6.2.1 Liquid Phase
For liquid-phase reactions, the density remains constant and consequently there is no change in either the volume V or the volumetric flow rate υ = υ0 during the course of the reaction. Therefore concentration is the preferred design variable. The mole balances derived in Chapter 1 (Table S-1) are now applied to each species for the generic reaction
![]()
The mole balances are then coupled to one another using the relative rates of reaction
![]()
Used to couple the mole balances.
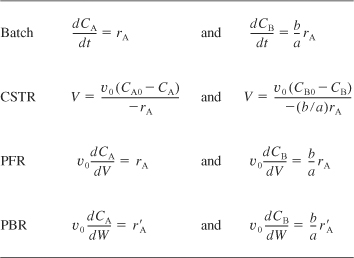
to arrive at Table 6-1, which gives the balance equations in terms of concentration for the four types of reactors we have been discussing. We see from Table 6-1 that we have only to specify the parameter values for the system (CA0, υ0, etc.) and for the rate law parameters (e.g., kA, α, β) to solve the coupled ordinary differential equations for either PFR, PBR, or batch reactors, or to solve the coupled algebraic equations for a CSTR.
Table 6-1. Mole Balances For Liquid-Phase Reactions
LIQUIDS