain one.
A special Polymath Web site (www.polymath-software.com/fogler) has been set up for this book by Polymath authors Cutlip and Shacham.
AspenTech. AspenTech is a process flow sheet simulator used in most senior chemical engineering design courses. It is now routinely introduced in earlier chemical engineering courses, such as thermodynamics, separations, and now in chemical reaction engineering (CRE). See the AspenTech Web site, www.aspentech.com. Like Polymath, AspenTech site licenses are available in most chemical engineering departments in the United States. Four AspenTech simulation examples specific to CRE are provided on the DVD-ROM with step-by-step tutorial screen shots.
As with Polymath programs, the input parameters can be varied to learn how they change the temperature and concentration profiles.
COMSOL.8 The COMSOL Multiphysics software is a partial differential equation solver that is used with DVD Chapter 15 to view both axial and radial temperature and concentration profiles. For users of this text, COMSOL has provided a special Web site that includes a step-by-step tutorial, along with examples. See www.comsol.com/ecre.
Further details of these three software packages can be found in Appendix E.
• Other DVD-ROM Resources
FAQs. The Frequently Asked Questions (FAQs) are a compilation of questions collected over the years from undergraduate students taking reaction engineering.
Visual Encyclopedia of Equipment. This section was developed by Dr. Susan Montgomery at the University of Michigan. Here, a wealth of photographs and descriptions of real and ideal reactors are given. Students with visual, active, sensing, and intuitive learning styles of the Felder/Solomon Index will particularly benefit from this section.
Reactor Lab (www.SimzLab.com). Developed by Professor Richard Herz at the University of California at San Diego, this interactive tool will allow students not only to test their comprehension of the CRE material, but also to explore different situations and combinations of reaction orders and types of reactions.
Green Engineering Home Problems. Green engineering problems for virtually every chapter have been developed by Professor Robert Hesketh at Rowan University and Professor Martin Abraham at the University of Toledo and these problems can be found at www.rowan.edu/greenengineering. These problems also accompany the book by David Allen and David Shonnard, Green Engineering: Environmentally Conscious Design of Chemical Processes (Prentice Hall, 2002).
Further information on how to use the DVD-ROM can be found in Appendix H.
E. The Web
The Web site (www.umich.edu/~essen or www.essentialsofCRE.com) will be used to update the text and the DVD-ROM. It will identify typographical and other errors in the first and later printings of Essentials of Chemical Reaction Engineering. In the near future, additional material may be added to include more solved problems, as well as additional Web Modules.
F. What’s New
A. Pedagogy. This book maintains all the strengths of the fourth edition of Elements of Chemical Reaction Engineering by using algorithms that allow students to learn chemical reaction engineering through logic rather than memorization. At the same time, it provides new resources that allow students to go beyond solving equations in order to get an intuitive feel and understanding of how reactors behave under different situations. This understanding is achieved through more than sixty interactive simulations provided on the DVD-ROM that is bound in the back of the book. The DVD-ROM has been greatly expanded to address the Felder/Solomon Inventory of Different Learning Styles9 through interactive Summary Notes and new and updated Interactive Computer Games (ICGs). For example, the Global Learner can get an overview of the chapter material from the Summary Notes; the Sequential Learner can use all the ![]() hot buttons; and the Active Learner can interact with the ICGs and use the
hot buttons; and the Active Learner can interact with the ICGs and use the ![]() hot buttons in the Summary Notes.
hot buttons in the Summary Notes.
A new pedagogical concept is introduced in this text through expanded emphasis on the example problems. Here, the students simply load the Living Example Problems (LEPs) onto their computers and then explore the problems to obtain a deeper understanding of the implications and generalizations before working the home problems for that chapter. This exploration helps students get an innate feel of reactor behavior and operation, as well as develop and practice their creative thinking skills. To develop critical thinking skills, instructors can assign one of the new home problems on troubleshooting, as well as ask the students to expand home problems by asking a related question that involves critical thinking using Tables P-1 and P-2. Creative thinking skills can be enhanced by exploring the example problems and asking “What if. . .” questions, by using one or more of the brainstorming exercises in Table P-3 to extend any of the home problems, and by working the open-ended problems. For example, in the case study on safety, students can use the DVD-ROM to carry out a post-mortem analysis on the nitroaniline explosion in Example 13-2 to learn what would have happened if the cooling had failed for five minutes instead of ten minutes. To this end, a new feature in the text is an Analysis paragraph at the end of each example problem. Significant effort has been devoted to developing example and home problems that foster critical and creative thinking.
B. Content. The following areas have received an a increased emphasis in Essentials over previous CRE editions by including thorough Example Problems and Home Problems on the following:
Safety: Three industrial explosions are discussed and modeled.
- Ammonium Nitrate CSTR Explosion (Chapters 12 and 13)
- Nitroaniline Batch Reactor Runaway (Chapter 13)
- T2 Laboratories Batch Reactor Runaway (Chapter 13)
- Resources from SAChE and CCPS (Chapter 12)
Solar Energy: Three examples of solar energy conversion are discussed.
- Solar Chemical Reactions (Chapter 3)
- Solar Thermal Reactors (Chapter 8)
- Solar Catalytic Water Spilling (Chapter 10)
Alternative Fuels:
- Production of Algae for Biomass (Chapter 9)
AspenTech:
An AspenTech tutorial for chemical reaction engineering and four example problems are provided on the DVD-ROM. The example problems are
- Production of Ethylene from Ethane
- The Pyrolysis of Benzene
- Adiabatic Liquid Phase Isomerization of Normal Butane
- Adabatic Production of Acetic Anhydride
G. Acknowledgments
There are so many colleagues and students who contributed to this book that it would require another chapter to thank them all in an appropriate manner. I again acknowledge all my friends, students, and colleagues for their contributions to the fourth edition of Elements of Chemical Reaction Engineering as well as this book, Essentials of Chemical Reaction Engineering (see Introduction, DVD-ROM). I give special recognition as follows.
First of all, I thank my colleague Dr. Nihat Gürmen, who coauthored the original CD-ROM and Web site. He has been a wonderful colleague to work with. I also would like to equally thank University of Michigan undergraduate student Maria Quigley. Maria has been working with me the last two-and-a-half years to convert the CD-ROM from the fourth edition of Elements of Chemical Reaction Engineering to the DVD-ROM in Essentials of Chemical Reaction Engineering. She also collected, typed, and organized more than 100 written reviews, critiques, and suggestions from the students who class-tested this book. Brendan Kirchner joined Maria the last eight months of the DVD development. Their hard work and suggestions are greatly appreciated, as is Mike Cutlip’s work to solve some critical issues as the DVD approached production.
Mike Cutlip, coauthor of Polymath, not only gave suggestions and a critical reading of many sections, but also, most importantly, provided continuous support and encouragement throughout the course of this project. Dr. Chau-Chyun Chen provided two AspenTech examples. Maria Quigley updated the AspenTech tutorial on the DVD-ROM, Professor Robert Hesketh of Rowan University provided an example for the DVD-ROM using COMSOL to solve partial differential equations with radial heat effects. Ed Fontes at COMSOL worked on and provided for the COMSOL Web site containing a tutorial and examples.
There are a number of people who need special mention. Bernard Goodwin, Prentice Hall publisher, was extremely encouraging, helpful, and supportive throughout. Julie Nahil, full-service production manager at Prentice Hall, provided encouragement, attention to detail, and a great sense of humor, which were greatly appreciated. Arjames Balgoa made a number of corrections to the first draft of this book, while Satinee Yindee provided a number of drawings for the various reactors. Vishal Chaudhary and Ravi Kapoor organized the first draft of the solutions manual during the summer of 2009. Manosij Basu, Akash Gupta, Sneh Shriyansh, and Utkarsh Prasad proofed and re-worked solutions for the solutions manual during the summer of 2010. Professor Carlos A. Ramírez of the University of Puerto Rico proofread the final draft of this book cover-to-cover and found many, many typographical errors. His attention to detail is a significant contribution to this book. Professor Lee Brown helped this project get off the ground with his support and input to the first edition of the Elements of Chemical Reaction Engineering.
I would like to thank Professor Alan Lane and the students at the University of Alabama for (1) class testing and providing comments on the draft copy of Essentials of Chemical Reaction Engineering, and (2) for the highly creative YouTube videos they developed on chemical reaction engineering, some of which we have linked to on the DVD-ROM. Professor David Doner and his students at the West Virginia University Institute of Technology also provided insightful comments and suggestions.
I am indebted to Ame and Catherine Vennema, whose gift of an endowed chair greatly helped in completing this project. The patience of all my Ph.D. students during the period in which this book was written, Hyun-Su Lee, Ryan Hartman, Kriangkrai Kraiwattanawong, Elizabeth Gorrepati, Michael Senra, Tabish Maqbool, Zhenyu Huang, Shanpeng Han, Michael Hoepfner, Nasim Haji Akbari Balou, and Oluwasegun Adegoke is greatly appreciated. Others I would like to thank for a variety of different reasons are Max Peters, Klaus Timmerhaus, Ron West, Joe Goddard, Jay Jorgenson, Stu Churchill, Emma Sundin, Susan Montgomery, Phil Savage, Suljo Linic, and the Starbucks staff at Arborland, where most of my editing of this book was accomplished.
Laura Bracken is very much a part of this book. I appreciate her excellent deciphering of equations and scribbles, her organization, her finding mistakes and inconsistencies, and her attention to detail in working with the galley and copyedited proofs. Through all this was her ever-present wonderful disposition. Thanks, Radar!!
Finally, to my wife Janet, love and thanks. She was a sounding board for so many things in this edition. For example, I would ask her, “Is this the correct phrase or word to use here” or “Is this sentence clear?” Sometimes she would reply, “Perhaps, but only if the reader happens to be clairvoyant.” Jan also helped me learn that creativity also involves knowing what to leave out. Without her enormous help and support the project would never have been possible.
HSF
Ann Arbor
For updates on the DVD and new and exciting applications, see the Web sites:
or

For typographical errors, click on Updates & FAQ on the Home page to find

