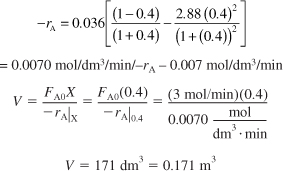4.2.3 Gas Phase Concentrations
In our previous discussions, we considered primarily systems in which the reaction volume or volumetric flow rate did not vary as the reaction progressed. Most batch and liquid-phase and some gas-phase systems fall into this category. There are other systems, though, in which either V or υ do vary, and these will now be considered.
A situation where one encounters a varying flow rate occurs quite frequently in gas-phase reactions that do not have an equal number of product and reactant moles. For example, in the synthesis of ammonia,
![]()
4 mol of reactants gives 2 mol of product. In flow systems where this type of reaction occurs, the molar flow rate will be changing as the reaction progresses. Because equal numbers of moles occupy equal volumes in the gas phase at the same temperature and pressure, the volumetric flow rate will also change.
In the stoichiometric tables presented on the preceding pages, it was not necessary to make assumptions concerning a volume change in the first four columns of the table (i.e., the species, initial number of moles or molar feed rate, change within the reactor, and the remaining number of moles or the molar effluent rate). All of these columns of the stoichiometric table are independent of the volume or density, and they are identical for constant-volume (constant-density) and varying-volume (varying-density) situations. Only when concentration is expressed as a function of conversion does variable density enter the picture.
Flow Reactors with Variable Volumetric Flow Rate
To derive the concentrations of each species in terms of conversion for a gas phase flow system, we shall use the relationships for the total concentration. The total concentration, CT, at any point in the reactor is the total molar flow rate, FT, divided by volumetric flow rate υ [cf. Equation (4-10)]. In the gas phase, the total concentration is also found from the gas law, CT = P/ZRT. Equating these two relationships gives
![]()
At the entrance to the reactor,
![]()
Taking the ratio of Equation (4-14) to Equation (4-15) and assuming negligible changes in the compressibility factor, Z, during the course of the reaction we have upon rearrangement
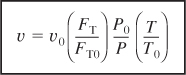
We can now express the concentration of species j for a flow system in terms of its flow rate, Fj, the temperature, T, and total pressure, P.

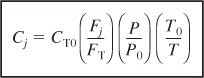
Use this concentration equation for membrane reactors (Chapter 6) and for multiple reactions (Chapter 8).
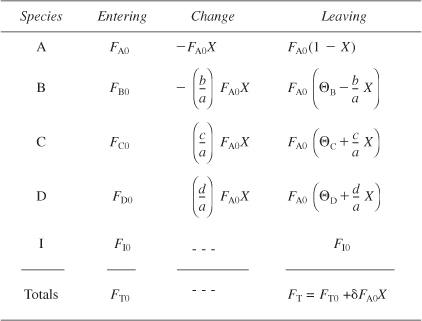
The total molar flow rate is just the sum of the molar flow rates of each of the species in the system and is
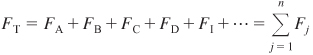
The molar flow rates, Fj, are found by solving the mole balance equations. The concentration given by Equation (4-17) will be used for measures other than conversion when we discuss membrane reactors (Chapter 6) and multiple gas-phase reactions (Chapter 8).
Now let’s express the concentration in terms of conversion for gas flow systems. From Table 4-2, the total molar flow rate can be written in terms of conversion and is
![]()
We divide Equation (4-19) through by FT0:
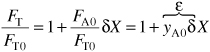
![]()
where yA0 is the mole fraction of A at the inlet, (i.e., (FA0/FT0)) and where δ is given by Equation (4-1) and ε is given by

Equation (4-21) holds for both batch and flow systems. To interpret ε, let’s rearrange Equation (4-20) at complete conversion, (i.e., X = 1 and FT = FTf)
![]()
![]()
Substituting for (FT/FTO) in Equation (4-16) for the volumetric flow rate, υ, we have
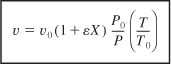
The concentration of species j in a flow system is
![]()
The molar flow rate of species j is
Fj = Fj0 + υj(FA0X) = FA0(Θj + υjX)
where Vi is the stoichiometric coefficient, which is negative for reactants and positive for products. For example, for the reaction
![]()
υA = –1, υB = –b/a, υC = c/a, υD = d/a and Θi = Fj0/FA0.
Substituting for υ using Equation (4-23) and for Fj, we have

Rearranging

Recall that yA0 = FA0/FT0, CA0 = yA0CT0, and ε is given by Equation (4-21) (i.e., ε = yA0δ).
The stoichiometric table for the gas-phase reaction (2-2) is given in Table 4-3.
Table 4-3. Concentrations in a Variable-Volume Gas Flow System

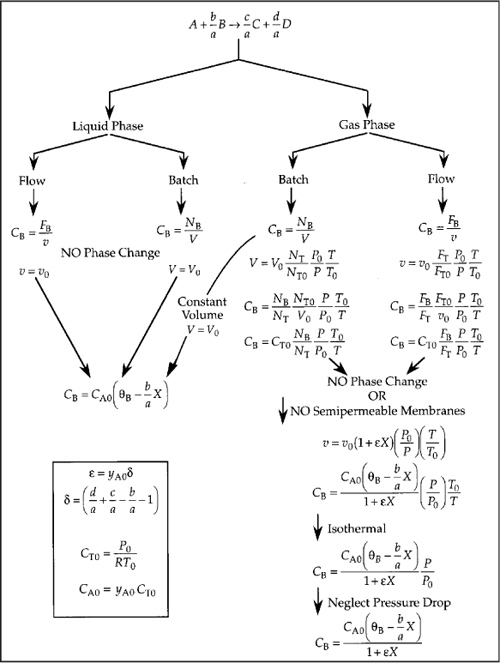
One of the major objectives of this chapter is to learn how to express any given rate law –rA as a function of conversion. The schematic diagram in Figure 4-3 helps to summarize our discussion on this point. The concentration of the key reactant, A (the basis of our calculations), is expressed as a function of conversion in both flow and batch systems, for various conditions of temperature, pressure, and volume.
Figure 4-3. Expressing concentration as a function of conversion.
Example 4-3. Determining Cj = hj (X) for a Gas-Phase Reaction
A mixture of 28% SO2 and 72% air is charged to a flow reactor in which SO2 is oxidized.
![]()
First, set up a stoichiometric table using only the symbols (i.e., Θi, Fi) and then prepare a second table evaluating the species concentrations as a function of conversion for the case when the total pressure is 1485 kPa (14.7 atm) and the temperature is constant at 227°C.
Taking SO2 as the basis of calculation, we divide the reaction through by the stoichiometric coefficient of our chosen basis of calculation:
![]()
The stoichiometric table is given as Table E4-3.1.
Table E4-3.1. Stoichiometric Table for ![]()
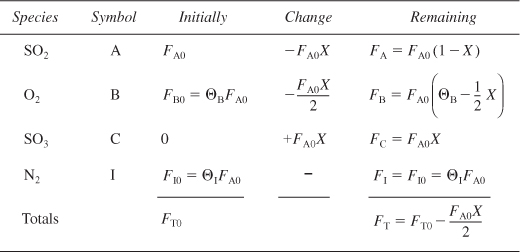
Initially, 72% of the total number of moles is air containing 21% O2 and 79% N2, along with 28% SO2.

From the definition of conversion, we substitute not only for the molar flow rate of SO2 (A) in terms of conversion but also for the volumetric flow rate as a function of conversion.
![]()
Recalling Equation (4-23), we have

Neglecting pressure drop in the reaction, P = P0, yields
![]()
If the reaction is also carried out isothermally, T = T0, we obtain
υ = υ0(1 + εX)

The inlet concentration of A is equal to the inlet mole fraction of A multiplied by the total inlet molar concentration. The total concentration can be calculated from an equation of state such as the ideal gas law. Recall that yA0 = 0.28, T0 = 500 K, and P0 = 1485 kPa.
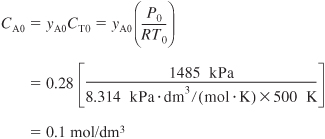
The total concentration is
![]()
![]()
We now evaluate ε.
![]()
Substituting for CA0 and ε in the species concentrations:
![]()
![]()
![]()
![]()
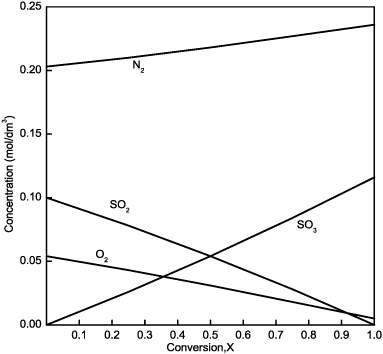
The concentrations of different species at various conversions are calculated in Table E4-3.1 and plotted in Figure E4-3.1. Note that the concentration of N2 is changing even though it is an inert species in this reaction!!
Table E4-3.2. Concentration as a Function of Conversion

Figure E4-3.1. Concentration as a function of conversion.
We are now in a position to express –rA as a function of X and use the techniques in Chapter 2. However, we will use a better method to solve CRE problems, namely the Polymath software, discussed in the next chapter.
Note: Because the volumetic flow rate varies with conversion, υ = υ0(1 – 0.14X), the concentration of inerts (N2) is not constant.
Now use techniques presented in Chapter 2 to size reactors.
Analysis: In this example, we formed a stoichiometric table in terms of molar flow rates. We then showed how to express the concentrations of each species in a gas phase reaction in which there is a change in the total number of moles. Next, we plotted each species concentration as a function of conversion and noted that the concentration of the inert, N2, was not constant but increased with increasing conversion because of the decrease in the total molar flow rate, FT, with conversion.
Example 4-4. Expressing the Rate Law for SO2 Oxidation in Terms of Partial Pressures and Conversions
The SO2 oxidation discussed in Example 4-3 is to be carried out over a solid platinum catalyst. As with almost all gas-solid catalytic reactions, the rate law is expressed in terms of partial pressures instead of concentrations. The rate law for this SO2 oxidation was found experimentally to be1

Where Pi (atm) is the partial pressure of species i. The reaction is to be carried out isothermally at 400°C. At this temperature the rate constant k, the adsorption constants for O2 (Ko2) and SO2 (Kso2) and the pressure equilibrium constant, KP, were found to be experimentally to be:
k = 9.7 mol SO2/atm3/2/h/g catalyst, Ko2 = 38.5 atm–1, Kso2 = 42.5 atm–1, and KP = 930atm–1/2
The total pressure and the feed composition (e.g., 28% SO2) are the same as in Example 4-3. Consequently, the entering partial pressure of SO2 is 4.1 atm. There is no pressure drop.
Write the rate law as a function of conversion.
No Pressure Drop and Isothermal Operation
For SO2
First we need to recall the relationship between partial pressure and concentration, followed by the relationship between concentration and conversion. Because we know how to express concentration as a function of conversion, we know how to express partial pressure as a function of conversion.
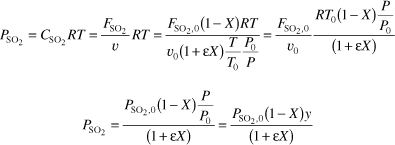
For no pressure drop P = P0, y = 1
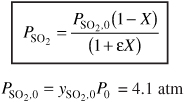
For SO3
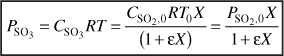
For O2

From Example 4-3
ΘB = 0.54
Factoring out ![]() in Equation (E4-4.5) gives
in Equation (E4-4.5) gives
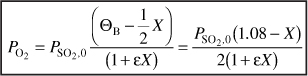
From Equation (E4-3.5)
![]()
Substitute for the partial pressure in the rate law Equation (E4-4.1)
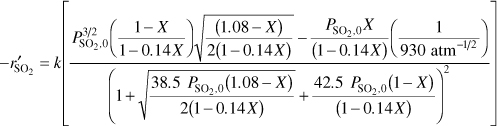
with k = 9.7 mol SO2/atm3/2/h/g cat PSO2, 0 = 4.1 atm, ![]()

We could now use a Levenspiel plot to find the catalyst weight W in a packed-bed reactor (PBR) to achieve a specified conversion.
![]()
Figure E4-4.1. Reciprocal rate of SO2 oxidation as a function of conversion.
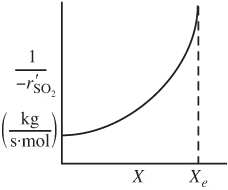
However, we will see in the next chapter there is a much, much better way to solve for the catalysis weight, W, by using numerical software packages. For example, we would couple Equation (E4-4.6) with Equation (2-17) and use an ordinary differential equation (ODE) solver, such as Polymath to find the conversion X as a function of catalyst weight W.
Analysis: In most heterogeneous catalytic reactions, rate laws are expressed in terms of partial pressures instead of concentration. However, we see that through the use of the ideal gas law we could easily express the partial pressure as a function of concentration then conversion in order to express the rate law as a function of conversion. In addition, for most all heterogeneous reactions you will usually find a term like (1 + KAPA + KBPB + . . .) in the denominator of the rate law, as will be explained in Chapter 10.
Thus far in this chapter, we have focused mostly on irreversible reactions. The procedure one uses for the isothermal reactor design of reversible reactions is virtually the same as that for irreversible reactions, with one notable exception. The maximum conversion that can be achieved at the reaction temperature is the equilibrium conversion, Xe. In the following example it will be shown how our algorithm for reactor design is easily extended to reversible reactions.
Example 4-5. Calculating the Equilibrium Conversion
The reversible gas-phase decomposition of nitrogen tetroxide, N2O4, to nitrogen dioxide, NO2,
![]()
is to be carried out at constant temperature. The feed consists of pure N2O4 at 340 K and 202.6 kPa (2 atm). The concentration equilibrium constant, KC, at 340 K is 0.1 mol/dm3 and the rate constant kN2O4 is 0.5min–1.
a. Calculate the equilibrium conversion of N2O4 in a constant-volume batch reactor.
b. Calculate the equilibrium conversion of N2O4 in a flow reactor.
c. Assuming the reaction is elementary, express the rate of reaction solely as a function of conversion for a flow system and for a batch system.
d. Determine the CSTR volume necessary to achieve 80% of the equilibrium conversion.
![]()
At equilibrium the concentrations of the reacting species are related by the relationship dictated by thermodynamics [see Equation (3-10) and Appendix C].
![]()
a. Batch System—Constant Volume, V = V0.
Table E4-5.1. Stoichiometric Table

For batch systems Ci = Ni/V,
E4-5.2
![]()
E4-5.3


At equilibrium, X = Xe, and we substitute Equations (E4-5.2) and (E4-5.3) into Equation (E4-5.1),
![]()
E4-5.4
![]()
We will use the software package Polymath to solve for the equilibrium conversion and let xeb represent the equilibrium conversion in a constant-volume batch reactor. Equation (E4-5.4) written in Polymath format becomes
f(xeb) = xeb – [kc*(1 – xeb)/(4*cao)] ^0.5
The Polymath program and solution are given in Table E4-5.2.
When looking at Equation (E4-5.4), you probably asked yourself, “Why not use the quadratic formula to solve for the equilibrium conversion in both batch and flow systems?” That is,
Batch: ![]()
Flow: 
The answer is that future problems will be nonlinear and require Polymath solutions; therefore, this simple exercise increases the reader’s ease in using Polymath.
There is a Polymath tutorial in the Summary Notes of Chapter 1.
Table E4-5.2. Polymath Program and Solution for Both Batch and Flow Systems

The equilibrium conversion in a constant-volume batch reactor is

Note: A tutorial on Polymath can be found in the summary notes of Chapter 1.
b. Flow system. The stoichiometric table is the same as that for a batch system except that the number of moles of each species, Ni, is replaced by the molar flow rate of that species, Fi. For constant temperature and pressure, the volumetric flow rate is υ = υ0(1 + εX), and the resulting concentrations of species A and B are
E4-5.5
![]()
E4-5.6
![]()
At equilibrium, X = Xe, we can substitute Equations (E4-5.5) and (E4-5.6) into Equation (E4-5.1) to obtain the expression

Simplifying gives
E4-5.7
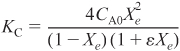
Rearranging to use Polymath yields
E4-5.8

For a flow system with pure N2O4 feed, ε = yA0 δ = 1(2 – 1) = 1.
We shall let Xef represent the equilibrium conversion in a flow system. Equation (E4-5.8) written in the Polymath format becomes
f(Xef) = Xef – [kc*(1 –Xef)*(1 + eps*Xef)/4/cao]^0.5
This solution is also shown in Table E4-5.2 ![]() .
.
Note that the equilibrium conversion in a flow reactor (i.e., Xef = 0.51), with no pressure drop, is greater than the equilibrium conversion in a constant-volume batch reactor (Xeb = 0.44). Recalling Le Châtelier’s principle, can you suggest an explanation for this difference in Xe?
c. Rate laws. Assuming that the reaction follows an elementary rate law, then
E4-5.9

- For a constant volume (V = V0) batch system.
Here CA = NA / V0 and CB = NB / V0. Substituting Equations (E4-5.2) and (E4-5.3) into the rate law, we obtain the rate of disappearance of A as a function of conversion:
E4-5.10
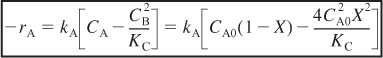
- For a flow system.
Here CA = FA/υ and CB = FB/υ with υ = υ0 (1 + εX). Consequently, we can substitute Equations (E4-5.5) and (E4-5.6) into Equation (E4-5.9) to obtain
E4-5.11

As expected, the dependence of reaction rate on conversion for a constant-volume batch system [i.e., Equation (E4-5.10)] is different than that for a flow system [Equation (E4-5.11)] for gas-phase reactions.
If we substitute the values for CA0, KC, ε, and kA = 0.5 min–1 in Equation (E4-5.11), we obtain –rA solely as a function of X for the flow system.

E4-5.12

We can now prepare our Levenspiel plot.
Figure E4-5.1. Levenspiel plot for a flow system.
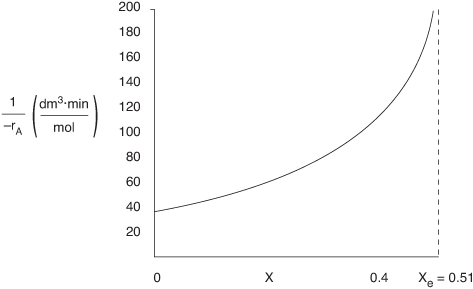
We see (1/–rA) goes to infinity as X approaches Xe.
d. CSTR volume. Just for fun (and this really is fun), let’s calculate the CSTR reactor volume necessary to achieve 80% of the equilibrium conversion of 51% (i.e., X = 0.8Xe = (0.8)(0.51) = 0.4) for a molar feed rate of A of 3 mol/min.
Analysis: The purpose of this example was to calculate the equilibrium conversion first for a constant volume batch system in part (a), and then for a constant pressure flow reaction in part (b). One notes that there is a change in the total number of moles in this reaction and, as a result, these two equilibrium conversions are not the same!! We next showed how to express –rA = f(X) for a reversible-gas-phase reaction. Finally, in Part (d) having –rA = f(X), we specified a molar flow rate of A (e.g., 3.0 mol A/min) and calculated the CSTR volume necessary to achieve 40% conversion. We did this calculation to give insight to the types of analyses we as chemical reaction engineers will carry out as we move into similar but more complex calculations in Chapters 5 and 6.
• Mole Balance, Ch 1
• Rate Law, Ch 3
• Stoichiometry, Ch 4
• Combine, Ch 5
• Evaluate, Ch 5
• Energy Balance, Ch 11
Summary
- The stoichiometric table for the reaction given by Equation (S4-1) being carried out in a flow system is
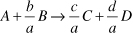
- In the case of ideal gases, Equation (S4-3) relates volumetric flow rate to conversion.

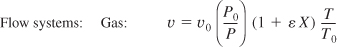

For the general reaction given by (S4-1), we have
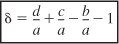

and
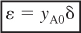
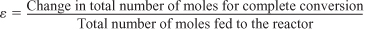
- For incompressible liquids υ = υ0, the concentrations of species A and C in the reaction given by Equation (S4-1) can be written as

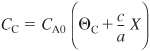
Equations (S4-7) and (S4-8) also hold for gas-phase reactions carried out at constant volume in batch systems.
- For gas-phase reactions, we use the definition of concentration (CA = FA/υ) along with the stoichiometric table and Equation (S4-3) to write the concentration of A and C in terms of conversion.
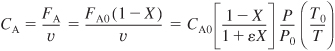

with

- In terms of gas-phase molar flow rates, the concentration of species i is
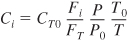
Equation (S4-11) must be used for membrane reactors (Chapter 6) and for multiple reactions (Chapter 8).
DVD-ROM Material
• Learning Resources
- Summary Notes for Chapter 4

- Interactive Computer Games
A. Quiz Show II


- Solved Problems
A. CDP4-BB Microelectronics Industry and the Stoichiometric Table
• Living Example Problems
- Example 4-5 Calculating the Equilibrium Conversion
• FAQ [Frequently Asked Questions]—In Updates/FAW icon section
• Professional Reference Shelf
Questions and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
![]()

a. List the important concepts that you learned from this chapter. What concepts are you not clear about?
b. Explain the strategy to evaluate reactor design equations and how this chapter expands on Chapters 2 and 3.
a. Example 4-1. Would the example be correct if water were considered an inert? Explain.
b. Example 4-2. How would the answer change if the initial concentration of glyceryl stearate were 3 mol/dm3?
c. Example 4-3. Under what conditions will the concentration of the inert nitrogen be constant? Plot Equation (E4-5.2) in terms of (1/–rA) as a function of X up to value of X = 0.99. What did you find?
d. Example 4-4. The entering flow rate of SO2 is 1,000 mol/h. Plot ![]() as a function of X to determine the PBR catalyst weight to achieve (1) 30% conversion, (2) 60% conversion, and (3) 99% conversion.
as a function of X to determine the PBR catalyst weight to achieve (1) 30% conversion, (2) 60% conversion, and (3) 99% conversion.
e. Example 4-5. Why is the equilibrium conversion lower for the batch system than the flow system? Will this always be the case for constant volume batch systems? For the case in which the total concentration CT0 is to remain constant as the inerts are varied, plot the equilibrium conversion as a function of mole fraction of inerts for both a PFR and a constant-volume batch reactor. The pressure and temperature are constant at 2 atm and 340 K. Only N2O4 and inert I are to be fed.
Load the Interactive Computer Games (ICG) Kinetic Challenge from the DVD-ROM. Play the game, and then record your performance number for the module which indicates your mastering of the material. Your professor has the key to decode your performance number. ICG Kinetics Challenge Performance # ______________.
Stoichiometry. The elementary gas reaction
4A + 2B → 2C
is carried out isothermally in a PFR with no pressure drop. The feed is equal molar in A and B and the entering concentration of A is 0.1 mol/dm3.
a. What is the entering concentration (mol/dm3) of B?
b. What are the concentrations of A and C (mol/dm3) at 25% conversion of A?
c. What is the concentration of B (mol/dm3) at 25% conversion of A?
d. What is the concentration of B (mol/dm3) at 100% conversion of A?
e. If at a particular conversion the rate of formation of C is 2 mol/min/dm3, what is the rate of formation of A at the same conversion?
Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction as a function of conversion, evaluating all constants (e.g., ε, Θ). Next, assume the reaction follows an elementary rate law, and write the reaction rate solely as a function of conversion, i.e., –rA = f(X).
a. For the liquid-phase reaction

the entering concentrations of ethylene oxide and water, after mixing the inlet streams, are 16.13 mol/dm3 and 55.5 mol/dm3, respectively. The specific reaction rate is k = 0.1 dm3/mol·s at 300 K with E = 12,500 cal/mol.
- After finding –rA = f(X), calculate the CSTR space-time, τ, for 90% conversion at 300 K and also at 350 K.
- If the volumetric flow rate is 200 liters per second, what are the corresponding reactor volumes?
b. For the isothermal, isobaric gas-phase pyrolysis
![]()
pure ethane enters the flow reactor at 6 atm and 1100 K, write –rA = f(X). How would your equation for the concentration and reaction rate, i.e., –rA = f (X), change if the reaction were to be carried out in a constant-volume batch reactor?
c. For the isothermal, isobaric, catalytic gas-phase oxidation
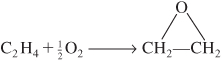
the feed enters a PBR at 6 atm and 260°C and is a stoichiometric mixture of only oxygen and ethylene.
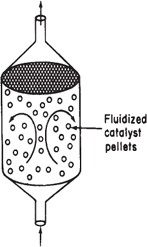
d. For the isothermal, isobaric, catalytic gas-phase reaction carried out in a fluidized CSTR
![]()
the feed enters at 6 atm and 170°C and is a stoichiometric mixture. What catalyst weight is required to reach 80% conversion in a fluidized CSTR at 170°C and at 270°C? The rate constant is defined with respect to benzene and υ0 = 50 dm3/min.
![]()
The formation of orthonitroanaline (an important intermediate in dyes—called fast orange) is formed from the reaction of orthonitrochlorobenzene (ONCB) and aqueous ammonia. (See Table 3-1 and Example 13-2.)

The liquid-phase reaction is first order in both ONCB and ammonia with k = 0.0017 m3/kmol · min at 188°C with E = 11,273 cal/mol. The initial entering concentrations of ONCB and ammonia are 1.8 kmol/m3 and 6.6 kmol/m3, respectively (more on this reaction in Chapter 13).

Cell growth takes place in bioreactors called chemostats.2 (cf. Chapter 9.)

A substrate such as glucose is used to grow cells and produce a product:
![]()
A generic molecule formula for the biomass is C4.4H7.3N0.86O1.2. Consider the growth of a generic organism on glucose
C6H12O6 + aO2 + bNH3 → c(C4.4H7.3N0.86O1.2) + dH2O + eCO2
Experimentally, it was shown that for this organism, the cells convert 2/3 of carbon substrate to biomass. (More on this in Chapter 9.)
a. Calculate the stoichiometric coefficients a, b, c, d, and e (Hint: carry out atom balances [Ans: c = 0.91]).
b. Calculate the yield coefficients YC/S (g cells/g substrate) and YC/O2 (g cells /g O2). The gram of cells are dry weight (no water—gdw) (Ans: YC/O2 = 1.77 gdw cells/g O2) (gdw = grams dry weight).
The gas-phase reaction
![]()
is to be carried out isothermally. The molar feed is 50% H2 and 50% N2, at a pressure of 16.4 atm and at a temperature of 227°C.
a. Construct a complete stoichiometric table.
b. What are CA0, δ, and ε? Calculate the concentrations of ammonia and hydrogen when the conversion of H2 is 60%. (Ans: = CH2 = 0.1 mol/dm3)
c. Suppose by chance the reaction is elementary with kN2 = 40 dm3/mol/s. Write the rate of reaction solely as a function of conversion for (1) a flow system and for (2) a constant volume batch system.
Calculate the equilibrium conversion and concentrations for each of the following reactions.
a. The liquid-phase reaction
![]()
with CA0 = CB0 = 2 mol/dm3 and KC = 10 dm3/mol.
b. The gas-phase reaction
![]()
carried out in a flow reactor with no pressure drop. Pure A enters at a temperature of 400 K and a pressure of 10 atm. At this temperature, KC = 0.25(mol/dm3)2.
c. The gas-phase reaction in part (b) carried out in a constant-volume batch reactor.
d. The gas-phase reaction in part (b) carried out in a constant-pressure batch reactor.
Consider a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring (Figure P4-10C). The reaction
![]()
with the rate expression
![]()

is taking place in this type of reactor.

Equal moles of A and B are present at t = 0
Initial volume: 0.15 ft3
Value of k1: 1.0 (ft3/lb mol)2·s–1
The relationship between the volume of the reactor and pressure within the reactor is
V = (0.1) (P) (V in ft3, P in atm)
Temperature of system (considered constant): 140°F
Gas constant: 0.73 ft3 · atm/lb mol · °R
a. Write the rate law solely as a function of conversion, numerically evaluating all possible symbols. (Ans.: –rA = 5.03 × 10–9 [(1 – X)3/(1 + 3X)3/2] lb mol/ft3· s.)
b. What is the conversion and rate of reaction when V = 0.2 ft3? (Ans.: X = 0.259, –rA = 8.63 × 10–10 lb mol/ft3·s.)
Additional information:
Equal moles of A and B are present at t = 0
Initial volume: 0.15 ft3
Value of k1: 1.0 (ft3/lb mol)2·s–1
The relationship between the volume of the reactor and pressure within the reactor is
V = (0.1) (P) (V in ft3, P in atm)
Temperature of system (considered constant): 140°F
Gas constant: 0.73 ft3·atm/lb mol·°R
What four things are wrong with this solution? The gas phase reaction
![]()
follows an elementary rate law as written and is carried out in a flow reactor operated isothermally at 427°C and 28.7 atmospheres. Pressure drop can be neglected. Express the rate law and the concentration of each species solely as a function of conversion. The specific reaction rate is 200 dm12/mol4·s and the feed is equal molar in A and B.
Solution
![]()
Because A is the limiting reactant, we divide through by its stoichiometry coefficient
![]()
So the elementary rate law is
![]()
Equal molar yA0 = 1: ε = yA0δ = 3 + 5 – 2 – 3 = 3
![]()
Equal molar feed in A and B, therefore
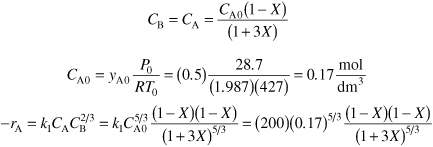
• Additional Homework Problems on DVD-ROM
A number of homework problems that can be used for exams or supplementary problems or examples are found on the DVD-ROM and on the CRE Website, http://www.engin.umich.edu/~cre. Also check out the Diet Coke YouTube video. There’s a link in the Chapter 5 Summary Notes.

New Problems on the Web
CDP4-New
From time to time new problems relating Chapter 4 material to everyday interests or emerging technologies will be placed on the web. Solutions to these problems can be obtained by emailing the author.
Supplementary Reading
For further elaboration of the development of the general balance equation, see not only the web site www.engin.umich.edu/~cre but also
FELDER, R. M., and R. W. ROUSSEAU, Elementary Principles of Chemical Processes, 3rd ed. New York: Wiley, 2000, Chapter 4.
HIMMELBLAU, D. M., and J. D. RIGGS, Basic Principles and Calculations in Chemical Engineering, 7th ed. Upper Saddle River, NJ: Prentice Hall, 2004, Chapters 2 and 6.
KEILLOR, GARRISON and TIM RUSSELL, Dusty and Lefty, the Lives of the Cowboys (Audio CD), St. Paul, MN: Highbridge Audio, 2006.